Abstract
The carbon-fluorine bond is the strongest covalent bond in organic chemistry, yet fluoroacetate dehalogenases can readily hydrolyze this bond under mild physiological conditions. Elucidating the molecular basis of this rare biocatalytic activity will provide the fundamental chemical insights of how this formidable feat is achieved. Here, we present a series of high-resolution (1.15–1.80 Å) crystal structures of a fluoroacetate dehalogenase, capturing snapshots along the defluorination reaction: the free enzyme, enzyme-fluoroacetate Michaelis complex, glycolyl-enzyme covalent intermediate and enzyme-product complex. We demonstrate that enzymatic defluorination requires a halide pocket that not only supplies three hydrogen bonds to stabilize the fluoride ion, but is also finely tailored for the smaller fluorine halogen atom to establish selectivity towards fluorinated substrates. We have further uncovered dynamics near the active site which may play pivotal roles in enzymatic defluorination. These findings may ultimately lead to the development of novel defluorinases that will enable the biotransformation of more complex fluorinated organic compounds, which in turn will assist the synthesis, detoxification, biodegradation, disposal, recycling and regulatory strategies for the growing markets of organofluorines across major industrial sectors.
Introduction
Fluorine forms the strongest single bond to carbon with a dissociation energy of up to 130 kcal/mol, the highest among all natural products.1,2 This unrivalled stability arises because fluorine is the most electronegative element, which introduces reinforcing ionic forces through strong polarization of this bond. The reactivity of the C-F bond is further lowered due to the poor accessibility to the valence electrons of the bonded fluorine atom.3,4
Fluorinated organic compounds are renowned for their unique features including inertness, hydrophobicity and even lipophobicity.5,6 Owing to these physicochemical properties, organofluorines are widely and increasingly used in numerous industries.1,2,6,7 For example, fluorinated drugs presently compose 20% of all pharmaceuticals, and up to 30% of all agrochemicals.8 However, the large-scale production and application of anthropogenic organofluorines have increasingly become a subject of debate due to their toxicity, global warming potential, ozone depletion, environmental persistence and bioaccumulation.4,9–11. A deeper understanding of the molecular mechanisms underlying the cleavage of the highly stable C-F bond is therefore of growing importance for the development of efficient strategies for degrading fluorinated organic compounds.4
Several microbial enzymes that break the C-F bond have been described.12,13 Among the firsts was the fluoroacetate dehalogenase (FAcD) discovered in a pseudomonad in 1965.1 It hydrolyzes various short-chain 2-haloacids with fluoroacetate (FAc) as its preferred substrate.14–17 FAc is very stable with an estimated half-life of over 47 years in water.18 It is the most common among the rare natural organofluorine compounds, synthesized by the thienamycin-producing actinobacterium Streptomyces cattleya as a secondary metabolite and by numerous plants as defense against grazing.19,20 To mammals, FAc is one of the most toxic poisons because of its close resemblance to acetate; it is transformed by ‘lethal synthesis’ in vivo into fluorocitrate, the source of a highly potent inhibitor blocking both the aconitase of the citric acid cycle and the citrate transport machinery across the mitochondrial membrane.19–21 For this, it also serves as a commercial rodenticide, codenamed ‘Compound 1080’, in Australia, Israel, Mexico, New Zealand and the U.S.A.22
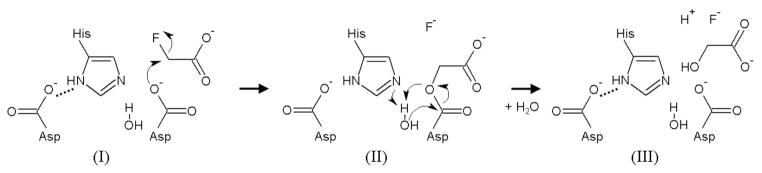
Despite the potential environmental and industrial applications of defluorinases, their mode of action has not been extensively characterized. In FAcDs, defluorination was proposed to involve an SN2 attack in which an aspartate nucleophile directly ejects the fluoride anion from the substrate (Figure 1).15,17 Although this mechanism parallels that of the structurally homologous, but non-defluorinating, haloalkane dehalogenases as well as the L-2-haloacid dehalogenases,15,23–26 the structural features that specifically confer defluorinating activity remain unclear. The crystal structure of the FAcD from Burkholderia sp. FA1 identified the active site containing the aspartate nucleophile. The nearby histidine and tryptophan residues were proposed to stabilize the leaving fluoride based on their binding to chloride.27 Subsequent theoretical studies predicted that an additional tyrosine residue participates in fluoride stabilization to assist the cleavage of the C-F bond.28 Interestingly, although the C-F bond is recognized as one of the strongest covalent bonds, activating this bond by employing acetate as nucleophile in an SN2 mechanism may require only ~20 kcal/mol.28 Given this relatively low activation energy, it is puzzling as to why most dehalogenating enzymes exhibit no defluorination activity. Illuminating the unique structural requirements which enable defluorination should be a first step towards the development of enzymes, possibly with the help of powerful emerging techniques such as de novo computational design,29 which eventually will be able to degrade even the notoriously persistent perfluorocarbons.
Figure 1. Proposed two-step reaction mechanism of fluoroacetate dehalogenases.
Catalysis involves the conserved aspartate-histidine-aspartate catalytic triad.15 The reaction cycles from the free enzyme, Michaelis complex (I), covalent ester intermediate (II), enzyme-product complex (III), and then back to the free enzyme. First, fluoride is displaced by the aspartate nucleophile in an SN2 attack. The resulting covalent intermediate is then hydrolyzed by a water molecule activated by the histidine base. This step is assisted by the second aspartate residue.
