Abstract
Spleen-preservation has recently been emphasized in benign and borderline malignant pancreatic diseases requiring distal pancreatectomy. Reports to suggest that laparoscopic distal pancreatectomy is feasible and safe have been increasingly published. Robotic surgical system has been introduced and is expected to provide unique advantages in laparoscopic surgery. However, robot-assisted pancreatic surgery has not yet been performed by many surgeons. A 45-year-old female patient with abdominal discomfort was found to have pancreatic cyst in the body of the pancreas. Mucinous cystic tumor of the pancreas was the most favourable preoperative diagnosis. She underwent spleen-preserving laparoscopic distal pancreatectomy by using da Vinci surgical robot system. Splenic artery and vein were so tightly adherent to the pancreatic cyst that segmental resection of splenic vessels was required. Postoperative course was uneventful. She was able to come home in 5 days after surgery. Postoperative follow up color doppler ultrasound scan, taken on 2 weeks after surgery, showed minimal fluid collection around surgical field and no evidence of splenic infarction with good preservation of splenic perfusion. Robot-assisted spleen preserving distal pancreatectomy is thought to be feasible and safe. Several unique advantages of robotic system are expected to enhance safer and more precise surgical performance in near future. More experiences are mandatory to confirm real benefit of robot surgery in pancreatic disease.
Keywords: Robot (da Vinci), laparoscopic, pancreatectomy
INTRODUCTION
Recently, cystic neoplasm of the pancreas has been much more frequently diagnosed, and its treatment seems to be different depending on the types of cystic tumor.1 The benign or borderline malignant cystic neoplasm of pancreas would be expected for long-term survival following surgical resection. Therefore, the value of spleen preservation needs to be emphasized because of life time risk of postspenectomy overwhelming sepsis as well as postoperative infectious morbidity.2 With the advance of laparoscopic experiences, technique, and instrument, the feasibility and safety of laparoscopic distal pancreatectomy has been reported since 1996.3,4 However, restricted ranges of motion and 2-dimentional operative field provided by laparoscopic surgery have restrained rapid spread of laparoscopic pancreatic surgery. However, recent advances in robotic surgical system with multi-articulated, master-slaved, and 3-dimentional surgical field allowed to overcome those obstacles in laparoscopic pancreatic surgery. Surgeons world-wide apparently have performed only a few tele-robotic pancreatic surgeries. Herein, we report our initial experience of da Vinci surgical robot (Intustive Surgical, Mountain View, CA, USA)-assisted laparoscopic spleen-preserving distal pancreatectomy with segmental resection of splenic vessels in mucinous cystic neoplasm of the pancreas.
CASE REPORT
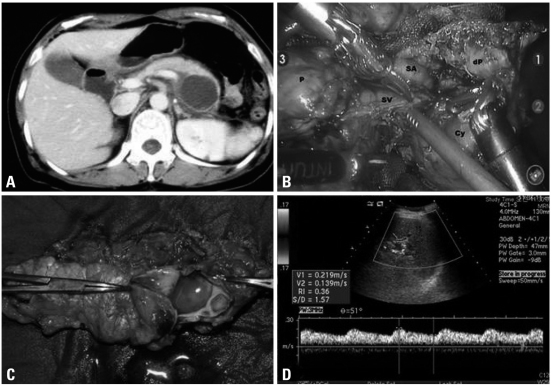
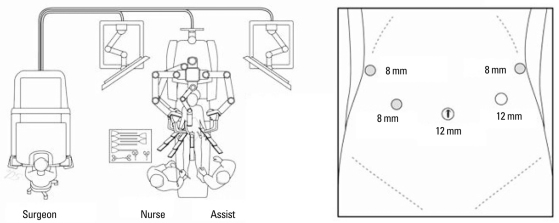
A 46-year-old female patient was admitted to our department due to a cystic tumor in pancreatic body and tail. Routine chemistry and tumor marker levels (CA 19-9, CEA, CA 125) were all within normal limits. Abdominal CT scan and EUS showed about 5×4 cm sized, slightly septated cystic tumor between the body and tail of the pancreas (Fig. 1A). There was no mural nodule, no calcification, and no eccentrically located mass within the cystic area. The benign mucinous cystic tumor of the pancreas was the most favorable preoperative diagnosis. The operation sequence was similar to usual laparoscopic distal pancreatectomy except trocar placement (Fig. 2). The robotic camera arm and other three instrument arms were then connected to their respective ports. At this point, the surgeon sat at the surgical console located about 3 m from the operating table. The assistant surgeon was