Abstract
As opposed to genetics, dealing with gene expressions by direct DNA sequence modifications, the term epigenetics applies to all the external influences that target the chromatin structure of cells with impact on gene expression unrelated to the sequence coding of DNA itself. In normal cells, epigenetics modulates gene expression through all development steps. When “imprinted” early by the environment, epigenetic changes influence the organism at an early stage and can be transmitted to the progeny. Together with DNA sequence alterations, DNA aberrant cytosine methylation and microRNA deregulation, epigenetic modifications participate in the malignant transformation of cells. Their reversible nature has led to the emergence of the promising field of epigenetic therapy. The efforts made to inhibit in particular the epigenetic enzyme family called histone deacetylases (HDACs) are described. HDAC inhibitors (HDACi) have been proposed as a viable clinical therapeutic approach for the treatment of leukemia and solid tumors, but also to a lesser degree for noncancerous diseases. Three epigenetic drugs are already arriving at the patient’s bedside, and more than 100 clinical assays for HDACi are registered on the National Cancer Institute website. They explore the eventual additive benefits of combined therapies. In the context of the pleiotropic effects of HDAC isoforms, more specific HDACi and more informative screening tests are being developed for the benefit of the patients.
Keywords: histone deacetylase inhibitors, epigenetic, clinical trials interpretation
Introduction
The transcriptional state of a eukaryotic gene is determined by the surrounding chromatin architecture, the state of DNA cytosine methylation in the promoter/first exons and the associated regulating microRNAs (miRNAs).
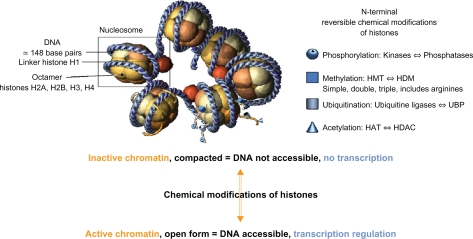
In the cell nucleus, the genome is packaged into a superstructure, the chromatin, whose elementary dynamic units are the nucleosomes. Each is made up of four associated dimers of core histones (H2A, H2B, H3, and H4) around which 147 base pairs of DNA are wrapped, the nucleosomes being finally linked together via the linker histone H1 (Figure 1). Chromatin is compacted around the DNA into a so-called “closed state” when cells are resting. It is opened into an “active state” to allow for gene transcription by adenosine triphosphate (ATP)-dependent protein complexes, which remodel the chromatin architecture. Theses complexes modify the accessibility of DNA regulatory sites through both repositioning (sliding) and ejecting nucleosomes. Modeling complexes include transcription co-activators, transcription factors, and epi-enzymes.1 Histone acetyltransferases (HATs), for instance, acetylate specific lysine residues of histones and convert them into an amide form, loosening histone contacts with DNA, resulting in exposed binding sites for the transcription machinery. On the other hand, other complexes function as gene silencers and deny the same machinery access to DNA. Repressive complexes include histone deacetylases (HDACs), which deacetylate specific lysine residues of the histones tails to induce tighter interactions between the now positively charged lysine (Nε protonated form) and the negatively-charged DNA phosphate groups.2 Beside acetylation, several other post-translational lysine modifications in histones have been described: methylation, phosphorylation, SUMOylation, and ATP-ribosylation.3,4
Figure 1.
The nucleosome unit and the histone tail chemical modification.
Abbreviations: HAT, histone acetyltransferase; HDM, histone demethylases; HDAC, histone deacetylase; HMT, histone methyltransferase; UBP, ubiquitin-specific protease.
