Abstract
Early HIV-1 infection is marked by rapid evolution of both CD8+ T lymphocyte (CTL) epitope targeting and viral sequences, while chronic infection demonstrates relative stability of these parameters. To examine the interactions of changing CTL targeting and viremia in early infection, we assessed CTL targeting and viremia levels in persons during early HIV-1 infection (estimated 15–271 days post-infection) who were placed on effective antiretroviral therapy. Pre-therapy, CTL targeting of viral proteins varied between persons depending on time after infection. Across individuals, increasing time after infection was associated with increasing Gag and Pol targeting, suggesting increasing targeting of conserved sequences. The intensity of Gag targeting correlated to lower viremia levels, while Env targeting correlated to higher viremia levels during early infection. This suggested that shifted targeting towards more conserved sequences is involved with the drop of viremia during early infection, consistent with prior observations of correlation between Gag targeting and lower viremia during chronic infection. After suppressive antiretroviral therapy, CTL targeting was generally static, indicating that HIV-1 replication and evolution drives the evolution of CTL targeting in early infection. Overall, these data suggest that early CTL targeting is directed towards more variable epitopes, causing escape and re-targeting until more conserved epitopes are recognized stably in chronic infection. Circumventing this natural history by pre-targeting CTL against more conserved epitopes with a vaccine could minimize the initial period of viral escape and immune damage during acute infection, improving long-term containment of HIV-1.
Introduction
The CD8+ T lymphocyte (CTL) response against HIV-1 is likely a key arm of immunity that contributes to partial control of viral replication within infected persons (reviewed in Ref. 1). Notably, during acute infection the development of virus-specific CTL temporally correlates to the drop in peak viremia to a quasi-stable set-point,2,3 and experimental CD8 depletion in vivo in the SIV-infected macaque model causes sharp rises in viremia.4–6 Such clinical observations have suggested a key protective role of CTL in the pathogenesis of infection, although the mechanisms for incomplete protection compared to other viruses such as cytomegalovirus remain unclear.
The targeting of CTL appears to be an important determinant of CTL antiviral efficacy in vivo. Notably, several studies have observed an inverse correlation between Gag-specific CTL and level of viremia but a positive correlation for Env-specific CTL,7–10 suggesting that targeting of Gag is generally beneficial compared to targeting of Env. The mechanism is unclear; Env-specific CTL can exert efficient suppression of HIV-1 replication similar to Gag-specific CTL in vitro,11,12 although some data suggest that circulating Env-specific CTL generally may be impaired in function.13
CTL targeting has been noted to shift between acute versus chronic HIV-1 infection.14 The reason for this evolution in targeting is not clear, but it may be related to rapidly evolving viral escape mutation that leads to decay of escaped CTL responses and subsequent replacement with other responses.15 After rapid flux in CTL responses and their targeted epitopes during acute infection, chronic infection is marked by relative stability of HIV-1-specific CTL responses and the epitopes that they target.16 Given that peak viremia in acute infection drops to a semi-stable “setpoint” in chronic infection, this suggests an interaction between evolution of the CTL response and the changing degree of immune containment over time.
We addressed two issues by examining a cohort of persons who were identified to have acute HIV-1 infection and initiated on combination antiretroviral therapy. First, we examined how CTL targeting differs depending on time after infection, and how the pattern of targeting correlates to changing viremia level after infection. Given the inverse correlation of Gag targeting to viremia in stable chronic infection, this addresses the question of whether changing Gag targeting during acute infection might contribute to changing viremia levels. Second, we examined the impact of antiretroviral treatment on the evolution of CTL targeting during acute infection. Because antigen drives CTL expansion and persistence, this addresses the hypothesis that changing CTL targeting is a response to HIV-1 sequence evolution and escape.
Materials and Methods
Research participants
All subjects were men recruited through the Acute Infection and Early Disease Research Program (AIEDRP) through Dr. Eric Daar's Los Angeles site. All men were infected in the Los Angeles area, where clade B HIV-1 is endemic. These participants were enrolled under Cedars-Sinai Medical Center and the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center IRB-approved protocols with informed consent.
Clinical monitoring
Standard certified laboratory testing of plasma HIV-1 RNA levels, peripheral blood CD4+ T cell counts, and other routine monitoring tests were performed at the clinical laboratory of Cedars-Sinai Medical Center.
