Abstract
Helicobacter pylori (H. pylori) is an important risk factor for chronic gastritis, peptic ulcer, and gastric cancer. Proteinase-activated receptor 2 (PAR2), subgroup of G-protein coupled receptor family, is highly expressed in gastric cancer, and chronic expression of cyclooxygenase-2 (COX-2) plays an important role in H. pylori-associated gastric carcinogenesis and inflammation. We previously demonstrated that H. pylori induced the expression of PAR2 and COX-2 in gastric epithelial cells. Present study aims to investigate whether COX-2 expression induced by H. pylori in Korean isolates is mediated by PAR2 via activation of Gi protein and Src kinase in gastric epithelial AGS cells. Results showed that H. pylori-induced COX-2 expression was inhibited in the cells transfected with antisense oligonucleotide for PAR2 or treated with Gi protein blocker pertussis toxin, Src kinase inhibitor herbimycin A and soybean trypsin inbitor, indicating that COX-2 expression is mediated by PAR2 through activation of Gi protein and Src kinase in gastric epithelial cells infected with H. pylori in Korean isolates. Thus, targeting the activation of PAR2 may be beneficial for prevention or treatment of gastric inflammation and carcinogenesis associated with H. pylori infection.
Keywords: Helicobacter pylori, cyclooxygenase-2, proteinase-activated receptor 2, AGS cells
H. pylori-associated acute and chronic antral inflammation has been associated with the expression of cyclooxygenase-2 (COX-2) in gastric epithelial cells,1 which is strongly correlated with the extent of chronic inflammatory cell infiltrate.2 Chronic expression of COX-2 is related to H. pylori-associated gastric carcinogenesis in addition to propagation of gastric inflammation since prostaglandins produced via COX-2 are reported to contribute to inflammation3 and carcinogenesis.4 Our previous study demonstrated that oxidant-sensitive transcription factor NF-κB mediates COX-2 expression, which may be related to cell proliferation in gastric epithelial cells.5 H. pylori-induced activation of NF-κB mediates the expression of several genes involved in inflammation, such as IL-86 and adhesion molecules intergrin α5, in gastric epithelial AGS cells.7 Proteinase-activated receptor 2 (PAR2) regulates cell proliferation and enhances COX-2 expression in human pancreatic cancer cells8 and integrin expression in H. pylori-infected gastric epithelial cells.9
Proteinase-activated receptors (PARs) are G protein-coupled receptors that are activated by the cleavage of their N-terminal domains by proteases.10,11 Proteinase-activated receptor 2 (PAR2) activation induces G protein-mediated signal transduction, generation of inositol triphosphate, mobilization of intracellular calcium, the activation of mitogen-activated protein kinase, cell growth, and the release of cytokines (IL-6, IL-8) and prostaglandins.12,13 PAR2 is activated by multiple trypsin-like serine proteases including trypsin and tryptase.13 Inflammatory cytokines (interleukin-1α, tumor necrosis factor-α) increase mRNA expression of PAR2.14 Invasive breast and pancreatic cancer cells express high levels of PAR2 compared with normal cells.15 These studies show the positive relationship between PAR2 expression and carcinogenesis. Previously, we demonstrated that H. pylori induced the expression and activation of PAR2 by stimulating the expression of trypsinogens and trypsin in gastric epithelial AGS cells.9 In addition, inhibition of PAR2 activation by a soyben trypsin inhibitor (SBTI) suppressed H. pylori-induced expression of integrins in gastric epithelial cells.9 Therefore, PAR2 seems to play an important role in H. pylori-associated gastric inflammation and/or carcinogenesis by mediating the induction of inflammatory and carcinogenic genes, including COX-2, in gastric epithelial cells.