All the modifications incurred by histones form the “histone code”. For example, K9 in H3 turns the chromatin into inactivity when methylated. Phosphorylation of serine 10 in the same H3 is required for methylation of K4 and acetylation of K9 and K14. Similar enzyme crosstalk has also been described, for ubiquitination of K120 in H2B prior to methylation of K79 in H3.5 Several epi-enzyme families are involved in the histones modifications. HATs and HDACs2 have balancing actions for histones acetylation. Methylation of histones6 is controlled by histones methyltransferases (HMTs) and histones demethylases (HDMs), while histones arginine methylation is catalyzed by the protein arginine N-methyltransferases family of enzymes.7–9 One, two, or three methylations are possible, with impact on gene expression/repression.10 The analysis of epigenetic marks at the genome-wide scale has shown that monomethylated H3K4 is associated with transcription factors binding to enhancers, trimethylated H3K4 with transcription start sites, and dimethylated H3K4 with both transcription start sites and enhancers.11
DNA methyltransferases (DNMTs) repress gene expression via DNA cytosine methylation, unfavorable to transcription factor binding. DNMTs are recruited and stabilized, on DNA, by HMTs and HDMs. Both are also able to recruit HDACs, methyl-binding proteins like methyl CpG binding protein 2 (MECP2), and several co-factors to further tune gene expression.12,13 Since DNMT co-factors are lacking in normal tissues, gene re-expression induced by DNMT inhibitors could be limited to tumor tissues to reduce “off-target” effects. DNA methylation in the epigenomes of human embryonic stem cells is an important field of research. The roles of DNA methylation in cancer genesis have also been extensively studied.12,13
Catalyzed by the ten-eleven translocation 1 (TET) family of enzymes, DNA hydroxymethylcytosine has been recently described as a step towards cytosine demethylation.14 Mutations and translocations of TET are present in myeloid malignancies.15 The role of hydroxymethylcytosine, if any, is not yet understood, but its existence questions all the results obtained so far when determining the cytosine methylation status.
In a further step of complexity, specialized miRNAs read the epi-code and target effectors genes to modulate their expression. MiRNAs are small non-coding RNAs of 20–22 nucleotides that inhibit gene expression when they engage either in imperfect base-pairing with their target mRNA 3′-untranslated region or affect its stability. MiRNA 29-a, -b and -c target DNMT3a and b directly and cell-specifically. HDAC4 is targeted by both miR-1 and miR-140, while miR-449-a targets HDAC1 in prostate malignant cells.16 Onco-proteins like promyelocyte leukemia retinoic acid receptor-α (PML-RARα) in promyelocytic leukemia and B-cell lymphoma 6 in non-Hodgkin’s lymphoma result from translocations. It is near the premiR-223 region that the t(8;21) translocation juxtaposes the Runt-related transcription factor 1 gene on chromosome 21 with the Cytochrome B Termination 1 gene on chromosome 8, generating the acute myeloid leukemia (AML)1-Eight Twenty One fusion gene.17 The recruitment, on this chimerical site, of DNMT, MeCP2, and HDAC1 repressor complexes, promotes leukemogenesis. Epi-miRNAs write their own epi-code when their cytosines are methylated. Downregulation of miR-124a induces an up regulation of its target, cyclin-dependent kinase 6 (CDK6), as well as phosphorylation of retinoblastoma, and contributes to the abnormal proliferation of acute lymphoblastic leukemia (ALL) cells both in vitro and in vivo.
Most epigenetic changes translate into either up regulation or silencing of gene expression.18 When inappropriate, they predispose the organism to more mutational events via increased genomic instability and aberrant cellular signaling. The field of epigenetic being extremely prolific, we have restricted our reference list to the essentials.