Mapping of HIV-1-specific CTL responses
Viably-cryopreserved peripheral blood mononuclear cells were utilized for interferon-γ ELISpot assays screening for CTL responses against the whole HIV-1 proteome as previously described.17–20 Briefly, CD8+ T lymphocytes were polyclonally expanded using a CD3:CD4 bi-specific monoclonal antibody (kind gift of Dr. Johnson Wong, Harvard Medical School) and interleukin-2 (NIH AIDS Research and Reagent Repository). This approach was previously shown to generate nonspecifically expanded CD8+ T lymphocytes that are >95% pure, which maintain frequencies of HIV-1-specific CTL that closely reflect those before expansion.17,21 Screening was performed with a library of peptides (NIH AIDS Research and Reagent Repository), spanning Gag, Pol, Env, Nef, Tat, Rev, Vpr, Vpu, Vif (catalog numbers 8116, 6208, 9487, 5189, 5138, 6445, 6447, 6444, 6446, all clade B consensus sequences with the exception of Env). These peptides were screened initially in 53 pools of 12 to 16 peptides each. Triplicate negative controls included CD8+ T lymphocytes alone, and a positive control included CD8+ T lymphocytes stimulated with anti-CD2/CD2R and anti-CD28 monoclonal antibodies (Becton Dickinson, San Jose, CA). After counting with an automated ELISpot counting system (Cellular Technologies Limited, Cleveland, OH), results against each peptide pool were calculated as spot-forming cells per million cells after subtracting the mean of the negative controls. Subsequent rounds of ELISpot screening were performed to identify responses against single 15-mer peptides. A spot forming cell (SFC) value of at least 50 per 106 CD8+ T lymphocytes that also exceeded two standard deviations above the mean of the negative control wells was considered positive for an individual peptide. Measurements that exceeded 5000 SFC/106 CD8+ T lymphocytes (1000 SFC/well) were rounded down to 5000/106 CD8+ T lymphocytes. A recognized isolated (nonconsecutive) peptide was assumed to contain a single epitope region, while consecutive recognized overlapping peptides were assumed to contain a single epitope region.
Results
Timing and characteristics of the HIV-1-infected participants
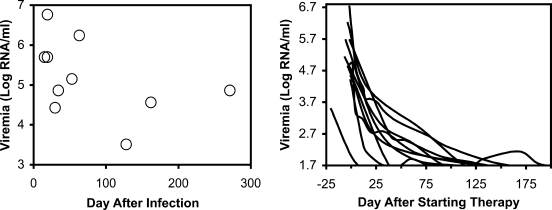
Ten HIV-1-infected persons in the Los Angeles area were identified based on documented HIV-1 seroconversion and/or acute febrile illness consistent with acute HIV-1 infection with high levels of viremia and previously negative HIV-1 serology, and effective antiretroviral treatment at varying time points after infection (Table 1). Eight of the ten reported a clinical syndrome consistent with acute infection, and plasma HIV-1 RNA testing and/or documented change in serostatus for HIV-1 supported the diagnosis. In these individuals, the estimated date of infection was set at 7 days prior to symptoms. Two other individuals reported no acute illness, but were documented to develop newly positive HIV-1-serologies within a window of less than 60 days; in these cases, the estimated date of infection was set at the midpoint between their last seronegative and first seropositive tests. Viremia levels (assessed just before starting antiretroviral therapy) tended to be higher in those persons who were more recently infected (Fig. 1),consistent with high viremia during acute infection and lower setpoint viremia during chronic infection. These data were consistent with the known pattern of peak viremia dropping to the setpoint during the transition between acute and chronic infection occurring in the first few weeks of infection. After institution of antiretroviral therapy, viremia levels dropped to the limit of detection (50 RNA copies/ml) in these subjects (Fig. 1).
Table 1.