positioned on the patient's left side for temporary use of laparoscopic instruments through Port 4. In spite of our previous experiences of robot surgical system, the cystic tumor and the remaining splenic vessels were so closely adherent that bleeding was frequently encountered, and most operation time was spent for this process. The risk of cyst rupture was also considered when we tried to separate and divide the small splenic vessels from pancreatic cyst (Fig. 1B). Therefore, concomitant segmental resection of splenic vessels was thought to be necessary for spleen preservation. Total operation took about 480 minutes, and about 800 mL of blood loss was noted without blood transfusion. The specimen was delivered through the umbilical port site with small vertical incision after putting into the plastic bag. The patient recovered without complication. She returned to an oral diet on the postoperative second day. After the drain was removed, she was discharged on the fifth day post-operation. The final pathologic examination revealed that the tumor was benign mucinous cystic neoplasm without any characteristics of malignant potential (Fig. 1C). A color doppler ultrasound scan obtained 14 days after surgery showed no evidence of splenic infarction and well preserved blood flow within the spleen without definitive peripancreatic fluid collection (Fig. 1D).
Fig. 1.
Perioperative data. Preoperative CT scan (A), Laparoscopic view. After division of pancreas, splenic vessels were noted to be tightly adherent to the pancreas. P, proximal pancreas; dP, distal pancreas; Cy, pancreatic cyst; SV, splenic vein; SA, splenic arery (B), Surgical pathology, About 4 cm sized cystic tumor containing clear mucin was removed by distal pancreatectomy. Pathologic examination revealed that this tumor was benign mucinous cystic tumor (C), and follow up color-doppler ultrasound san showed good perfusion to the spleen without any spleen-related complications (D).
Fig. 2.
The schema for port placement and operation room.
DISCUSSION
Robot assisted pancreatic surgery seems to be rarely performed by surgeons world-wide. According to a review of published literatures, robot assisted spleen-preserving distal pancreatectomy is believed to be rare surgical procedure. Since Melvin, et al.5 first reported "robotic resection of pancreatic neuroendocrine tumor" in 2003, Giulianotti, et al.6 reported 13 pancreatic resections in his early series. They reported that the average time to spend was 490 minutes (range, 360-660 minutes) for eight robot assisted laparoscopic pancreaticoduodenectomy and 270 minutes (range, 210-360 minutes) for five left pancreatectomy. Among 5 cases of distal pancreatectomy, only two patients (1 for insulinoma and 1 for benign cystic lesion) received a spleen-preserving resection.
Since pancreas is naturally deep seated (retroperitoneal) solid organ with sufficient blood supply from adjacent great vessels, laparoscopic surgery has been launched relatively late, compared with other surgical field. Laparoscopic distal pancreatectomy is accepted as a feasible and safe minimally invasive procedure in benign and borderline malignant disease of the pancreas. Recently, many reports to suggest favorable results of laparoscopic distal pancreatectomy have been published.
Theoretically, robot can greatly increase the ability to deal with small and delicate branches of the splenic vessels due to superior visualization and precision offered by the da Vinci surgical system. Even though both splenic vessels could not be preserved in this case due to severe inflammatory adhesion between splenic vessels and pancreatic cyst/pancreas and also due to the risk of cyst rupture, free movement of instrument and 3-dimentional surgical field provided very comfortable laparoscopic environment, compared to conventional spleen preserving laparoscopic distal pancreatectomy with segmental resection of both splenic vessels.