HDACs
HDACs remove the acetyl group from an N-ε-acetyl lysine located near the amino terminus of a core histone, cleaving an amide bond and increasing the positive charge of the histone. The removal of acetyl groups from the histones tails stabilizes nucleosomal DNA-histones interactions by its subsequent change in electrostatic charges. It is the basis for HDAC-mediated transcriptional repression via chromatin condensation.19 HDACs have been categorized into four classes. Class I HDACs (HDAC1, 2, 3, and 8) are nuclear proteins with ubiquitous expression involved in regulating cell proliferation.20 HDAC2 has been shown to suppress apoptosis in tumor cells not only via both the intrinsic/mitochondrial and the extrinsic/death-receptor pathways, but also via mitotic failure and autophagic cell death, while HDAC3 is involved in bone structure and S-phase check point.21,22 Class II HDACs have a tissue-specific expression and can shuttle between the nucleus and the cytoplasm. They are divided into two subclasses: IIa with HDAC4, 5, 7, and 9. HDAC4 represses chondrocyte hypertrophy. HDAC7 functions in the down regulation and apoptosis of T-cells.20 HDAC9 is involved in cardiomyocyte differentiation.23 Class IIb includes HDAC10 and HDAC6. The latter contains two tandem catalytic domains: one is for histones deacetylation and the other for deacetylation of α-tubulin. HDAC6 has also the capacity to bind directly to ubiquitinated proteins through an ubiquitin-binding domain, to target cargo proteins for subsequent processing. HDAC6-specific effects on cell motility and the proteasome are thought to be responsible for much of the toxicity of HDACs inhibitors (HDACi). HDAC 10 and 9 are required for chromosome homologous recombination.24 Class III HDACs include 7 different members of the sirtuin (SIRT) family. They are dependent on nicotinamine adenine dinucleotide (NAD+) to remove the acetyl group from lysine residues in histones and nonhistone substrates. Resveratrol from grapes and red wine is a SIRT1 activator.25 HDAC11 is the only member of Class IV.
Thus, it appears that HDAC activity depends on isoform types, sub cellular localization, association into multi-protein complexes and even post-translational modifications. HDACs are also able to deacetylate nonhistone proteins such as transcription factors, chaperone proteins and effectors of DNA repair, cell-signaling and metabolism. The ongoing concept is that deacetylation stabilizes these proteins. HDACs have different developmental functions, as shown by the different phenotypes obtained in knockout mice.26 Disruption of HDAC1 causes early embryonic lethality. HDAC2 knockout mice are viable but present fatal multiple cardiac defects. Germline HDAC3-deficiency causes embryonic lethality. HDAC3 conditional knockout mice gave severe deficits in membranous and endochondral bone formation. Germline deletion of HDAC4 causes premature ossification of the developing bones. HDAC6-deficiency slightly enhances trabecular bone formation. HDAC7 knockout gave vascular defects. HDAC8 is essential for neural crest progenitor cell differentiation and skull bone formation. HDAC9 knockout mice are viable at birth but have a myocardial hypertrophy.
HDACs and their inhibitors
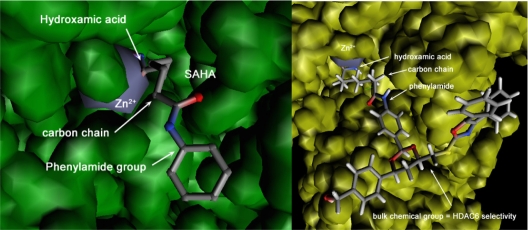
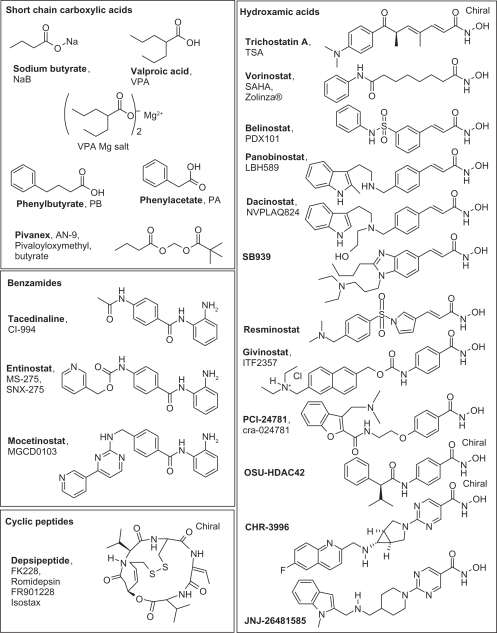
In tumor cells, deletion of a single HDAC is not sufficient to induce cell death but leads to nuclear bridging and fragmentation, and ‘in fine’ to cell mitotic catastrophe. This suggests that inhibition of HDAC may be sufficient for anticancer activity and provides a rational incentive for the development of HDACi.27 In the 1970s, seminal experiments showed that treatment of cells with the short-chain fatty acid NaB (sodium butyrate) caused hyperacetylation of histone octamers and led to the discovery of HDACs.28 The zinc-dependent HDACs of classes I, II, and IV are now known to have a common active site made of a tubular hydrophobic channel with a zinc atom (Zn2+) at its end, forming the enzyme catalytic pocket.29 The acetyl part of the lysine substrate in histones/proteins bind to the zinc atom while the protein four carbon lysine chains fits into the catalytic pocket, and deacetylation then follows. An HDACi is designed to block the HDAC catalytic activity. Several possibilities exist: irreversible or reversible binding to the enzyme catalytic site, competition with the enzymatic substrate, and deformation of the enzyme. Accordingly and as shown in Figure 2, the pharmacophore model for HDACi includes a zinc-binding group, competing somehow with the natural acetyl lysine substrate, a hydrophobic cap interacting with the external surface of the active site (generally aromatic) generating specificity, and a short linker connecting these two elements, which fits in the catalytic pocket. The zinc-binding groups can be a carboxylic acid in valproic acid (VPA), a hydroxamic acid in Vorinostat, benzamide in Entinostat, sulfhydryl in Romidepsin,30 and a ketone in Trapoxin. The linkers can be simple carbon chains, like in Vorinostat, or aromatic groups, like in Entinostat. HDACi in clinical trials are reported in Figure 3. Trichostatine (TSA) from Streptomyces hygroscopius was isolated as an antifungal antibiotic and was incidentally shown to have an anti-proliferative activity on murine leukemia cells. Further studies demonstrated that it was a pan-HDACi. The hydroxamate portion at the end of the molecule acts as a zinc-binding group. Because of toxic side effects, it is not used clinically but participates in the rational conception of HDACi via molecular modeling, as shown in Figure 2.
Figure 2.
Left panel: X-ray crystallographic data for SAHA bound to HDAC8. The zinc atom in the HDAC active site is shown in grey. The hydroxamic acid group in SAHA is bound to Zn2+, the phenylamide group is outside the enzyme active site, and these two elements are linked by a short carbon chain. Right panel: Modeled tubulin bound to HDAC6. Zn2+in the active site is shown in grey. The hydroxamic acid group in tubulin is bound to the Zn2+, the phenylamide group is outside the enzyme active site and these two elements are linked by a short carbon chain. A bulk chemical entity has been grafted onto the phenylamide part of SAHA to obtain selectivity towards HDAC6 due to specific S-pi interactions from sulfur atom (in yellow).
Abbreviations: HDAC, histone deacetylase; SAHA, suberoylanilide hydroxamicacid.
Figure 3.
Histone deacetylase (HDAC) inhibitors used in clinical trials arranged by chemical classes.
Besides its HDAC inhibition activity, Vorinostat (Figure 2, left), deriving from TSA, has a complex and not yet fully characterized activity leading to the accumulation of acetylated histones and non histone proteins. First generation HDACi are not selective except for a partial selectivity achieved31,32 in rare cases using bulk chemical groups to generate specific interactions with the external surface of the active site of the enzyme, like in tubacin.33 Sulfur-based zinc-binding groups also showed some selectivity in compounds like Largazole, a potent and selective HDACi for HDAC1 and 2. It is a densely functionalized macrocyclic peptide isolated from the Cyanobacterium symploca sp. by Luesch and coworkers.34 Entinostat and Mocetinostat have selectivity for HDAC1-3 and also against HDAC11 for the latter. Valproate and sodium butyrate (NaB) better target HDAC I and IIa. For sirtuins35 inhibition is based on NAD+competitive binding with attempts to propose a pharmacophore, according to the various inhibitor structures described.36
SIRT1 activation is the novel therapeutic approach to treat chronic inflammatory diseases, and enzyme activators are therefore sought. Many screening tests to search for HDACi use short histone peptides, capturing baits and engineered cells. All have their limitations because, in vivo, HDAC are parts of mega Daltons modeling chromatin complexes that may change within each cell type.