In addition, the genetic differences of H. pylori isolates play a role in the clinical outcome of the infection, particularly H. pylori-virulence associated genes such as vacA, cagA, and iceA genes.16 Infection by cagA strain is more likely to result in peptic ulceration, atrophic gastritis, and gastric carcinoma.17,18 Presence of cagA or vacA in H. pylori strain showed different expression of genes as compared to cagA negative or vacA negative H. pylori in gastric epithelial AGS cells.19 The studies suggest that the presence of virulence factors (vacA, cagA, and iceA) and isotypes of each virulence factor (vacA s1b m2, vacA s1c m1, iceA1, iceA2 etc.) in H. pylori strain are important to determine disease incidence related to H. pylori infection. Since the predominant genotype of H. pylori in Korea has been reported to be cagA positive and vacA positive genotype,20 H. pylori in Korean isolates may be used to determine the pathogenic mechanism of H. pylori-induced gastric diseases in Korea.
In the present study, we investigated whether H. pylori-induced COX-2 expression is mediated by PAR2 via activation of Gi protein and Src kinase in gastric epithelial AGS cells. Thus, the cells were transfected with sense oligonucleotide (S ODN) and antisense oligonucleotide (AS ODN) for PAR2 and cultured in the presence of H. pylori. In other sets of experiments, the cells were treated with Gi protein blocker pertussis toxin, Src kinase inhibitor herbimycin A or soybean trypsin inhibitor for 1 hour and cultured in the presence of H. pylori. Subsequently, mRNA and protein expression of COX-2 were determined by RT-PCR analysis and Western blot analysis, respectively. Pertussis toxin inhibits Gi protein by coupling the inhibitory receptors to the adenylate cyclase system.21 Herbimycin A is known to be an irreversible inhibitor of Src kinase.22
We used HP99 which was isolated from gastric antral mucosa of Korean patients with gastric and duodenal ulcer, and identified it as cagA+, vacA s1b, m2, iceA1 H. pylori strain.23 H. pylori was added to human gastric epithelial AGS cells (ATCC CRL 1739, American Type Culture Collection, Manassas, Virginia, USA) at a bacterium/cell ratio of 100 : 1 in a 3 mL volume. The cells were transfected with S ODN and AS ODN for PAR2 for 16 hours and cultured in the presence of H. pylori. In other sets of experiments, the cells were treated with Gi protein blocker pertussis toxin (400 ng/mL), Src kinase inhibitor herbimycin A (10 µM), or soybean trypsin inhibitor (1, 2, 5 nM) for 1 hours and cultured in the presence of H. pylori. mRNA and protein expression of COX-2 were determined by reverse transcription-polymerase chain reaction (RT-PCR) analysis (at 12 hours) and Western blot analysis (at 24 hours), respectively. For ODN preparation, single-stranded ODNs were prepared commercially (GIBCO-BRL, New York, USA). ODNs were phosphorothioate-modified to reduce intracellular nuclease digestion. AS ODN and S ODN target the ATG start codon of the PAR2 mRNA. The sequence of PAR-2 AS ODN was 5'-TCCGCATCCTCCTGGAA-3', and that of PAR2 S ODN was 5'-TTCCAGGAGGATGC GGA-3'. For RT-PCR analysis, the primers used were: PAR2, forward 5'-GATGGCACATCCCACGTC-3', reverse 5'-GGCATGTATGTGATAGGC-3', giving a 288 bp PCR product; COX-2, forward 5'-TTCAAATGAGATTGTGGGAAAATTGCT-3', reverse 5'-AGATCATCTCTGCCTGAGTATCTT-3', giving a 296 bp PCR produc; β-actin, forward 5'-ACCAACTGGGACGACATGGAG-3', reverse 5'-GTGAGGATCTTCATGAGGTAGTC-3', giving a 354 bp PCR product. The PCR products were amplified and visualized by UV transilumination.23 For Western blot analysis, the proteins were detected with polyclonal antibodies for PAR2 (Catalog # SC-13504) and COX-2 (Catalog # SC-19999), perchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), at 1 : 1000 dilation and the immunoreactive proteins were visualized by enhanced chemiluminescence.24
Since mRNA and protein expressions of COX-2 were evident at 12 hours and 24 hours in our previous study,24 in the following experiments using transfection with S ODN and AS ODN for PAR2 or treatment with Gi protein blocker pertussis toxin, Src kinase inhibitor herbimycin A and SBTI, respectively, the time points of 12 hours and 24 hours for mRNA and protein expressions of COX-2, respectively, were used. β-Actin (for mRNA) and actin (for protein) were constitutively expressed in the cells and not changed with culture period.