To explore the molecular basis of enzymatic defluorination and the preferential hydrolysis of the C-F bond over weaker carbon-halogen bonds by FAcDs, we have structurally and biochemically characterized the FAcD RPA1163. This defluorinase was discovered in a functional genomic screen of the photosynthetic bacterium Rhodopseudomonas palustris CGA009,30 which is well known for its metabolic versatility and bioremediation potential. Our high-resolution crystal structures reveal for the first time the structures of intermediates for each step of the defluorination reaction (Table 1).
Table 1.
Summary of crystal structures of RPA1163 and mutants
| Catalytic event | Free enzyme | Michaelis complex | Covalent intermediate | Product complex | ||
|---|---|---|---|---|---|---|
| Enzyme/ligand | WT/apo | D110N/FAc | D110N/ClAc | D110N/BrAc | H280N/FAc | WT/GOA |
| PDB code | 3R3U | 3R3V | 3R3W | 3R3X | 3R3Y | 3R3Z |
| Resolution (Å) | 1.60 | 1.50 | 1.60 | 1.80 | 1.15 | 1.70 |
| R/Rfree (%) | 17.2/20.2 | 18.6/22.3 | 18.1/22.3 | 19.2/23.3 | 15.3/18.7 | 17.9/21.8 |
The structures of the complexes were produced by soaking experiments. FAc, ClAc, BrAc and GOA denote fluoroacetate, chloroacetate, bromoacetate and glycolate, respectively.
Results and Discussion
Overall Structure of RPA1163
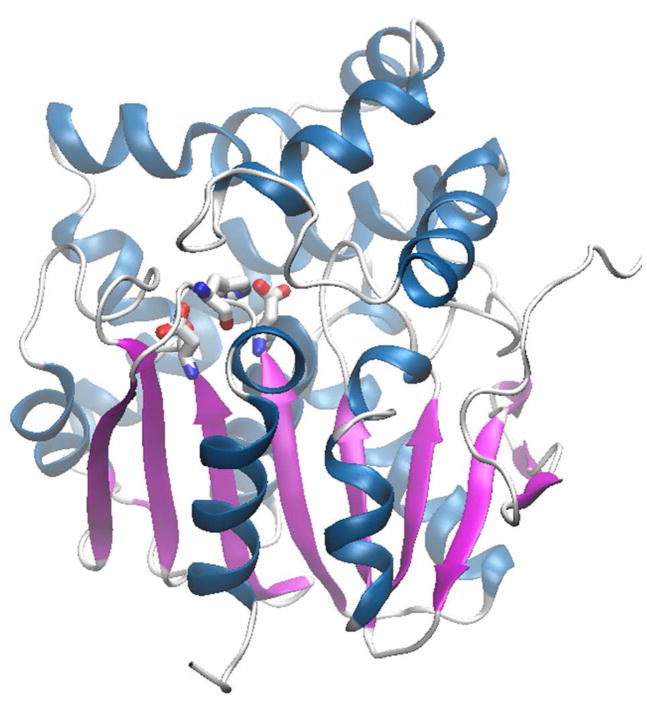
The apo-structure of WT RPA1163 shows a homodimeric protein with an α/β hydrolase fold,23 comprising an α/β/α core linked to an α-helical cap (Figure 2). The conserved catalytic triad (Asp110 nucleophile, His280 base and Asp134 carboxylate) marks the active site location, which is buried in the domain interface and accessible only via an 11 Å-long channel (Figure 2).
Figure 2. Overall structure of RPA1163.
The upper and lower domains are the α-helical cap and α/β/α core, respectively. The catalytic triad Asp110-His280-Asp134 protruding from the core domain is shown as sticks. The arrow indicates the opening of the 11 Å-long channel leading to the active site.
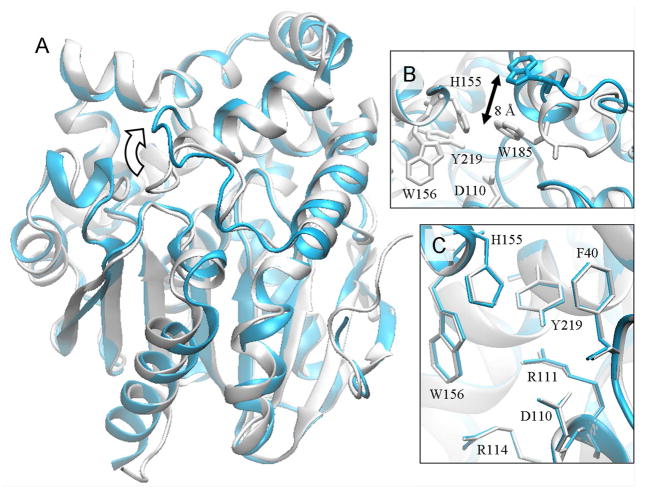
When compared to the Burkholderia FAcD (42% sequence identity; Supporting Information, Figure S1), the overall folds of the two enzymes are highly similar with an rmsd of 1.1 Å for all Cα atoms (Figure 3A). Both FAcDs share an active site identical in composition, which also comprises Phe40, Arg111, Arg114, His155, Trp156, Trp185 and Tyr219 (RPA1163 numbering). Moreover, the spatial arrangement of these residues is strikingly well conserved; a least-squares fit of the 77 non-hydrogen atoms from seven of these residues (Asp110, Arg111, Arg114, His155, Trp156, Tyr219, and Phe40) yields an rmsd of only 0.19 Å (Figure 3C). This suggests that defluorination requires a highly precise arrangement of active site residues and bonding geometries.
Figure 3. Structural comparison between RPA1163 and the Burkholderia FAcD.
The secondary structural elements of RPA1163 (grey) superpose well onto those of the Burkholderia FAcD (cyan). (A) At the active site entrance, the cap domain loop which carries Trp185 displays the largest conformational difference (curved arrow). (B) The active site cavity viewed along the access tunnel. The corresponding tryptophan side chain in the Burkholderia FAcD is located ~8 Å further away from the active site. (C) Superposition of seven catalytically important residues reveals a remarkably conserved active site architecture.