HDAC inhibitors metabolism
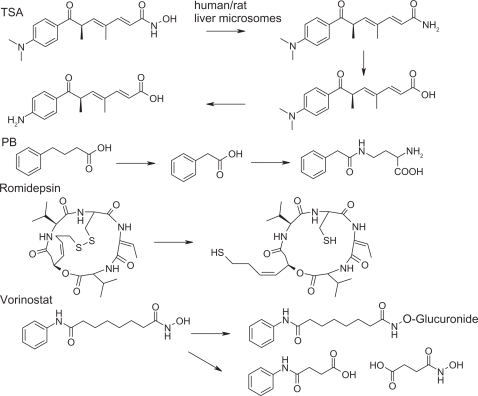
The metabolism of HDACi is an important concern during clinical assays. It is studied to determine the correlation between HDACi blood concentration, effective biological effects and eventual drug interactions. The known metabolisms of some HDACi are reported in Figure 4. TSA is metabolized as the inactive trichostatic acid, which is further demethylated37 for rapid clearance (Figure 4). Phenyl butyrate (PB) metabolism has been described in several contexts.38 PB is β-oxidized to phenylacetate, and cleared out upon glutamine addition. Vorinostat is also oxidized to 4-anilino-4-oxobutanoic acid and glucuronylated.39,40 Romidepsin is a disulfide prodrug. The real active form corresponds to the free thiol metabolite,41 produced in vivo; the butenthiol part being thought to be the zinc-binding group. A glutathione conjugate has also been described,42 which is metabolized in vivo by the cytochrome P450s43 with slow and high acetylating subjects. Other HDACi stabilities have been investigated.44,45
Figure 4.
Metabolic processes for some histone deacetylase inhibitors.
Abbreviations: PB, phenyl butyrate; TSA, trichostatin.
In-vitro effects of HDAC inhibitors
DNA chips studied the transcriptome of cells treated with Vorinostat and Romidepsin,46 revealing that the expression of 40% of all genes was affected over a period of 16 hours. A Belinostat mRNA signature of 25 genes was sufficient to assess the overall gene modulation. Panobinostat modulated cell cycles and angiogenic genes.47 Tumor antigen expression modulation and major histo-compatibility antigen (MHC) molecule induction48 have been observed with Dacinostat.49 Mice bearing human tumor xenografts treated with Belinostat showed a modulation of the expression of genes active in the cellular G2/M phase. This was different from what was seen with 5-fluorouracil (5-FU), Cisplatin, Paclitaxel, or Thiotepa. Synergistic effects were obtained when combining HDACi and DNA demethylating agents,50 or HDACi and all-trans retinoic acid (ATRA), a cell-differentiating agent used to treat acute promyelocytic leukemia (APL).51 The influence of epigenetic modulators to modify stem cell fate and its relevance for curing diseases has been reviewed.52 Successful therapeutic use of HDACi may thus depend on the cellular environment, the specific HDAC targeted, and the relative dependence of the tumor on the unique set of pathways influenced by a specific HDAC. Results are summarized in Table 1.53
Table 1.
