Abstract
Previously, we reported that epigallocatechin 3-O-gallate (EGCg) has growth-inhibitory effect on clinical isolates of Candida species. In this study, we investigated the antifungal activity of EGCg and antifungal agents against thirty-five of dermatophytes clinically isolated by the international guidelines (M38-A2). All isolates exhibited good susceptibility to EGCg (MIC50, 2-4 µg/mL, MIC90, 4-8 µg/mL, and geometric mean (GM) MICs, 3.36-4 µg/mL) than those of fluconazole (MIC50, 2-16 µg/mL, MIC90, 4-32 µg/mL, and GM MICs, 3.45-25.8 µg/mL) and flucytosin (MIC50, MIC90, and GM MICs, >64 µg/mL), although they were less susceptible to other antifungal agents, such as amphotericin B, itraconazole, and miconazole. These activities of EGCg were approximately 4-fold higher than those of fluconazole, and were 4 to 16-fold higher than flucytosin. This result indicates that EGCg can inhibit pathogenic dermatophyte species. Therefore, we suggest that EGCg may be effectively used solely as a possible agent or combined with other antifungal agents for antifungal therapy in dermatophytosis.
Keywords: Epigallocatechin 3-O-gallate, Dermatophytes, Microsporum canis, Trichophyton mentagrophytes, Trichophyton rubrum, Susceptibility
Dermatophytosis, mycotic infections, is one of among the most common and widespread worldwide infectious diseases and represent an important public health problem yet unresolved. It can be caused by keratinophilic and keratinolytic dermatophytes, particularly Microsporum canis (M. canis), Trichophyton mentagrophytes (T. mentagrophytes), and Trichophyton rubrum (T. rubrum).1-8 Although many antifungal agents have been developed during the last decades and have become available for dermatophytosis, they are confined to a relatively few chemical groups. In addition, the occurrence of resistance or side effects in clinically isolated strains leads to failure in the treatment of mycosis.9 Thus, effective antifungal agents, which are highly effective and safe, are necessary and important for the extermination of antibiotic-susceptible and -resistant strains.
In recent years, there are several reports on antifungal activity of natural products.10-14 Green tea polyphenols have been reported to have an antimicrobial effect against oral, intestinal, and food-borne bacteria, antitoxicity against various bacterial haemolysins, and antiviral activity.15-20 The main polyphenol component of green tea, epigallocatechin 3-O-gallate (EGCg), with direct antibacterial properties, can decrease bacterial invasion by inhibition of bacterial gelatinase activities.21,22 Okubo, et al.23 reported that black tea extract inhibited the growth of Trichophyton mentagrophytes and Trichophyton rubrum, but it was not by EGCg only. Recently, we showed that EGCg has growth-inhibitory effect on clinical isolates of Candida species, but not on dermatophytes.24 In this study, therefore, we investigated the antifungal activity of EGCg and five antifungal agents, such as amphotericin B, flucytosin, fluconazole, itraconazole, and miconazole against clinical isolates of dermatophyte species by determination of minimum inhibitory concentration (MIC).
As described in our previous study,24 all tests performed in RPMI-1640 medium (Sigma, St. Louis, MO, USA) with L-glutamine and low glucose (2 mg/mL), without phenol red and sodium bicarbonate, buffered with 0.165 M 3-(N-morpholino) propanesulfonic acid, pH adjusted to 7.0 with NaOH and sterilized. EGCg was also kindly supplied by Pharma Foods International Co. Ltd. (Kyoto, Japan), and the purity of EGCg exceeded 97%, and the concentration of EGCg from 0.06 to 32 µg/mL was used. Dry plates including five antifungal agents such as amphotericin B (AMPH; 0.03-16 µg/mL), flucytosin (5FC; 0.125-64 µg/mL), fluconazole (FLCZ; 0.125-64 µg/mL), itraconazole (ITCZ; 0.015-8 µg/mL), and miconazole (MCZ; 0.06-32 µg/mL), were purchased from Eiken Chemical Co., LTD. (Tokyo, Japan). Thirty-five clinical isolates of three dermatophyte species (thirteen M. canis strains; IFM 41048, 41054, 41061, 41115, 46053, 53789, 53817, 54153, 54155, 54156, 54157, 54158, 54199, eleven T. mentagrophytes strains; IFM 46600, 46609, 46634, 47174, 47176, 53815, 53931, 55190, 55191, 55192, 55193, and eleven T. rubrum strains; IFM 45623, 45625, 47162, 47163, 47164, 47165, 47166, 47167, 47170, 55188, 55189), and three Candida strains, Candida albicans (ATCC 90028), C. parapsilosis (ATCC 90018), and C. krusei (ATCC 6258) for quality control were obtained from the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University in Japan. All dermatophytes were cultured on potato dextrose agar (PDA) slant at 35℃ for 10 to14 days, whereas three Candida strains were cultured on PDA slant at 35℃ for 24 hours and passaged twice at a 48 hours interval before use.