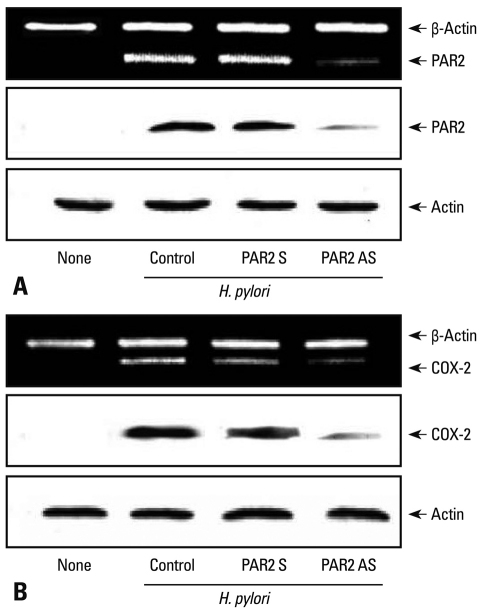
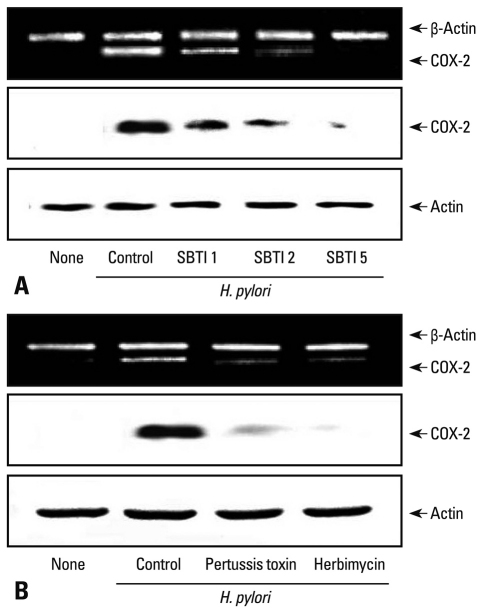
To determine direct involvement of PAR2 in the expression of COX-2, the cells were transfected with PAR2 AS ODN or S ODN and cultured in the presence of H. pylori for 12 hours (mRNA) or 24 hours (protein) (Fig. 1). Transfection efficiency of ODN was determined by observing mRNA and protein levels of PAR2 in the cells transfected (Fig. 1A). H. pylori-induced PAR2 expression was inhibited in the cells transfected with AS ODN, but not changed in those transfected with S ODN. H. pylori-induced expression of COX-2 was similarly inhibited in the cells transfected with AS ODN compared to those in the cells transfected with S ODN (Fig. 1B). Since protein level and the activity of trypsin increased in H. pylori-infected AGS cells,9 trypsin may activate PAR2 in AGS cells. Previously, we showed the increase of intracellular Ca mobilization by PAR2 activation in H. pylori-infected AGS cells,25 which was inhibited by SBTI (data not shown), demonstrating that SBTI in AGS cells suppresses PAR activation induced by H. pylori. Using SBTI, we determined whether SBTI suppresses H. pylori-induced expression of COX-2 in AGS cells. As seen in Fig. 2A, H. pylori-induced expression of COX-2 was inhibited dose-dependently by SBTI. G protein coupled receptor (GPRC) signalings have been shown to be inhibited by pertussis toxin, which inactivates ADP-ribosylation of α subunits of the Gi class in various cells,21,26 and Src family non-receptor tyrosine kinases play important roles in PAR-dependent GPCR signalings.27,28 Therefore, using Gi protein blocker pertussis toxin and Src kinase inhibitor herbimycin A, we examined the involvement of Gi protein and Src kinase in H. pylori-induced COX-2 expression in AGS cells. Fig. 2B shows that pertussis toxin and herbimycin A inhibited the expression of COX-2 in HP99-infected AGS cells. These results demonstrated that Gi protein and Src kinase are involved in PAR2-mediated COX-2 expression in H. pylori-infected gastric epithelial cells. Gi protein is known to mediate the activation of NF-κB in lung cancer cells29 and keratonocytes,30 and the connection of Src kinase to NF-κB has been reported in toll-like receptor-activated signaling31 and epidermal growth factor-induced cell proliferation.32 Since H. pylori induces the activation of NF-κB whose binding site is located in the promoter region of COX-2 gene, both Gi protein and Src kinase may be related to COX-2 expression via NF-κB pathway in addition to PAR2 pathway in H. pylori-infected gastric epithelial cells.