Study Participants and Timing of CTL Assessments
| Subject | Year infected | Sx | Last SN | First SP | Pre-Tx CTL | Pre-Tx VL (viremia, RNA/mL) | Tx start | Tx ARV | First post-Tx CTL | Second post-Tx CTL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1999 | Y | 14 | 15 | 15 | 15 (500,000) | 17 | ZDV/3TC/NFV | 106 | 182 |
| 2 | 1998 | Y | 14 | 25 | 19 | 19 (5,799,200) | 21 | D4T/3TC/IDV | 98 | 180 |
| 3 | 1999 | Y | 14 | 19 | 19 | 19 (500,000) | 25 | D4T/3TC/ABC/NVP/HU | 133 | 232 |
| 4 | 1998 | N | −24 | 24 | 30 | 30 (27,018) | 31 | D4T/3TC/NVP | 102 | 186 |
| 5 | 1998 | Y | 15 | 17 | 37 | 34 (73,010) | 37 | D4T/3TC/Rit-SQV | 111 | 230 |
| 6 | 2000 | Y | N/A | 32 | 53 | 53 (141,697) | 60 | ZDV/3TC/ABC/NVP | 153 | 231 |
| 7 | 1999 | Y | N/A | 63 | 63 | 63 (1,731,500) | 67 | D4T/DDI/NFV | 138 | 257 |
| 8 | 1999 | Y | N/A | 97 | 128 | 128 (3,256) | 149 | ZDV/3TC/EFV | 210 | 296 |
| 9 | 2000 | N | −9 | 9 | 135 | 162 (36,713) | 163 | ZDV/3TC/EFV | 294 | 357 |
| 10 | 2000 | Y | 13 | 26 | 271 | 271 (72,790) | 275 | ZDV/3TC/EFV | 355 | 421 |
For each participant, the timing of HIV-1 infection was estimated to be 7 days prior to symptoms consistent with acute HIV-1 infection syndrome in association with documented (Subjects 1, 2, 3, 5, 10) or suspected seroconversion (Subjects 6, 7, 8), or the midpoint in the interval between the last seronegative and first seropositive HIV-1 antibody test in persons who did not have symptoms (subjects 4 and 9). All values are listed as days in relationship to this estimated time of infection. “Sx” = presence or absence of an acute flu-like illness consistent with acute infection; “Last SN” = time of last documented negative HIV-1 serology; “First SP” = time of first documented positive HIV-1 serology; “Pre-Tx CTL” = time of PBMC collection for pre-treatment analysis of HIV-1-specific CTL responses; “Pre-Tx VL” = time of last viremia measurement before starting treatment; “Tx Start” = time combination antiretroviral therapy was started; “Tx ARV” = antiretroviral therapy regimen (ABC, abacavir; D4T, stavudine; DDI, didanosine; EFV, efavirenz; HU, hydroxyurea; NFV, nelfinavir; NVP, nevaripine; Rit-SQV, ritonavir-boosted saquinavir; 3TC, lamivudine; ZDV, zidovudine); “First Post-Tx CTL” and “Second Post-Tx CTL” = times of CTL measurements after starting therapy.
FIG. 1.
Baseline viremia levels and impact of antiretroviral therapy. For the 10 study participants, pre-treatment viremia levels measured between 1 and 21 days of initiating treatment are plotted against the estimated time since infection (left panel), and serial viremia levels are plotted against the time since starting combination antiretroviral therapy (right panel).
Varying distribution of cellular immune targeting of HIV-1 proteins correlated to time after infection
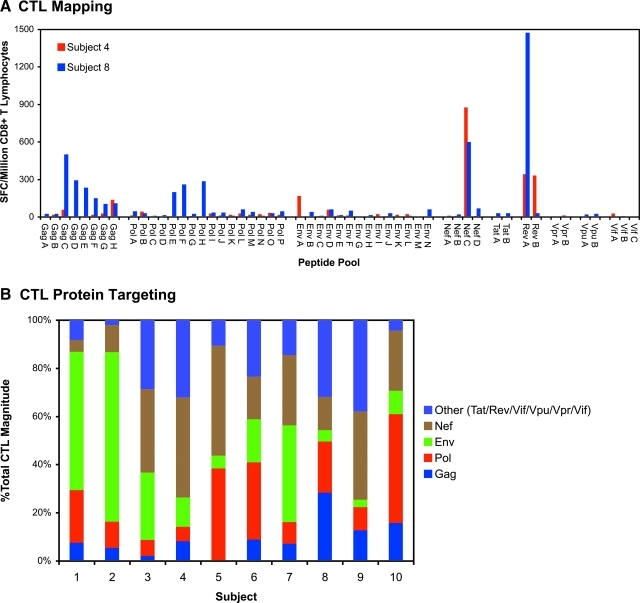
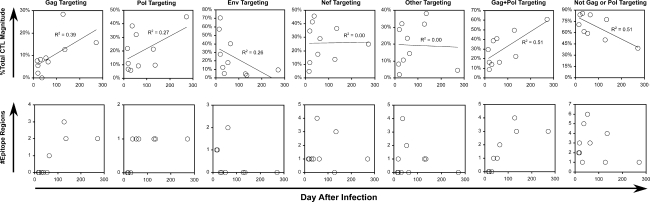
HIV-1-specific CD8+ T lymphocyte (CTL) measurements were assessed using peripheral blood mononuclear cells obtained coincident with the viremia measurements before starting antiretroviral therapy. By interferon-γ ELISpot analysis using 53 pools of peptides spanning the entire proteome, HIV-1-specific CTL responses were detectable in all persons (Fig. 2A). Examining the targeting of these responses, the distribution of protein targeting varied between individuals (Fig. 2B). Comparing CTL protein targeting to the duration of infection across persons, there were apparent patterns consistent with changing targeting over time. The percentage of the total CTL response (Fig. 3, top row) targeting Gag appeared to be higher in persons with later infection. This correlation was stronger when considering the sum of Gag and Pol targeting. A similar pattern was seen for the absolute number of epitope regions targeted (Fig. 3, bottom row). These findings suggested a shift from CTL targeting towards the most conserved proteins (Gag and Pol) within the first year after HIV-1 infection.