In-vitro modulation of gene expression by HDACi
| HDACi |
Gene expression |
|
|---|---|---|
| Upregulated (cell type) | Downregulated (cell type) | |
| Vorinostat Romidepsin |
TOB1, BTG1, BTG2, MX11, MAD, MLX, TIEG, ID2, ID3, CDKs, DRAK1, DRAK2, DAPK3, GADD45β, GADD153. | MYC, MCM-3, MCM-5, MCM-7. |
| Vorinostat (in CDK2 expressing cells) | PKCA, PAK1, TRAF1. | ZAK, BCL2, B-MYC, GRIM19, PDC4, P2RX1, CD27. |
| Belinostat | CTGF, DHRS2, DNAJB1, H1F0, MAP1 LC3B, ODC, SAT, TACC1. | ABL1, CTPS, EIF4G2, KPNB1, CAD, RAN, TP53, TYMS, TD-60. |
| Panobinostat | p-FLT-3, FLT-3, Bcr-Abl, p-AKT, phospho-ERK1/2. TS (in HCT116 cells). | |
| Apicidin | CDKN1A, gelsolin. | |
Abbreviations: TOB1, transducer of ERBB2; BTG1, B-cell translocation gene 1; BTG2, B-cell translocation gene 2; MX11, MAX dimerization protein 1; MAD, mitotic arrest deficient-like; MLX, MAX-like protein X; TIEG, Kruppel-like factor 10; ID2, inhibitor of DNA binding 2; ID3, inhibitor of DNA binding 3; CDKs, cyclin-dependent kinase; DRAK1, serine/threonine kinase; DRAK2, serine/threonine kinase 17b; DAPK3, death-associated protein kinase 3; GADD45β, growth arrest and DNA-damage-inducible 45 beta; GADD153, DNA-damage-inducible transcript 3; MYC, v-myc myelocytomatosis viral oncogene homolog tumor necrosis factor; MCM-3-7, minichromosome maintenance complex component 3-5-7; PRKCA, Protein kinase C a; PAK1, p21 protein (Cdc42/Rac)-activated kinase 1; TRAF1, TNF receptor-associated factor 1; ZAK, zinc finger protein 33A; BCL2, B-cell CLL/lymphoma 2; B-MYC, c-myc binding protein; GRIM19, NADH dehydrogenase 1 alpha subcomplex; PDC4, DEP domain containing 4; P2RX1, purinergic receptor; P2X, ligand-gated ion channel1; D27, CD27 molecule; CTGF, connective tissue growth factor; DHRS2, dehydrogenase/reductase S2; DNAJB1, DnaJ (Hsp40) homolog; subfamily B; member 1; H1F0, H1 histone family; member 0; MAP1LC3B, microtubule-associated protein 1 light chain 3 beta; ODC, ornithine decarboxylase 1; SAT, spermidine/spermine N1-acetyltransferase 1; TACC1, transforming acidic coiled-coil containing protein 1; ABL1, c-abl oncogene 1; CTPS, CTP synthase; EIF4G2, eukaryotic translation initiation factor 4 gamma; KPNB1, karyopherin beta; CAD, carbamoyl-phosphate synthetase 2; RAN, member RAS oncogene family; TP53, tumor protein p53; TYMS, thymidylate synthetase; TD-60, regulator of chromosome condensation 2; FLT-3, fms-related tyrosine kinase 3; Bcr-Abl, c-abl oncogene 1; non-receptor tyrosine kinase; p-AKT, v-akt murine thymoma viral oncogene homolog 1; TS, Thymidilate synthase; CDKN1A, cyclin-dependent kinase inhibitor 1A; CDK2, cyclin-dependent kinase inhibitor 2A.
Clinical trials with zinc-dependent HDACi
This part of the review describes the HDACi that have been or are being investigated in clinical trials. In Table 2, all current trials are recapitulated. In Table 3, and for each molecule, some data related to epigenetic measurements are summarized.
Table 2.
Clinical trials for epigenetic drugs
| Safety study of CHR-3996, in patients with advanced solid tumours |
| Safety and Tolerability of CHR-2845 to treat hematological diseases or lymphoid malignancies |
| A safety and dose-finding study of JNJ-26481585 for patients with advanced refractory leukemia or myelodysplastic syndrome |
| Phase II study of Givinostat in very high-risk relapsed/refractory Hodgkin’s lymphoma patients |
| Phase II study of Givinostat followed by Mechlorethamine in relapsed/refractory Hodgkin’s lymphoma patients |
| Phase II study of Givinostat in refractory/relapsed lymphocytic leukemia |
| Phase II study of Givinostat in combination with hydroxyurea in polycythemia vera |
| Clinical trial of Belinostat in patients with advanced multiple myeloma |
| Belinostat to treat tumors of the thymus at an advanced stage |
| Belinostat in relapsed or refractory peripheral T-cell lymphoma |
| Belinostat in treating patients with MSD |
| Safety and efficacy of Belinostat when used with standard of care chemotherapy for untreated NSCLC |