The largest conformational difference between RPA1163 and the Burkholderia FAcD is found in a loop of the α-helical cap domain (Figure 3A). This loop harbours Trp185 (RPA1163 numbering), which is in close proximity to the fluoride pocket (discussed below). In RPA1163, this loop makes several contacts with the α/β/α core domain. In the Burkholderia FAcD, however, it is lifted outward and becomes more exposed to solvent. As a result, the corresponding tryptophan side chain in the Burkholderia FAcD is driven ~8 Å further away from the fluoride pocket (Figure 3B). This cap domain loop in FAcD can therefore exhibit significant mobility.
Structure of the Enzyme-Substrate Complex
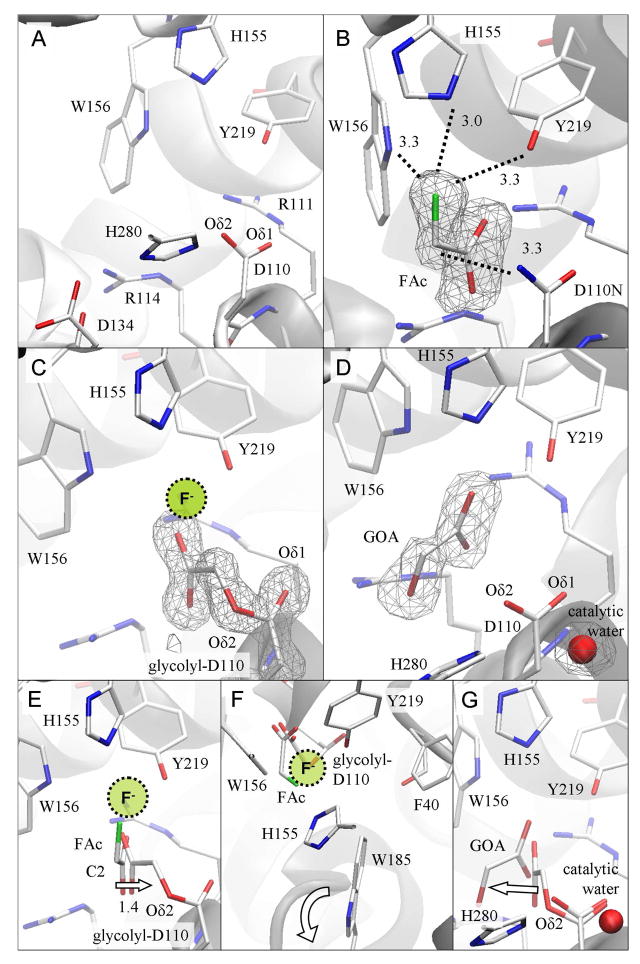
The binding of substrate is illustrated by the Michaelis complex in which the intact FAc molecule is trapped in the active site of the catalytically inactive nucleophile mutant Asp110Asn (Figure 4B). The carboxylate oxygen atoms of FAc are coordinated to Arg111 (O1-Nη1 2.7 Å) and Arg114 (O2-Nε 2.8 Å and O2-Nη1 3.0 Å), and through a hydrogen bond to Tyr219 (O1-Oη 2.8 Å). The fluorine atom is bound by His155 (F-Nε2 distance of 3.0 Å), Trp156 (F-Nε1 3.3 Å) and Tyr219 (F-Oη 3.2 Å), suggesting their contributions to weakening the C-F bond and stabilizing the displaced fluoride ion. Further destabilization of the C-F bond is likely accomplished by placing the fluorine-bearing carbon atom (C2) of FAc close (~3 Å) to Oδ2 of the Asp110 nucleophile in WT RPA1163, which has been substituted by Nδ2 in this mutant. The C-F bond forms a 99° angle with C2--Nδ2 (or 105° with C2--Oδ2 when superposing the FAc molecule onto the WT structure) which is far from the anticipated 180° angle. The altered shape and electrostatics of the active site have apparently kept C2 of FAc slightly (~1.4 Å, see next section) out of its near-attack conformation. Nevertheless, given the largely unmodified bonding network in the active site and the highly similar ligand binding modes among the structures of the various reaction intermediates, this enzyme-substrate complex probably closely represents the active site organization prior to the SN2 nucleophilic attack.
Figure 4. Structural comparison of reaction intermediates reveals the progress of enzymatic defluorination in RPA1163.
(A) Active site structure of the free WT FAcD. The cavity comprises the catalytic triad (Asp110-His280-Asp134), the carboxylate binding site (Arg111 and Arg114) and the fluoride pocket (His155, Trp156 and Tyr219). (B) The Asp110Asn/FAc Michaelis complex. The bound FAc reveals the key enzyme-substrate interactions (dashed lines with distances in Å). (C) The His280Asn/FAc covalent intermediate. The location where the displaced fluoride ion may be transiently bound is shown. (D) The WT/glycolate (GOA) product complex. (E) The initial SN2 attack is deduced from the superposition of FAc as found in the Michaelis complex onto the structure of the covalent intermediate. The arrow shows the displacement of the substrate’s C2 atom as this occurs. The location where the expelled fluoride ion may be transiently bound is indicated. (F) The same structural comparison viewed down the line of the atoms involved in the nucleophilic attack (i.e. from fluorine to the substrate’s C2 to Asp110’s Oδ2). The halide stabilizing residues (His155, Trp156 and Tyr219) form a ‘claw’ reaching for the leaving fluoride. Phe40 and Trp185 are also sufficiently close to participate in catalysis. The ‘flapping’ motion of the indole side chain of Trp185 is indicated by the curved arrow. (G) The subsequent hydrolysis of the covalent intermediate is inferred from the superposition of the glycolyl-Asp110 residue as found in the covalent intermediate onto the enzyme-product complex. This step employs the catalytic water activated by His280. The arrow indicates how the product glycolate (GOA) detaches from its former covalent bonding partner. The omit Fo−Fc electron density maps are contoured at 3 δ.