FIG. 2.
Assessment of HIV-1-specific CTL targeting. Standard IFN-γ ELISpot assays were performed using CD8+ T lymphocytes screened against 53 pools of peptides (consecutive 15-mer peptides overlapping by 11 amino acids, 16 or fewer per pool) spanning the entire HIV-1 proteome. (A) Data are shown for baseline pre-treatment evaluations of subjects 4 and 8, plotted as spot-forming cells (SFC) per million CD8+ T lymphocytes. Subsequent rounds of ELISpot were performed to determine single recognized 15-mer peptides within peptide pools with positive responses (not shown). (B) CTL targeting against each HIV-1 protein was determined by summing the SFC for all peptide pools spanning each protein, as a percentage of total SFC against all peptide pools for all proteins. Color images available online at www.liebertonline.com/aid.
FIG. 3.
Relationship of HIV-1-specific CTL targeting versus time after infection (before antiretroviral therapy). Top row: For each of the indicated HIV-1 proteins or combinations of proteins, the percentage of targeted SFC compared to the total (as depicted in Fig. 2) is plotted against the estimated time after infection of each subject. Significant correlations (linear regression) included Gag (p = 0.054) and Gag+Pol/Not Gag or Pol (p = 0.020). Bottom row: the number of epitope regions targeted within Gag, Pol, Env, Nef, and other proteins is plotted against the estimated time after infection for each subject. Significant correlations (Spearman rank) included Gag (p = 0.010), Pol (p = 0.009), and Gag+Pol (p = 0.005).
Increased CTL targeting of Gag is associated with reduced viremia in early HIV-1 infection
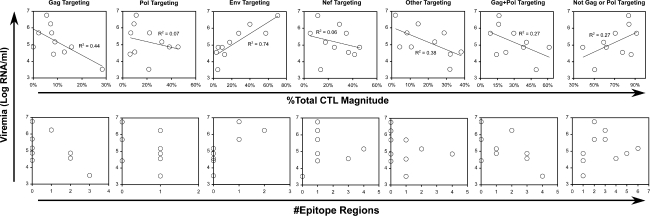
To assess whether reduction in viremia to setpoint during early infection might be related to changing CTL targeting, targeting was compared to viremia level across subjects. The percentage of the total CTL response (Fig. 4, top row) targeting Gag showed a negative correlation to viremia in these subjects, while targeting of Env showed a positive correlation. In contrast to the pattern of changing targeting over time, Pol targeting did not show a clear correlation to viremia, and combined Gag–Pol targeting also was not better correlated. Similarly, the absolute number of Env epitope regions targeted was positively correlated to viremia, with a trend suggesting lower viremia with broader Gag targeting (Fig. 4, bottom row). Overall, these results suggested that the increasing Gag targeting seen during early infection is correlated to dropping viremia, while Env targeting has the opposite effect, and Pol targeting appears to have little influence.
FIG. 4.