| A Phase I study of Belinostat in combination with Cisplatin and Etoposide in adults with SCLC and other advanced cancers |
| Vorinostat for locally advanced NSCLC |
| Vorinostat in treating patients with metastatic and/or locally advanced or locally recurrent thyroid cancer |
| A Study of the efficacy of Vorinostat in patients with polycythemia verae and essential thrombocythemia |
| Study of Vorinostat Plus Capecitabine and Cisplatin for 1st Line Treatment of metastatic or recurrent gastric cancer |
| Vorinostat with Capecitabine Using a new weekly dose regimen for advanced breast cancer |
| Study of Vorinostat combination with Bortezomib in patients with multiple myeloma |
| Study of Vorinostat and Gefitinib in relapsed/or refractory patients with advanced NSCLC |
| Proteasome Inhibitor NPI-0052 (marizomlib, salinosporamide A) and Vorinostat in patients with NSCLC, pancreatic cancer, melanoma or lymphoma |
| Vorinostat combined with Gemtuzumab Ozogamicin, Idarubicin and Cytarabine in acute myeloid leukemia |
| Trial for locally advanced Her2 positive breast cancer using Vorinostat and Paclitaxel, Trastuzumab, Doxorubicin and Cyclophasmide on a weekly basis |
| Sirolimus and Vorinostat in advanced cancer |
| Temsirolimus and Vorinostat in treating patients with metastatic prostate cancer |
| Vorinostat, Carboplatin and Gemcitabine plus Vorinostat maintenance in women with recurrent, Platinum-sensitive epithelial ovarian, Fallopian tube, or peritoneal cancer |
| Vorinostat and Gemcitabine in treating patients with metastatic or unresectable solid tumors |
| Vorinostat and Lenalidomide in treating patients with relapsed or refractory Hodgkin lymphoma or non-Hodgkin lymphoma |
| Hydroxychloroquine + Vorinostat in advanced solid tumors |
| Vorinostat in combination with palliative radiotherapy for patients with NSCLC |
| Vorinostat in combination with radiation therapy and infusional Fluorouracil (5-FU) in patients with locally advanced adenocarcinoma of the pancreas |
| Study of 5-azacytidine in combination with Vorinostat in patients with relapsed or refractory diffuse large B cell lymphoma |
| An Investigational Study of Vorinostat Plus Targretin (Bexaroten) in cutaneous T-cell lymphoma patients |
| Phase II Trial of Vorinostat and Tamoxifen for patients with breast cancer |
| Oral Panobinostat in relapsed or refractory CLL and MCL (non-Hodgkin’s lymphoma) |
| Panobinostat in Phase II in SCLC |
| Panobinostat in treating patients with relapsed or refractory acute lymphoblastic leukemia or acute myeloid leukemia |
| Study of Oral Panobinostat in adult patients with refractory/resistant cutaneous T-cell lymphoma |
| Study of Bortezomib and Panobinostat in treating patients with relapsed/refractory peripheral T-cell lymphoma or NK/T-cell Lymphoma |
| Study of Panobinostat to treat malignant brain tumors |
| Study of Oral Panobinostat in patients with cutaneous T-cell lymphoma and adult T-cell leukemia/lymphoma |
| Panobinostat in adult patients with advanced solid tumors or cutaneous T-cell lymphoma |
| A study of Panobinostat as second-line therapy in patients with chronic graft-versus-host disease |
| Panobinostat treatment for refractory clear cell renal carcinoma |
| A Study to investigate the effect of food on oral Panobinostat absorption in patients with advanced solid tumors |
| ERB-B4 after treatment with Panobinostat in ER+ Tamoxifen refractory breast cancer |
| Panobinostat in addition to corticosteroids in patients with acute graft versus host disease |
| Panobinostat and Imatinib Mesylate in treating patients with previously treated chronic phase chronic myelogenous leukemia |
| Study of Imatinib, a Platelet-derived Growth Factor Receptor Inhibitor, and Panobinostat, in the treatment of newly diagnosed and recurrent chordoma |
| Oral Panobinostat in combination with Carboplatin and Paclitaxel in advanced solid tumors |