Cleavage of the C-F Bond by Nucleophilic Attack
The SN2 reaction is initiated by the Oδ2 atom of Asp110 attacking C2 of FAc to expel the fluoride, with inversion of stereochemistry at C2. The cleavage of the C-F bond results in the simultaneous formation of the glycolyl-enzyme covalent intermediate (Figure 1). This structure is captured using the His280Asn mutant which no longer has the histidine base to activate the catalytic water required for hydrolyzing the covalent intermediate (Figure 4C). Compared with the Michaelis complex, the C2 of the substrate moiety (formerly FAc) is shifted 1.4 Å deeper into the active site to covalently link to Oδ2 of Asp110 (Figure 4E). This newly formed O-C bond points directly towards a cavity lined by His155, Trp156 and Tyr219. With SN2 reactions imposing a collinear alignment from the nucleophile (Oδ2), electrophile (C2) to the leaving atom (fluoride), this geometric arrangement further implicates the role of these three aromatic residues in stabilizing the fluoride ion (Figure 4E,F). Soaking of His280Asn/FAc crystals in as much as 0.1 M NH4F produced no additional electron density inside the fluoride pocket. This low affinity for free fluoride suggests that the displaced fluoride is irreversibly shuttled to the bulk solvent. This process may be assisted by the highly dynamic Trp185 in the proximity of the fluoride pocket (discussed below), possibly through a ‘flapping’ motion (Figure 4F).
It is worth noting that the line of the SN2 attack (i.e. F--C2--Oδ2) appears nearly coplanar with FAc’s carboxylate group. This is distinct from the anticipated orthogonal orientation in free solution, which according to ab initio calculations allows FAc’s carboxylate group to assist transition state stabilization by delocalizing its π-electrons into the adjacent orbitals of the breaking C-F and forming C-O bonds.31 Although it is not clear why FAcDs do not harness the stabilization energy intrinsic to this attack conformation, one could argue that the molecular evolution of FAcDs has yet to be perfected. The strongest evolutionary pressure for further acceleration of C-F bond cleavage, however, may have well been removed considering that this step is not rate-limiting in FAc hydrolysis,28 and that the current catalytic rates are already sufficient to ensure the survival of the host organism.
Hydrolysis of the Covalent Intermediate
Subsequently, the glycolyl-Asp110 ester intermediate is hydrolyzed to complete the catalytic cycle. As shown in the WT/glycolate product complex (Figures 4D,G), this involves an attack on Cγ of Asp110 by the nearby water molecule present in most structures (Cγ-O 3.1±0.1 Å) (Figure 4D). This is likely facilitated by the simultaneous abstraction of a proton from the catalytic water by the His280 base (O-Nε2 3.1 Å), assisted in turn by Asp134 (Nδ1-Oδ1 2.6 Å). Hydrolysis of the ester intermediate is also expected to proceed via a tetrahedral intermediate, in which the transient negative charge on Oδ1 of Asp110 is stabilized by an oxyanion hole formed by the backbone amides of Phe40 (Oδ1-N 2.8 Å) and Arg111 (Oδ1-N 2.8 Å).
In the WT/glycolate product complex, the carboxylate group of glycolate is coordinated to Arg111 and Arg114 resembling the substrate in the Michaelis complex (Figure 4B,D). Interestingly, one of the carboxylate oxygen atoms is hydrogen-bonded to the halide pocket (His155, Trp156 and Tyr219). The hydroxyl group of glycolate is located close (3.4 Å) to Oδ2 of Asp110 (Figure 4G). Since this hydroxyl group is derived from Oδ2 of Asp110, this complex probably reveals the initial departure of glycolate before it completely dissociates from the enzyme.
Structural Basis for Halide Selectivity and Enzymatic Defluorination
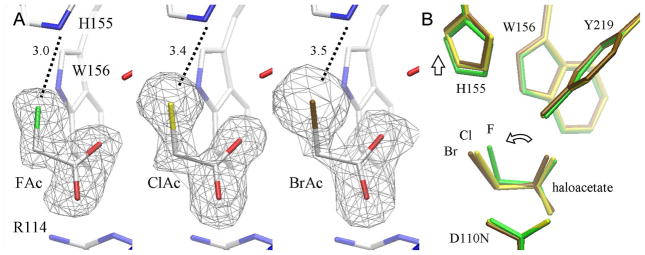
RPA1163 displays a remarkable preference for FAc hydrolysis over that of ClAc or BrAc despite FAc containing the stronger carbon-halogen bond (Table 2 and Supporting Information, Figure S8). This trend appears to correlate with the increase in size of the halide atom (van der Waals radii: F=1.47 Å, Cl=1.75 Å and Br=1.85 Å). To rationalize the structural basis of the selectivity for FAc, the Michaelis complex structures with ClAc and BrAc were also determined (Table 1 and Figure 5A). The comparison of the RPA1163 active sites in the three complexes suggests that only His155 has the elasticity to yield additional space in the halide pocket. Compared to the Asp110Asn/FAc complex, the imidazole ring of His155 is retracted 0.30 Å and 0.36 Å from the halide cavity in the ClAc and BrAc complexes, respectively (Figure 5B). However, the motional freedom of His155 alone seems insufficient for allowing the FAcD to readily accommodate larger haloacetates since the larger halogen atoms are kept further out of the halide pocket, apparently withholding the substrate from binding deeper within the active site (Figure 5B). Therefore, the lower activity of FAcD towards larger haloacetates (despite their weaker C-X bonds) is likely caused by the increased energy barrier required to overcome the steric hindrance from ‘squeezing’ the larger halide into too small a pocket in order to achieve acceptable reaction geometry.