Relationship of viremia to CTL targeting (before antiretroviral therapy). CTL targeting was compared to viremia level at the baseline assessment performed 1–21 days before initiating therapy. As described in Table 1, baseline viremia level (plotted in Fig. 1) and CTL (plotted in Fig. 2) measurements were assessed on samples from the same days, with the exception of Subject 9 for which CTL assessment was 27 days earlier than viremia. Top Row: The protein targeting of CTL (percentage of SFC versus total SFC) is plotted against viremia level. Significant correlations (linear regression) included Gag (p = 0.038) and Env (p = 0.001). Bottom Row: The number of epitope regions identified in the indicated proteins or protein combinations is plotted against viremia level. Significant correlations (Spearman rank) included only Env (p = 0.009).
Suppressive antiretroviral therapy blunts the evolution of CTL targeting in early infection
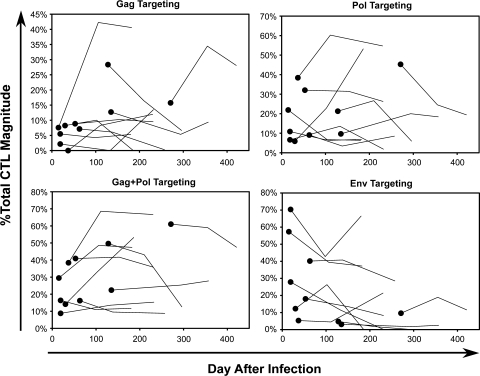
The effect of early suppressive antiretroviral therapy (reducing viremia to <50 RNA copies/ml) on CTL targeting was assessed. Longitudinal assessment of treated subjects revealed that the percentage of CTL magnitude targeting Gag, Pol Gag and Pol, or Env showed no consistent patterns of change over time after treatment (Fig. 5),in contrast to the data across subjects before treatment (Fig. 3). With a few exceptions, the earliest treated persons generally showed flat percentages of Gag, Pol, and Env targeting that did not correspond to the increases in Gag and Pol and decrease in Env targeting seen across individuals over time in the absence of treatment. These results suggested that reduced viral replication on treatment halted the evolution of CTL targeting in early infection, through the retardation of viral sequence evolution.
FIG. 5.
Impact of antiretroviral therapy on evolution of CTL targeting. For each protein or protein combination, CTL targeting is plotted against estimated time after infection. The solid dots represent baseline values just before initiation of treatment (plotted in Fig. 3), and the connected lines represent two subsequent CTL measurements after treatment (at time points indicated in Table 1).
Discussion
Although there is a temporal correlation of formation of HIV-1-specific CTL responses to the decline of viremia from its peak during acute infection,2,3 there is a significant lag between the earliest detectable responses and the drop in viremia to the setpoint seen during chronic infection. One explanation could be that CTL magnitude takes time to build to sufficient levels to slow and then reduce viral replication. Another explanation could be that the CTL response requires time to adapt before viral replication can be effectively reduced. Supporting the second hypothesis, acute infection is marked by frequent mutational escape in CTL epitopes.22–26 By the time of chronic infection, CTL targeting and epitope sequences reach relative stability,16 because previously subdominant CTL are now able to expand27 after the decay of escaped CTL.24,28 Taken together, these observations suggest that targeting of variable epitopes by early immunodominant CTL allows viral escape from CTL antiviral activity, and that eventual re-targeting of CTL against more conserved epitopes reduces escape and leads to the quasi-stable setpoint interaction between CTL and viral replication.
In support of this scenario, our data demonstrate diminishing targeting of Env and increasing targeting of Gag and Pol during the transition from acute to chronic infection. Env is the most variable HIV-1 protein, while Gag and Pol are the most conserved,29 suggesting that a shift from variable to conserved epitope targeting is the mechanism for the observed shifts in targeting and reduction of viremia achieved at the end of early infection. This is consistent with the observation that targeting of Gag and Env are negatively and positively correlated respectively to viremia during chronic infection,7–10 and further implies that the degree of this targeting evolution may be a determinant of the final setpoint reached after early infection. Further evidence for this scenario comes from the observation that antiretroviral therapy interrupts this trend in CTL targeting; this implies that the evolution of targeting is driven by the evolution of HIV-1. This is consistent with prior data examining CTL responses in persons receiving treatment in early infection.30 Thus, this bi-directional interaction is interrupted by antiretroviral therapy that reduces viral replication and therefore viral mutation.