All standard antifungal susceptibility testing was performed according to the document M38-A2, published by the Clinical and Laboratory Standards Institute (CLSI; formerly National Committee for Clinical Laboratory Standards, or NCCLS).25 As described in our previous study,24 fungal cell suspensions were spectrophotometrically standardized to a turbidity equivalent to that of a 0.5 McFarland standard. The suspensions were prepared from 10 to 14 day cultures grown on PDA, and the density was 2×105 colony forming unit (cfu)/mL. The final inoculum was adjusted to approximately 2×104 cfu/mL. All tests were performed in 96-wells plate. Aliquots of one hundred microliters of suspension were inoculated into each well containing diluted EGCg or five antifungal agents. Drug-free controls and growth controls were included for each assay. The plates were incubated at 35℃ and read visually after 4 to 7 days. The MICs of EGCg and five antifungal agents were defined as the lowest drug concentrations that resulted in a 50% and 90% reduction in growth compared with that of the drug-free growth control, as recommended by the CLSI.25 Geometric mean (GM) MICs were determined to facilitate comparisons of the activities of EGCg and five antifungal agents. Data shown are from three separate tests and were analyzed statistically by calculating means and S.D of the means.
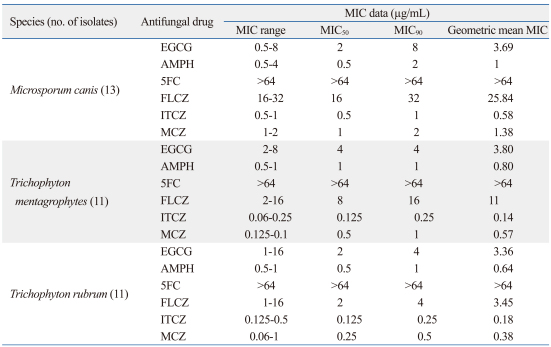
MICs were determined after 7 days of culture because the growth of some strains for 4 days was insufficient. MIC50 and MIC90, the MIC values that inhibited 50 and 90% of isolate growth, as well as the MIC range of EGCg and antifungal agents, AMPH, FLCZ, 5FC, ITCZ, and MCZ, against thirty-five clinical isolates of three dermatophyte species are summarized in Table 1. Although, the susceptibilities were different depending on the types of strains and species, the dermatophytes used in this study were in general susceptible to EGCg. The MIC ranged from 0.5 to 16 µg/mL with all isolates, and the GM MICs were 3.69 µg/mL in M. canis, 3.80 µg/mL in T. mentagrophytes, and 3.36 µg/mL in T. rubrum species. Moreover, all isolates exhibited better susceptibilities to EGCg (MIC50, 2-4 µg/mL, MIC90, 4-8 µg/mL, and GM MICs, 3.36-4 µg/mL) than to FLCZ (MIC50, 2-16 µg/mL, MIC90, 4-32 µg/mL, and GM MICs, 3.45-25.8 µg/mL) and 5FC (MIC50, MIC90, and GM MICs, >64 µg/mL). As shown in Table 1, however, they were slightly less susceptible to other antifungal agents. The GM MICs of EGCg were approximately 7- to 17-fold in M. canis, 3- to 17-fold in T. mentagrophytes, and 1- to 19-fold in T. rubrum higher than those of FLCZ and 5FC. Among EGCg and five antifungal agents tested in this study, only the GM MICs of FLCZ were different among the dermatophyte species tested; As shown in Table 1, M. canis (25.84 µg/mL), T. mentagrophytes (11 µg/mL), and T. rubrum (3.45 µg/mL). Among the antifungal agents tested, ITCZ (MIC50, <0.125-0.5 µg/mL, MIC90, <0.25-1 µg/mL, and GM MICs, 0.14-0.58 µg/mL) had the strongest antifungal activities regardless of the strain.
Table 1.
Antifungal Activities of EGCg and Antifungal Agents Against Clinical Isolates of Dermatophytes
EGCG, Epigallocatechin 3-O-gallate; AMPH, amphotericin B; 5FC, flucytosine; FLCZ, fluconazole; ITCZ, itraconazole; MCZ, miconazole.
Recently, Weitzman and Summerbell1 reported that proliferation of new classes of drugs, such as terbinafine and itraconazole, represents the most remarkable trend in dermatophytosis therapy. Itraconazole have been used effectively.26 However, treatment with these agents for prolonged period requires periodic monitoring of liver activity.27 Moreover, these agents may have drug interactions with other medications.28 In this context, therefore, new antifungal agents from natural products could be useful alternatives for the treatment of dermatophytosis, because they have some advantages, such as reduced risk of side-effects and lower cost, and there has recently been growing interest in the use of medicinally important plants and their compounds to cure some diseases.