Table 2.
Steady-state kinetics of RPA1163 and mutants
| Fluoroacetate | Chloroacetate | |||||||
|---|---|---|---|---|---|---|---|---|
| Enzyme | kcat (min−1) | % | Km (mM) | kcat/Km (s−1M−1) | kcat (min−1) | % | Km (mM) | kcat/Km (s−1M−1) |
| WT* | 6.7 ± 0.6 | 100 | 3.3 ± 0.2 | 33 | 1.38 ± 0.07 | 21 | 1.6 ± 0.5 | 14 |
| Phe40Ala | 0.21 ± 0.04 | 3.1 | 4.4 ± 0.8 | 0.79 | 0.029 ± 0.009 | 0.44 | 1.5 ± 0.4 | 0.32 |
| His155Asn | 0.70 ± 0.06 | 11 | 2.9 ± 0.2 | 4.1 | 5.3 ± 0.5 | 79 | 2.0 ± 0.3 | 45 |
| Trp156His | 0.11 ± 0.01 | 1.7 | 8 ± 3 | 0.22 | 0.10 ± 0.02 | 1.5 | 6 ± 1 | 0.27 |
| Trp185Phe | 0.59 ± 0.04 | 8.8 | 3.3 ± 0.6 | 2.9 | 0.32 ± 0.04 | 4.7 | 1.0 ± 0.3 | 5.0 |
| Tyr219Phe | 0.035 ± 0.009 | 0.53 | 1.7 ± 0.2 | 0.34 | 0.42 ± 0.04 | 6.3 | 1.00 ± 0.07 | 7.0 |
The parameters were determined from at least triplicate measurements and the standard deviations are shown. The % activity normalizes all kcat to FAc hydrolysis by WT.
Data reported previously.30
Figure 5. RPA1163 Asp110Asn mutant in complex with fluoro-, chloro- and bromoacetate.
(A) The binding of FAc, BrAc and ClAc in the active site of the Asp110Asn mutant. FAc is bound at full occupancy, but only ~75% saturation for ClAc and BrAc are observed. The residual electron densities on O2 of ClAc and BrAc are both modelled as a chloride ion at ~25% occupancy (Supporting Information, Figure S3). The omit Fo−Fc electron density maps are contoured at 4.5 (FAc), 3.0 (ClAc) and 2.8 (BrAc) σ. (B) The superposition of the FAc, ClAc and BrAc complexes (in green, yellow and brown, respectively) using all Cα atoms of the protein subunits. Only His155 is displaced, albeit minimally, by the increasingly larger halide of the substrate (vertical arrow). The binding of the larger halogen atom forces a slight tilt to the entire haloacetate substrate thereby keeping the halogen atom further out of the active site (curved arrow).
Steady-state kinetics measurements for FAc hydrolysis by RPA1163 mutants reveal that His155Asn, Trp156His, and Tyr219Phe all have a significantly reduced kcat but a generally unaffected Km (Table 2). This is consistent with the proposed role of these residues in fluoride stabilization. Mutations to the nearby Phe40 and ‘flapping’ Trp185 also disrupt dehalogenation activity, but the precise functions of these two amino acids are still speculative.
RPA1163 displays 5-fold higher activity (kcat) towards FAc than to ClAc (Table 2). Intriguingly, this substrate preference can be reversed by the His155Asn and Tyr219Phe mutations. In His155Asn, the mutation enlarges the halide pocket to relieve steric constraints without disrupting the electrostatic environment. This enhances ClAc turnover 4-fold compared to WT, which contributes significantly to the 8-fold higher activity on ClAc than FAc. In Tyr219Phe, deleting the hydroxyl group enlarges the halide pocket at the cost of a polar contact, thereby removing both steric constraints and electrostatic stabilization. Although this slows both defluorination and dechlorination activities, it is the much larger 190-fold drop in FAc turnover that accounts for the 12-fold higher ClAc activity over FAc. These results suggest that steric effects dictate the halide selectivity, whereas electrostatic stabilization determines the overall catalytic efficiency of the enzyme.
Dynamics of RPA1163
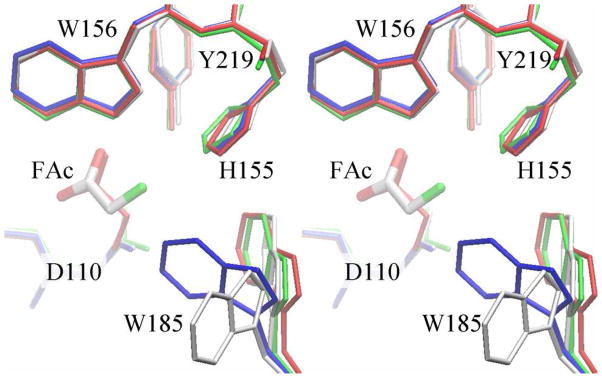
The initial comparison between RPA1163 and the Burkholderia FAcD suggests the possibility of large conformational freedom of the active site loop which bears Trp185 (Figure 3A,B, discussed above). This is consistent with the elevated B-factors and the survey of all Trp185 conformations in 20 RPA1163 structures (including additional reference structures presented in Supporting Information), which apparently reveals a ‘flapping’ motion in which the indole side chain sweeps by the cavity of the halide pocket (Supporting Information, Figure S2B,C).