Although targeting evolves towards both the conserved Gag and Pol proteins in acute infection, the major correlate of immune control appears to be Gag and not Pol, in agreement with correlations observed during stable chronic infection.7–10 This indicates that sequence conservation is not the only factor in CTL efficacy. A potential explanation is the lower expression of Pol compared to Gag due to the ribosomal frameshift required for Pol translation from the single gag-pol transcript;31 this can result in reduced levels of Pol compared to Gag epitopes32 and less antiviral efficacy of Pol-specific CTL.11,12
The delay in targeting conserved epitopes may have significant ensuing repercussions for CTL efficacy in general. Early immunodominance of CTL targeting more variable epitopes may limit the ability to re-target efficiently against more conserved epitopes due to “original antigenic sin”,33 and there likely is severe and poorly reversible total body depletion of CD4+ helper cells through direct viral infection during this early period of poor immune containment,34–36 leading to lack of help to maintain CTL function.37 Thus, accelerating this early evolution of CTL targeting, or pre-determining it by vaccination, could offer an avenue to improve immune containment of HIV-1. Supporting this idea, protective HLA types appear to be associated with early CTL targeting of highly conserved epitopes similar to those targeted in chronic infection, for which escape carries a high fitness cost.38
It should be noted that the antigenic unit of CTL is the epitope, rather than whole proteins such as Gag and Env. Thus, these observed trends for protein targeting and their relationship to containment of viremia likely represent effects of epitope properties and not necessarily the properties of the whole proteins from which the epitopes are derived. Because Gag is more conserved than Env overall, Gag epitopes on average will be more conserved than Env epitopes, and thus a selective pressure for more conserved epitope targeting will manifest as an overall trend for more Gag-specific and fewer Env-specific CTL. However, there are stretches of variable sequences in Gag, and conserved sequences in Env29 and CTL against these epitopes may be selected based on these properties rather than targeting of Gag or Env. Thus, while the properties of epitopes may be influenced by the protein from which they are derived, important functional characteristics of epitopes such as sequence constraint may vary considerably between epitopes from the same protein. This is supported by the observation that protective CTL epitope targeting can be observed not only in Gag, but also in generally more variable proteins such as Vpr and Nef.39
Finally, it should be noted that the cross-sectional nature of our study is a caveat to interpreting the evolution of CTL targeting over time. With the relatively small numbers of subjects studied, we cannot exclude the possibility that the trends seen across persons could be due to random factors such as differences in targeting associated with differing HLA types between individuals, rather than changes in targeting over time. Although our data are suggestive, detailed longitudinal studies would be required to demonstrate time-specific evolution of CTL targeting definitively.
In summary, early HIV-1 infection appears to be marked by evolution of CTL targeting from less conserved to more conserved proteins, and this is likely driven by viral escape and CTL re-targeting. Shifting of targeting to more conserved and highly expressed epitopes likely plays a role in the eventual ability of the CTL response to reduce peak viremia to a quasi-stable setpoint during early chronic infection. These data suggest that addressing this early process of early escape from CTL could offer an intervention to improve immune control.
Acknowledgments
We are indebted to the participants of this study for their donation of blood samples. We would like to thank Jacqueline A. Pitt for her help with study coordination, and Janis Giorgi for her original involvement in starting this project. This work was supported by PHS Grants AI043203 (OOY), M01-RR00424 (ESD), AI069424 (ESD), CH05-SD-0607-005 (ESD), and AI058845 (BDJ).
Author Disclosure Statement
The authors have no institutional or commercial affiliations that pose any conflicts of interest.
Sequence Data
Not applicable.