EGCg, a main component of tea catechins present in green tea, is known to possess antibacterial activity and the effects of certain antibiotics.29-31 Hirasawa and Takada32 reported the susceptibility of Candida albicans to catechins including EGCg. Recently, we examined anticandidal effect of EGCg under an in vitro condition, and we demonstrated in this study, potent antifungal activity of EGCg against clinical isolates of pathogenic dermatophytes in vitro compared with the other antifungal agents tested, FLCZ and 5FC. Among the dermatophyte species tested, T. rubrum was the most susceptible to EGCg: they were more susceptible than to 5FC and were similar to those of FLCZ, although they were less susceptible than to ITCZ, MCZ, and AMPH. GM MICs of EGCg towards M. canis and T. mentagrophytes were lower and they were more susceptible to EGCg than to FLCZ and 5FC. These results suggest that EGCg can be used as an antifungal agent or adjuvant with antifungal agents in dermatophytosis and can be applied in the field of antifungal therapy, although the dermatophytes were slightly less susceptible to it than to other antifungal agents, such as ITCZ, MCZ, and AMPH. Furthermore, EGCg does not develop resistances and it may avoid the side effects.21,24,30,31
In conclusion, it would seem that EGCg may be used as an antifungal agent solely or in combination with other antifungal agents as previously reported24,32 or as an adjuvant. Although, higher concentration of EGCg than that used in the present study may be needed to treat human dermatophytosis patients, the mechanism of antifungal effects in dermatophytes has not yet been defined, and more studies such as in vivo or ex vivo experiments are needed to verify these possibilities. Nevertheless, the present study showed that EGCg can be applied as an alternate antifungal agent to overcome resistance to other reported antifungal agents, and it may be effectively used as a possible agent or adjuvant for the treatment of dermatophytosis.
ACKNOWLEDGEMENTS
This study was supported by Cooperative Research Program of Medical Mycology Research Center, Chiba University [06-10] in Japan.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Weitzman I, Summerbell RC. The dermatophytes. Clin Microbiol Rev. 1995;8:240–259. doi: 10.1128/cmr.8.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das S, Goyal R, Bhattacharya SN. Laboratory-based epidemiological study of superficial fungal infections. J Dermatol. 2007;34:248–253. doi: 10.1111/j.1346-8138.2007.00262.x. [DOI] [PubMed] [Google Scholar]
- 3.Ginter-Hanselmayer G, Weger W, Ilkit M, Smolle J. Epidemiology of tinea capitis in Europe: current state and changing patterns. Mycoses. 2007;50(Suppl 2):6–13. doi: 10.1111/j.1439-0507.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 4.Hernández-Salazar A, Carbajal-Pruneda P, Fernández Martínez R, Arenas R. [Dermatophytosis due to Trichophyton rubrum. Ten-year period (1996-2006) data collection in Dermatology Department in Mexico City] Rev Iberoam Micol. 2007;24:122–124. doi: 10.1016/s1130-1406(07)70026-8. [DOI] [PubMed] [Google Scholar]
- 5.Porro AM, Yoshioka MC, Kaminski SK, Palmeira Mdo C, Fischman O, Alchorne MM. Disseminated dermatophytosis caused by Microsporum gypseum in two patients with the acquired immunodeficiency syndrome. Mycopathologia. 1997;137:9–12. doi: 10.1023/a:1006806304125. [DOI] [PubMed] [Google Scholar]
- 6.Seyfarth F, Ziemer M, Gräser Y, Elsner P, Hipler UC. Widespread tinea corporis caused by Trichophyton rubrum with non-typical cultural characteristies--diagnosis via PCR. Mycoses. 2007;50(Suppl 2):26–30. doi: 10.1111/j.1439-0507.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarifakioglu E, Seçkin D, Demirbilek M, Can F. In vitro antifungal susceptibility patterns of dermatophyte strains causing tinea unguium. Clin Exp Dermatol. 2007;32:675–679. doi: 10.1111/j.1365-2230.2007.02480.x. [DOI] [PubMed] [Google Scholar]
- 8.Robert R, Pihet M. Conventional methods for the diagnosis of dermatophytosis. Mycopathologia. 2008;166:295–306. doi: 10.1007/s11046-008-9106-3. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Rossi NM, Peres NT, Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia. 2008;166:369–383. doi: 10.1007/s11046-008-9110-7. [DOI] [PubMed] [Google Scholar]