Trp185 appears most dynamic in the absence of substrate as suggested by its poorly defined electron density and its multiple conformations (Figure 6). In one major conformation, Trp185’s indole ring is swung into the active site cavity, analogous to a door open inwards (see also Figure 3B). In the other major conformation, the side chain blocks access to the active site channel. Trp185 adopts the former (tucked in/open) conformation in both the Michaelis complex and the covalent intermediate, but an intermediary conformation in the WT/glycolate product complex (Figure 6). We speculate that Trp185 is initially tucked in during substrate binding to help occlude water molecules near the halide pocket from the otherwise larger active site cavity. If Trp185 were mobile during the catalytic cycle, its side chain could come as close as ~2.8 Å (Cη2--F) to the fluoride ion suggesting a potential interaction. The non-identical kcat of FAc and ClAc hydrolysis in the Trp185Phe mutant (Table 2) also seem to indicate Trp185’s connection to the halide. However, whether this imparts substrate recognition, C-X bond cleavage, or halide dissociation remains to be determined.
Figure 6. Stereoview comparison of different Trp185 conformations in various defluorinase complexes.
The active sites residues from WT/apo (grey), Asp110Asn/FAc Michaelis complex (green), His280Asn/FAc covalent intermediate (red) and WT/GOA product complex (blue) are shown.
Additional marked structural variations and disorder are found in a second loop formed by residues 251–259 which covers part of the active site entrance (Supporting Information, Figure S2A). We speculate that this segment may regulate substrate entry and product dissociation.
Conclusion
SN2 reactions, despite being a staple in organic chemistry, are rather few in the biological realm; many are based on the widely used group transfer reactions involving S-adenosyl methionine or its derivatives.32 Nevertheless it is a reaction strategy employed by many dehalogenases including haloalkane dehalogenases, fluoroacetate dehalogenases, L-2-haloacid dehalogenases, D- and DL-2-haloacid dehalogenases, haloalcohol dehalogenases and dichloromethane dehalogenases. However, only the fluoroacetate dehalogenases and a few novel L-2-haloacid dehalogenases have acquired defluorination activity.30 It was proposed that FAcDs can defluorinate because they supply three electrostatic contacts or hydrogen bonds to activate the C-F bond.28 This contrasts with the non-defluorinating dehalogenases, which appear to use fewer such contacts for breaking the weaker C-X bonds.26,33–37 However, our results suggest that defluorination further requires the close and most precise placement of the binding residues in order to effectively stabilize the small fluoride ion. Interestingly, nucleophilic substitution is also employed by some halogenases to catalyze the reverse reaction,32 but only the Streptomyces cattleya enzyme can form the C-F bond.38 The halide pocket of this fluorinase is similar to that of FAcDs in that it is small and forms three contacts with the fluoride.39–41 This appears to be a common strategy for directing fluorine biochemistry in addition to establishing fluoride selectivity.
Nature has also evolved other enzymes which can break strong chemical bonds such as N≡N, C≡N, C≡C, C=C, or C=O. However, these enzymes are mostly oxidoreductases (e.g. nitrogenases or CO dehydrogenases) that require high energy input in the form of ATP and/or low-potential electrons. A few organofluorines appear to be defluorinated anaerobically and may act as electron acceptors for certain reductive dehalogenases, but the underlying catalytic mechanism is still unknown, and it is unclear whether reductive dehalogenases can cleave the C-F bond.12,13 In contrast, RPA1163 and other related defluorinases are able to break this strong bond without the need for external energy input.
The high-resolution crystal structures of RPA1163 provide a detailed molecular view of biocatalytic defluorination, which requires three closely and precisely positioned polar contacts/hydrogen bonds to activate and stabilize the small fluorine atom, and protein motions with a potential functional relevance are identified. These results lay the foundations for future biotechnological developments, with a view towards viable sustainable management and disposal practices for the valuable but persistent fluorinated organic products.
Materials and Methods
Materials
The Ni-NTA resin and QIAprep spin miniprep kit were purchased from Qiagen (Mississauga, Canada) and the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, USA). Lysogeny broth and terrific broth pellets were obtained from EMD Chemicals (Gibbstown, USA); agar, ampicillin, kanamycin, glycerol, HEPES, IPTG and Tris from BioShop (Burlington, Canada); sodium fluoroacetate, chloroacetic acid and bromoacetic acid from Sigma-Aldrich (St. Louis, USA); and bromothymol blue from Fisher Scientific (Ottawa, Canada). The oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, USA). Paratone N was purchased from Hampton Research (Aliso Viejo, USA). All purchased chemicals were of the highest grades commercially available.
Protein Purification
Protein purification was performed as described.30 In brief, the protein was purified from the E. coli cell-free extract by Ni-affinity chromatography, followed by cleavage of the His6-tag using TEV protease and a second round of Ni-affinity chromatography. The protein was further purified by size exclusion chromatography in 150 mM NaCl and 50 mM Tris-H2SO4 pH 8.5, buffer-exchanged into 50 mM Tris-H2SO4 pH 8.5 and finally flash-frozen in liquid nitrogen. Protein concentrations were determined from their absorbance at 280 nm using extinction coefficients derived by ProtPARAM.42
Site-directed Mutagenesis
Site-directed mutagenesis was performed using QuikChange according to the manufacturer’s instructions.
Crystallization
Crystals in space group P212121 were produced by hanging drop, in 16–20% PEG3350, 200 mM NH4Cl and 100 mM sodium cacodylate pH 6.5, using as seed WT parent crystals grown with the supplementation of 4% sucrose. P21 crystals were grown in 15–24% PEG3350, 100–200 mM CaCl2 and 100 mM Tris-HCl pH 7.5. All enzyme-ligand complexes were produced by soaking crystals in mother liquor supplemented with the respective ligand (Supporting Information, Table S1).