References
- 1.Yang OO. Will we be able to 'spot' an effective HIV-1 vaccine? Trends Immunol. 2003;24:67–72. doi: 10.1016/s1471-4906(02)00034-0. [DOI] [PubMed] [Google Scholar]
- 2.Borrow P. Lewicki H. Hahn BH. Shaw GM. Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koup RA. Safrit JT. Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin X. Bauer DE. Tuttleton SE, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matano T. Shibata R. Siemon C. Connors M. Lane HC. Martin MA. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz JE. Kuroda MJ. Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 7.Rolland M. Heckerman D. Deng W, et al. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS ONE. 2008;3:e1424. doi: 10.1371/journal.pone.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiepiela P. Ngumbela K. Thobakgale C, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 9.Masemola A. Mashishi T. Khoury G, et al. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: Correlation with viral load. J Virol. 2004;78:3233–3243. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riviere Y. McChesney MB. Porrot F, et al. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retroviruses. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 11.Yang OO. Kalams SA. Rosenzweig M, et al. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang OO. Kalams SA. Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: Evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H. Piechocka-Trocha A. Miura T, et al. Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. J Virol. 2009;83:3138–3149. doi: 10.1128/JVI.02073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goulder PJ. Altfeld MA. Rosenberg ES, et al. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J Exp Med. 2001;193:181–194. doi: 10.1084/jem.193.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang OO. Aiming for successful vaccine-induced HIV-1-specific cytotoxic T lymphocytes. AIDS 30. 2008;22:325–331. doi: 10.1097/QAD.0b013e3282f29491. [DOI] [PubMed] [Google Scholar]
- 16.Koibuchi T. Allen TM. Lichterfeld M, et al. Limited sequence evolution within persistently targeted CD8 epitopes in chronic human immunodeficiency virus type 1 infection. J Virol. 2005;79:8171–8181. doi: 10.1128/JVI.79.13.8171-8181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibarrondo FJ. Anton PA. Fuerst M, et al. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J Virol. 2005;79:4289–4297. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balamurugan A. Lewis MJ. Kitchen CM, et al. Primary human immunodeficiency virus type 1 (HIV-1) infection during HIV-1 Gag vaccination. J Virol. 2008;82:2784–2791. doi: 10.1128/JVI.01720-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang OO. Church J. Kitchen CM, et al. Genetic and stochastic influences on the interaction of human immunodeficiency virus type 1 and cytotoxic T lymphocytes in identical twins. J Virol. 2005;79:15368–15375. doi: 10.1128/JVI.79.24.15368-15375.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang OO. Daar ES. Jamieson BD, et al. Human immunodeficiency virus type 1 clade B superinfection: Evidence for differential immune containment of distinct clade B strains. J Virol. 2005;79:860–868. doi: 10.1128/JVI.79.2.860-868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones N. Agrawal D. Elrefaei M, et al. Evaluation of antigen-specific responses using in vitro enriched T cells. J Immunol Methods. 2003;274:139–147. doi: 10.1016/s0022-1759(02)00510-0. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y. McNevin J. Cao J, et al. Selection on the human immunodeficiency virus type 1 proteome following primary infection. J Virol. 2006;80:9519–9529. doi: 10.1128/JVI.00575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borrow P. Lewicki H. Wei X, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 24.Price DA. Goulder PJ. Klenerman P, et al. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci USA. 1997;94:1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones NA. Wei X. Flower DR, et al. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J Exp Med. 2004;200:1243–1256. doi: 10.1084/jem.20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen TM. Altfeld M. Geer SC, et al. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol. 2005;79:13239–13249. doi: 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Most RG. Sette A. Oseroff C, et al. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- 28.Jamieson BD. Yang OO. Hultin L, et al. Epitope escape mutation and decay of human immunodeficiency virus type 1 specific cytotoxic T lymphocyte responses. J Immunol. 2003;171:5372–5379. doi: 10.4049/jimmunol.171.10.5372. [DOI] [PubMed] [Google Scholar]
- 29.Yang OO. Candidate vaccine sequences to represent intra- and inter-clade HIV-1 variation. PLoS ONE. 2009;4:e7388. doi: 10.1371/journal.pone.0007388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altfeld M. Rosenberg ES. Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacks T. Power MD. Masiarz FR. Luciw PA. Barr PJ. Varmus HE. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 32.Tsomides TJ. Aldovini A. Johnson RP. Walker BD. Young RA. Eisen HN. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–1293. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klenerman P. Zinkernagel RM. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394:482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 34.Veazey RS. DeMaria M. Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 35.Mattapallil JJ. Douek DC. Hill B. Nishimura Y. Martin M. Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 36.Brenchley JM. Schacker TW. Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med S. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalams SA. Buchbinder SP. Rosenberg ES, et al. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J Virol. 1999;73:6715–6720. doi: 10.1128/jvi.73.8.6715-6720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang YE. Li B. Carlson JM, et al. Protective HLA class I alleles restricting acute-phase CD8+ T cell responses are associated with viral escape mutations located in highly conserved regions of HIV-1. J Virol. 2009;83:1845–1855. doi: 10.1128/JVI.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heckerman D. Frahm N. Pereyra F, et al. Vaccine-induced targeting of epitopes associated with spontaneous control of HIV viral replication is associated with lower set-point viral loads in HIV-infected participants from the STEP trial. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (2009); Montreal, Canada. 2009. [Google Scholar]