With regard to managing pancreatic stump in laparoscopic distal pancreatectomy, we used endo-GIA because this method is simple, quick and known to be safe alternative to the standard suture closure technique.7 By using robot surgical system, the alternative methods seem to be available as an potential effort to reduce pancreatic leak, such as isolation and ligation of main pancreatic duct8,9 and covering of stapled pancreatic remnants with a jejunal seromuscular patch.10 Therefore, laparoscopic distal pancreatectomy can be done by the same surgical procedure as done in open distal pancreatectomy with the help of robotic surgical system, which might lead to lower postoperative morbidity, compared to conventional laparoscopic surgery using endo-GIA only. Further experiences are expected to confirm such possibility in near future.
Several disadvantages of robot surgery were also noted. The surgeons who control robot in surgical console can not feel any touch sensation of grasping tissue. They have to rely only on visual feed-back. In our case, easy bleeding was noted during grasping or traction of pancreas during the trial of division of small splenic vessels from the pancreas. The relatively longer operation time is also potential disadvantage for patients. Surgeons need also an another learning-curve period for adapting themselves to the new surgical system. Another important criticism is higher cost, compared to conventional laparoscopic surgery. During the last two decades, surgeons already established safe laparoscopic technique based on their own experiences. Surgeons may already have overcome the limitation of conventional laparoscopic surgery. In fact, it may not be exaggerating to state that almost all general surgery could be performed by laparoscopic approach in these days. The pancreatic surgery using surgical robot is an emerging technique. The potential advantages provided by surgical robot system may allow for safe and effective laparoscopic distal pancreatectomy. Nevertheless, it remains to be confirmed whether the real clinical benefit of robot assisted pancreatic surgery does exist in current far advanced laparoscopic era. We need more experiences.
ACKNOWLEDGEMENTS
This case was presented as video presentation, "The Korean first experience of Robot assisted spleen-preserving laparoscopic distal pancreatectomy in mucinous cystic tumor of the pancreas" in MIRA 2008 held in Rome, Italy (Jan 25th, 2008).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sarr MG, Murr M, Smyrk TC, Yeo CJ, Fernandez-del-Castillo C, Hawes RH, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417–428. doi: 10.1016/s1091-255x(02)00163-4. [DOI] [PubMed] [Google Scholar]
- 2.Shoup M, Brennan MF, McWhite K, Leung DH, Klimstra D, Conlon KC. The value of splenic preservation with distal pancreatectomy. Arch Surg. 2002;137:164–168. doi: 10.1001/archsurg.137.2.164. [DOI] [PubMed] [Google Scholar]
- 3.Sussman LA, Christie R, Whittle DE. Laparoscopic excision of distal pancreas including insulinoma. Aust N Z J Surg. 1996;66:414–416. doi: 10.1111/j.1445-2197.1996.tb01222.x. [DOI] [PubMed] [Google Scholar]
- 4.Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051–1054. doi: 10.1016/s0039-6060(96)80054-7. [DOI] [PubMed] [Google Scholar]
- 5.Melvin WS, Needleman BJ, Krause KR, Ellison EC. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A. 2003;13:33–36. doi: 10.1089/109264203321235449. [DOI] [PubMed] [Google Scholar]
- 6.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777–784. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 7.Takeuchi K, Tsuzuki Y, Ando T, Sekihara M, Hara T, Kori T, et al. Distal pancreatectomy: is staple closure beneficial? ANZ J Surg. 2003;73:922–925. doi: 10.1046/j.1445-2197.2003.02821.x. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–196. doi: 10.1002/bjs.4032. [DOI] [PubMed] [Google Scholar]
- 9.Pannegeon V, Pessaux P, Sauvanet A, Vullierme MP, Kianmanesh R, Belghiti J. Pancreatic fistula after distal pancreatectomy: predictive risk factors and value of conservative treatment. Arch Surg. 2006;141:1071–1076. doi: 10.1001/archsurg.141.11.1071. discussion 6. [DOI] [PubMed] [Google Scholar]
- 10.Oláh A, Issekutz A, Belágyi T, Hajdú N, Romics L., Jr Randomized clinical trial of techniques for closure of the pancreatic remnant following distal pancreatectomy. Br J Surg. 2009;96:602–607. doi: 10.1002/bjs.6620. [DOI] [PubMed] [Google Scholar]