Fig. 1.
H. pylori-induced expressions of PAR2 and COX-2 are inhibited in AGS cells transfected with PAR2 AS ODN. AGS cells were seeded in 6-well culture plates at 5×105 cells per well transfected with S or AS ODNs for PAR-2 for 16 h. The bacterial cells were added to the cultured cells at a bacterium/cell ratio of 100 : 1 for 12 h (for mRNA) or 24 h (for protein). The expressions of mRNA and protein for PAR2 (A) and COX-2 (B) were determined by RT-PCR and Western blotting, respectively. PAR2, proteinase-activated receptor 2; COX-2, cyclooxygenase-2.
Fig. 2.
H. pylori-induced expression of COX-2 is inhibited in AGS cells treated with soybean trypsin inhibitor (SBTI), pertussis toxin and herbimycin A. AGS cells in 6-well culture plates were treated with SBTI (1, 2, 5 nM) (A), or pertussis toxin (400 ng/mL) or herbimycin A (10 µM) (B) for 1 h and cultured in the presence of H. pylori at a bacterium/cell ratio of 100 : 1 for 12 h (for mRNA) or 24 h (for protein). COX-2 mRNA expression levels were determined by RT-PCR and at protein level by Western blotting. COX-2, cyclooxygenase-2.
Recent studies demonstrate that COX-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor.33 COX-2 expression is not associated with the risk of cervical cancer in Korean population.34 Therefore, gastric carcinogenesis or inflammation may not be fully dependent on COX-2 expression in gastric epithelial cells or gastric mucosal tissues. Further study should be performed to determine the role of COX-2 in gastric carcinogenesis or inflammation at molecular and cellular levels. Recent study showed that advanced oxidation protein products were increased in serum of gastric cancer patients.35 However, the activities of antioxidant enzyme catalase in serums were not different between H. pylori-positive and negative patients. Since oxygen radicals are important in transcription of COX-2, the relation between oxidative stress and PAR2-mediated signaling should be investigated. The novel finding of the present study is that H. pylori in Korean isolates induced COX-2 expression, which is mediated by PAR2 through activation of Gi protein and Src kinase in gastric epithelial cells. Specific targeting of the activation of PAR2 may be beneficial for prevention or treatment of H. pylori-associated gastric inflammation and carcinogenesis in Korea.
ACKNOWLEDGEMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0001669) (to H Kim) and the Korea Research Foundation Grant funded by Korea Government (MOEHRD) (KRF-2006-353-F00019) (to H-Y Chung). H Kim is grateful to Brain Korea 21 Project, College of Human Ecology, Yonsei University.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Tsukui T, Miyake K, et al. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000;46:782–789. doi: 10.1136/gut.46.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol. 1999;94:1218–1223. doi: 10.1111/j.1572-0241.1999.01070.x. [DOI] [PubMed] [Google Scholar]
- 3.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556–2560. [PubMed] [Google Scholar]
- 5.Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001;81:349–360. doi: 10.1038/labinvest.3780243. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001;36:706–716. doi: 10.1080/003655201300191969. [DOI] [PubMed] [Google Scholar]
- 7.Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int J Biochem Cell Biol. 2003;35:1284–1296. doi: 10.1016/s1357-2725(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 8.Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- 9.Seo JH, Lim JW, Yoon JH, Kim H. Proteinase-activated receptor-2 mediates the expression of integrin alpha5 and beta1 in helicobacter pylori-infected gastric epithelial AGS cells. Digestion. 2009;80:40–49. doi: 10.1159/000216353. [DOI] [PubMed] [Google Scholar]
- 10.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci U S A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, et al. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998;94:356–362. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003;111:25–27. doi: 10.1172/JCI17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton JR, Frauman AG, Cocks TM. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res. 2001;89:92–98. doi: 10.1161/hh1301.092661. [DOI] [PubMed] [Google Scholar]