Structure Determination and Manipulation
Diffraction data were collected at cryogenic temperature, using paratone N as cryoprotectant. Data were reduced with XDS43 and the structures refined iteratively with the help of Refmac5 in the CCP4i crystallographic software package and Coot.44–46 Anisotropic B factors were refined for datasets with resolutions better than 1.2 Å. The phases were initially solved by molecular replacement with Phaser47 using a homology model built by Phyre48 from the Burkholderia FAcD structure (PDB code: 1Y37). The PRODRG web server was used for generating the coordinates and restraint parameters files of ligands and modified amino acid residues.49 The secondary structure matching algorithm (SSM) aligning all Cα atoms was employed in all structural comparisons unless specified otherwise.50 Graphical representations of protein structures were generated in VMD.51 The atomic coordinates and structure factors have been deposited into the RCSB Protein Data Bank under PDB codes listed in Table 1 and Supporting Information, Table S1.
Isothermal Microcalorimetry
Steady-state kinetics were measured by microcalorimetry in at least triplicates in 100 mM Tris-H2SO4 pH 8.5 at 30°C using the VP-ITC microcalorimeter (MicroCal, LLC, Northampton, USA). The principles and procedures have been described.30,52 The nature of the reaction (i.e. product inhibition and irreversibility) was first characterized by consecutive single-injection experiments. The apparent reaction enthalpy (ΔHapp) for each substrate was calculated directly from the peak areas without consideration of equilibrium because the reaction was shown to be virtually irreversible.30 Multiple-injection experiments were performed because of product inhibition. Substrate was injected as 6 × 3 μl, 6 × 10 μl and 6 × 32 μl at approximately 4 min intervals and the final concentrations accumulated approximately from Km/10 to 10xKm whenever achievable. The kcat and Km were extracted by non-linear regression using the software package Origin (MicroCal, LLC).
Colorimetric Dehalogenation Assays
Dehalogenation activity was monitored through the proton released in a weakly buffered system, which is coupled to the color change of a pH indicator for detection.53 The reaction was performed in a volume of 1 ml. The final concentrations of the assay components were 10 mM sodium haloacetate, 1 mM Tris-H2SO4 pH 8.5, 20 μg/ml bromothymol blue and 150 μg/ml WT RPA1163. The absorbance at 616 nm was recorded. The background drift of A616 was measured for 2 min before the addition of enzyme. The enzymatic reaction was then monitored for 3 minutes and the net change in slope was used for calculating the reaction rate. The final values are averaged from triplicate runs.
Supplementary Material
Acknowledgments
We thank Aled M. Edwards for a critical review of the manuscript, and Aiping Dong, Yan Liu, Xiaohui Xu and Hong Zheng for their technical assistance. We are also grateful to the beamline staff at BioCARS and the Structural Biology Center (SBC) at Argonne National Laboratory for their help during data collection. The results shown in this report are derived from work performed at Argonne National Laboratory, BioCARS and SBC at the Advanced Photon Source. Argonne is operated by the University of Chicago Argonne, LLC, for the U.S. Department of Energy, Office of Biological and Environmental Research under contract DE-AC02-06CH11357. Use of the BioCARS Sector 14 was supported by the National Institutes of Health, National Center for Research Resources, under grant No. RR007707. The research was supported by the Natural Sciences and Engineering Research Council of Canada through a graduate scholarship (P.W.Y.C.) and operating grants (E.A.E. and E.F.P.), and by the Canada Research Chairs Program (E.F.P.).
Footnotes
Supporting Information Available: Table of crystallographic statistics, sequence alignment of FAcDs, structural variations and ligand binding of RPA1163, structural influence of mutations and crystal packing, pH-based assays of haloacetates, and thermodynamics of hydrolytic defluorination. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Goldman P. Science. 1969;164:1123–30. doi: 10.1126/science.164.3884.1123. [DOI] [PubMed] [Google Scholar]
- 2.Lemal DM. J Org Chem. 2004;69:1–11. doi: 10.1021/jo0302556. [DOI] [PubMed] [Google Scholar]
- 3.O’Hagan D. Chem Soc Rev. 2008;37:308–19. doi: 10.1039/b711844a. [DOI] [PubMed] [Google Scholar]
- 4.Douvris C, Ozerov OV. Science. 2008;321:1188–90. doi: 10.1126/science.1159979. [DOI] [PubMed] [Google Scholar]
- 5.Smart BE. J Fluor Chem. 2001;109:3–11. [Google Scholar]
- 6.Ritter SK. Chem Eng News. 2010;88:12–7. [Google Scholar]
- 7.Key BD, Howell RD, Criddle CS. Environ Sci Technol. 1997;31:2445–2454. [Google Scholar]
- 8.Muller K, Faeh C, Diederich F. Science. 2007;317:1881–6. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 9.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Environ Health Perspect. 2007;115:1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Environ Sci Technol. 2006;40:3463–73. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- 11.Shine KP, Sturges WT. Science. 2007;315:1804–5. doi: 10.1126/science.1141677. [DOI] [PubMed] [Google Scholar]
- 12.Natarajan R, Azerad R, Badet B, Copin E. J Fluor Chem. 2005;126:424–435. [Google Scholar]
- 13.Murphy CD. Biotechnol Lett. 2010;32:351–9. doi: 10.1007/s10529-009-0174-3. [DOI] [PubMed] [Google Scholar]