- 10.Mondello F, De Bernardis F, Girolamo A, Salvatore G, Cassone A. In vitro and in vivo activity of tea tree oil against azole-susceptible and -resistant human pathogenic yeasts. J Antimicrob Chemother. 2003;51:1223–1229. doi: 10.1093/jac/dkg202. [DOI] [PubMed] [Google Scholar]
- 11.Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavermicocca P, Valerio F, Visconti A. Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl Environ Microbiol. 2003;69:634–640. doi: 10.1128/AEM.69.1.634-640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertsch J, Tobler RT, Brun R, Sticher O, Heilmann J. Antifungal, antiprotozoal, cytotoxic and piscicidal properties of Justicidin B and a new arylnaphthalide lignan from Phyllanthus piscatorum. Planta Med. 2003;69:420–424. doi: 10.1055/s-2003-39706. [DOI] [PubMed] [Google Scholar]
- 14.Hellio C, Pons AM, Beaupoil C, Bourgougnon N, Gal YL. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int J Antimicrob Agents. 2002;20:214–219. doi: 10.1016/s0924-8579(02)00172-3. [DOI] [PubMed] [Google Scholar]
- 15.Sakanaka S, Kim MJ, Taniguchi M, Yamamoto T. Antibacterial substances in Japanese green tea extract against Streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 1989;53:2307–2311. [Google Scholar]
- 16.Hirasawa M, Takada K, Makimura M, Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res. 2002;37:433–438. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 17.Ahn YJ, Sakanaka S, Kim MJ, Kawamura T, Fujisawa T, Mitsuoka T. Effect of green tea extract on growth of intestinal bacteria. Microb Ecol Health Dis. 1990;3:335–338. [Google Scholar]
- 18.Hara Y, Ishigami T. Antibacterial activities of tea polyphenols against foodborne pathogenic bacteria. J Jpn Soc Food Sci Tech. 1989;36:996–999. [Google Scholar]
- 19.Okubo S, Ikigai H, Toda M, Shimanura T. The anti-haemolysin activity of tea and coffee. Lett Appl Microbiol. 1989;9:65–66. [Google Scholar]
- 20.Nakane H, Ono K. Differential inhibitory effects of some catechin derivatives on the activities of human immunodeficiency virus reverse transcriptase and cellular deoxyribonucleic and ribonucleic acid polymerases. Biochemistry. 1990;29:2841–2845. doi: 10.1021/bi00463a029. [DOI] [PubMed] [Google Scholar]
- 21.Blanco AR, La Terra Mule S, Babini G, Garbisa S, Enea V, Rusciano D. (-)Epigallo-catechin-3-gallate inhibits gelatinase activity of some bacterial isolates from ocular infection, and limits their invasion through gelatine. Biochim Biophys Acta. 2003;1620:273–281. doi: 10.1016/s0304-4165(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 22.Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004;48:1968–1973. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okubo S, Toda M, Hara Y, Shimamura T. [Antifungal and fungicidal activities of tea extract and catechin against Trichophyton] Nippon Saikingaku Zasshi. 1991;46:509–514. doi: 10.3412/jsb.46.509. [DOI] [PubMed] [Google Scholar]
- 24.Park BJ, Park JC, Taguchi H, Fukushima K, Hyon SH, Takatori K. Antifungal susceptibility of epigallocatechin 3-O-gallate (EGCg) on clinical isolates of pathogenic yeasts. Biochem Biophys Res Commun. 2006;347:401–405. doi: 10.1016/j.bbrc.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 25.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved standard-second edition, CLSI document. Wayne, PA, USA: CLSI/NCCLS; 2008. p. M38-A2. [Google Scholar]
- 26.Tejasvi T, Sharma VK, Sethuraman G, Singh MK, Xess I. Invasive dermatophytosis with lymph node involvement in an immunocompetent patient. Clin Exp Dermatol. 2005;30:506–508. doi: 10.1111/j.1365-2230.2005.01839.x. [DOI] [PubMed] [Google Scholar]
- 27.Zapata Garrido AJ, Romo AC, Padilla FB. Terbinafine hepatotoxicity. A case report and review of literature. Ann Hepatol. 2003;2:47–51. [PubMed] [Google Scholar]
- 28.Huang DB, Ostrosky-Zeichner L, Wu JJ, Pang KR, Tyring SK. Therapy of common superficial fungal infections. Dermatol Ther. 2004;17:517–522. doi: 10.1111/j.1396-0296.2004.04055.x. [DOI] [PubMed] [Google Scholar]
- 29.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 30.Jodoin J, Demeule M, Beliveau R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim Biophys Acta. 2002;1542:149–159. doi: 10.1016/s0167-4889(01)00175-6. [DOI] [PubMed] [Google Scholar]
- 31.Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2002;85:57–72. doi: 10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirasawa M, Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J Antimicrob Chemother. 2004;53:225–229. doi: 10.1093/jac/dkh046. [DOI] [PubMed] [Google Scholar]