- 15.Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001;61:5933–5940. [PubMed] [Google Scholar]
- 16.Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996;5:191–202. [PubMed] [Google Scholar]
- 17.Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 18.Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995;87:1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- 19.Bach S, Makristathis A, Rotter AM, Hirschl M. Gene expression profiling in AGS cells stimulated with Helicobacter pylori isogenic strains (cagA positive or cagA negative) Infect Immun. 2002;70:988–992. doi: 10.1128/IAI.70.2.988-992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moss J, Bruni P, Hsia JA, Tsai SC, Watkins PA, Halpern JL, et al. Pertussis toxin-catalyzed ADP-ribosylation: effects on the coupling of inhibitory receptors to the adenylate cyclase system. J Recept Res. 1984;4:459–474. doi: 10.3109/10799898409042567. [DOI] [PubMed] [Google Scholar]
- 22.Uehara Y, Fukazawa H, Murakami Y, Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989;163:803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- 23.Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49–62. doi: 10.1038/sj.labinvest.3700010. [DOI] [PubMed] [Google Scholar]
- 24.Cho SO, Lim JW, Kim KH, Kim H. Involvement of Ras and AP-1 in Helicobacter pylori-induced expression of COX-2 and iNOS in gastric epithelial AGS cells. Dig Dis Sci. 2010;55:988–996. doi: 10.1007/s10620-009-0828-y. [DOI] [PubMed] [Google Scholar]
- 25.Seo JH, Kim KH, Kim H. Role of proteinase-activated receptor-2 on cyclooxygenase-2 expression in H. pylori-infected gastric epithelial cells. Ann N Y Acad Sci. 2007;1096:29–36. doi: 10.1196/annals.1397.067. [DOI] [PubMed] [Google Scholar]
- 26.LaMorte VJ, Harootunian AT, Spiegel AM, Tsien RY, Feramisco JR. Mediation of growth factor induced DNA synthesis and calcium mobilization by Gq and Gi2. J Cell Biol. 1993;121:91–99. doi: 10.1083/jcb.121.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- 28.Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 29.Seo M, Nam HJ, Kim SY, Juhnn YS. Inhibitory heterotrimeric GTP-binding proteins inhibit hydrogen peroxide-induced apoptosis by up-regulation of Bcl-2 via NF-kappaB in H1299 human lung cancer cells. Biochem Biophys Res Commun. 2009;381:153–158. doi: 10.1016/j.bbrc.2009.01.188. [DOI] [PubMed] [Google Scholar]
- 30.Goon Goh F, Sloss CM, Cunningham MR, Nilsson M, Cadalbert L, Plevin R. G-protein-dependent and -independent pathways regulate proteinase-activated receptor-2 mediated p65 NFkappaB serine 536 phosphorylation in human keratinocytes. Cell Signal. 2008;20:1267–1274. doi: 10.1016/j.cellsig.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol. 2009;182:3522–3529. doi: 10.4049/jimmunol.0802280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh HL, Tung WH, Wu CY, Wang HH, Lin CC, Wang TS, et al. Thrombin induces EGF receptor expression and cell proliferation via a PKC(delta)/c-Src-dependent pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009;29:1594–1601. doi: 10.1161/ATVBAHA.109.185801. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology. 2009;50:834–843. doi: 10.1002/hep.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TS, Jeon YT, Kim JW, Park NH, Kang SB, Lee HP, et al. Lack of association of the cyclooxygenase-2 and inducible nitric oxide synthase gene polymorphism with risk of cervical cancer in Korean population. Ann N Y Acad Sci. 2007;1095:134–142. doi: 10.1196/annals.1397.017. [DOI] [PubMed] [Google Scholar]
- 35.Noyan T, Guducuoglu H, Ilhan M. A study of oxidative stress parameters in anti-helicobacter pylorus immunoglobulin G positive and negative gastric cancer patients. Yonsei Med J. 2009;50:677–682. doi: 10.3349/ymj.2009.50.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]