- 14.Au KG, Walsh CT. Bioorg Chem. 1984;12:197–205. [Google Scholar]
- 15.Kurihara T, Esaki N. Chem Rec. 2008;8:67–74. doi: 10.1002/tcr.20141. [DOI] [PubMed] [Google Scholar]
- 16.Kurihara T, Yamauchi T, Ichiyama S, Takahata H, Esaki N. J Mol Cat B: Enz. 2003;23:347–355. [Google Scholar]
- 17.Liu JQ, Kurihara T, Ichiyama S, Miyagi M, Tsunasawa S, Kawasaki H, Soda K, Esaki N. J Biol Chem. 1998;273:30897–902. doi: 10.1074/jbc.273.47.30897. [DOI] [PubMed] [Google Scholar]
- 18.TOXNET. Hazardous Substances Data Bank. National Library of Medicine (US); Bethesda (MD): [Google Scholar]
- 19.O’Hagan D, Harper BD. J Fluor Chem. 1999;100:127–133. [Google Scholar]
- 20.Murphy CD, Schaffrath C, O’Hagan D. Chemosphere. 2003;52:455–61. doi: 10.1016/S0045-6535(03)00191-7. [DOI] [PubMed] [Google Scholar]
- 21.Peters R, Wakelin RW. Proc R Soc Lond B Biol Sci. 1953;140:497–507. doi: 10.1098/rspb.1953.0004. [DOI] [PubMed] [Google Scholar]
- 22.Proudfoot AT, Bradberry SM, Vale JA. Toxicol Rev. 2006;25:213–9. doi: 10.2165/00139709-200625040-00002. [DOI] [PubMed] [Google Scholar]
- 23.Holmquist M. Curr Protein Pept Sci. 2000;1:209–35. doi: 10.2174/1389203003381405. [DOI] [PubMed] [Google Scholar]
- 24.Verschueren KH, Seljee F, Rozeboom HJ, Kalk KH, Dijkstra BW. Nature. 1993;363:693–8. doi: 10.1038/363693a0. [DOI] [PubMed] [Google Scholar]
- 25.de Jong RM, Dijkstra BW. Curr Opin Struct Biol. 2003;13:722–30. doi: 10.1016/j.sbi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Schmidberger JW, Wilce JA, Tsang JS, Wilce MC. J Mol Biol. 2007;368:706–17. doi: 10.1016/j.jmb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Jitsumori K, Omi R, Kurihara T, Kurata A, Mihara H, Miyahara I, Hirotsu K, Esaki N. J Bacteriol. 2009;191:2630–7. doi: 10.1128/JB.01654-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamachi T, Nakayama T, Shitamichi O, Jitsumori K, Kurihara T, Esaki N, Yoshizawa K. Chemistry. 2009;15:7394–403. doi: 10.1002/chem.200801813. [DOI] [PubMed] [Google Scholar]
- 29.Siegel JB, Zanghellini A, Lovick HM, Kiss G, Lambert AR, St Clair JL, Gallaher JL, Hilvert D, Gelb MH, Stoddard BL, Houk KN, Michael FE, Baker D. Science. 2010;329:309–13. doi: 10.1126/science.1190239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan WY, Wong M, Guthrie J, Savchenko AV, Yakunin AF, Pai EF, Edwards EA. Microb Biotechnol. 2010;3:107–20. doi: 10.1111/j.1751-7915.2009.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach RD, Coddens BA, Wolber GJ. J Org Chem. 1986;51:1030–1033. [Google Scholar]
- 32.O’Hagan D, Schmidberger JW. Nat Prod Rep. 2010;27:900–18. doi: 10.1039/b919371p. [DOI] [PubMed] [Google Scholar]
- 33.Bohac M, Nagata Y, Prokop Z, Prokop M, Monincova M, Tsuda M, Koca J, Damborsky J. Biochemistry. 2002;41:14272–80. doi: 10.1021/bi026427v. [DOI] [PubMed] [Google Scholar]
- 34.Schmidberger JW, Wilce JA, Weightman AJ, Whisstock JC, Wilce MC. J Mol Biol. 2008;378:284–94. doi: 10.1016/j.jmb.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 35.Marsh A, Ferguson DM. Proteins. 1997;28:217–26. doi: 10.1002/(sici)1097-0134(199706)28:2<217::aid-prot10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.de Jong RM, Kalk KH, Tang L, Janssen DB, Dijkstra BW. J Bacteriol. 2006;188:4051–6. doi: 10.1128/JB.01866-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong RM, Tiesinga JJ, Rozeboom HJ, Kalk KH, Tang L, Janssen DB, Dijkstra BW. Embo J. 2003;22:4933–44. doi: 10.1093/emboj/cdg479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong C, Huang F, Deng H, Schaffrath C, Spencer JB, O’Hagan D, Naismith JH. Nature. 2004;427:561–5. doi: 10.1038/nature02280. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Robinson DA, McEwan AR, O’Hagan D, Naismith JH. J Am Chem Soc. 2007;129:14597–604. doi: 10.1021/ja0731569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eustaquio AS, Pojer F, Noel JP, Moore BS. Nat Chem Biol. 2008;4:69–74. doi: 10.1038/nchembio.2007.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blasiak LC, Drennan CL. Acc Chem Res. 2009;42:147–55. doi: 10.1021/ar800088r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Methods Mol Biol. 1999;112:531–52. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 43.Kabsch W. J Appl Cryst. 1993;26:795–800. [Google Scholar]
- 44.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr D Biol Crystallogr. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 45.Emsley P, Lohkamp B, Scott WG, Cowtan K. Acta Crystallogr D Biol Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CCP4. Acta Crystallogr D Biol Crystallogr. 1994;50:760–3. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 47.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley LA, Sternberg MJ. Nat Protoc. 2009;4:363–71. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 49.Schuttelkopf AW, van Aalten DM. Acta Crystallogr D Biol Crystallogr. 2004;60:1355–63. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- 50.Krissinel E, Henrick K. Acta Crystallogr D Biol Crystallogr. 2004;60:2256–68. doi: 10.1107/S0907444904026460. [DOI] [PubMed] [Google Scholar]
- 51.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33–8. 27–8. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 52.Todd MJ, Gomez J. Anal Biochem. 2001;296:179–87. doi: 10.1006/abio.2001.5218. [DOI] [PubMed] [Google Scholar]
- 53.Holloway P, Trevors JT, Lee H. J Microbiol Meth. 1998;32:31–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.