| Safety and efficacy studies of Panobinostat and Bicalutamide in patients with recurrent prostate cancer after castration |
| Panobinostat and Everolimus in treating patients with recurrent multiple myeloma, non-Hodgkin lymphoma, or Hodgkin lymphoma |
| Panobinostat and Fluorouracil followed by Leucovorin Calcium in treating patients with stage IV colorectal cancer who did not respond to previous |
| Fluorouracil-based chemotherapy |
| Sorafenib and Panobinostat in hepatocellular carcinoma |
| A Safety study of Panobinostat and Everolimus to stabilize kidney cancer |
| Phase I/II Study of Panobinostat and Erlotinib for advanced aerodigestive tract cancers |
| Use of Panobinostat with or without Rituximab to treat B-cell non-Hodgkin lymphoma |
| Entinostat in treating patients with advanced solid tumors or lymphoma |
| Entinostat in treating patients with hematologic cancer |
| Safety and efficacy study of Entinostat a new chemotherapy agent to treat metastatic melanoma |
| Entinostat and Sorafenib Tosylate in treating patients with advanced or metastatic solid tumors or refractory or relapsed acute myeloid leukemia |
| Entinostat and Isotretinoin in treating patients with metastatic or advanced solid tumors or lymphoma |
| Entinostat and Azacitidine in treating patients with myelodysplastic syndromes, chronic myelomonocytic leukemia, or acute myeloid leukemia |
| Entinostat and Clofarabine in treating patients with newly diagnosed, relapsed, or refractory poor-risk acute lymphoblastic leukemia or bilineage/biphenotypic leukemia |
| A Phase II Study of Entinostat, in combination with GM-CSF treating relapsed and refractory myeloid malignancies |
| Azacitidine with or without Entinostat in treating patients with MSD, chronic myelomonocytic leukemia, or acute myeloid leukemia |
| Interleukin 2, Aldesleukin and Entinostat for kidney cancer |
| Safety and Efficiency Study of Valproic Acid In HAM/TSP |
| Valproic Acid as an effective therapy for chronic lymphocytic leukemia |
| Valproic Acid and Its effects on HIV latent reservoirs |
| Valproic Acid in treating patients with previously treated non-Hodgkin lymphoma, Hodgkin lymphoma, or chronic lymphocytic Leukemia |
| Bevacizumab, chemotherapy and Valproic Acid in advanced sarcoma |
| Combined therapy with Valproic Acid, All-trans Retinoic Acid (ATRA) and Cytarabine in acute myelogenous leukemia |
| Valproic Acid with Temozolomide and radiation therapy to treat brain tumors |
| Azacytidine and Valproic Acid in patients with advanced cancers |
| Phase II study of 5-Azacytidine Plus Valproic Acid and eventually ATRA in intermediate II and high risk MDS |
| Phase I/II Trial of Valproic Acid and Karenitecin for melanoma |
| 5-azacytidine, Valproic Acid and ATRA in acute myeloid leukemia and high risk MDS |
| Hydralazine and Valproate added to chemotherapy for breast cancer |
| Hydralazine and Valproate plus Cisplatin chemoradiation in cervical cancer |
| A Pilot study of Pivanex in patients with chronic lymphocytic leukemia |
| A Pilot study of Pivanex in patients with malignant melanoma |
| Comparative trial of Pivanex and Docetaxel vs Docetaxel Monotherapy in Patients with advanced NSCLC |
| Study of SB939 in Subjects with myelofibrosis |
| SB939 in treating patients with locally advanced or metastatic solid tumors |
| SB939 in treating patients with recurrent or metastatic prostate cancer |
| Phase 2 study of Azacitidine vs MGCD0103 vs combination in elderly subjects with newly diagnosed AML or MDS |
| AR-42 (OSU-HDAC42) in treating patients with advanced or relapsed multiple myeloma, chronic lymphocytic leukemia, or Lymphoma |
| Resminostat in relapsed or refractory Hodgkin’s lymphoma |
| Study of the safety and tolerability of PCI-2478 in patients with lymphoma |
| Safety and tolerability study of PCI-24781 in subjects with cancer |
| Phase I Study of gene induction mediated by sequential Decitabine/Depsipeptide infusion with or without concurrent Celecoxib in subjects with pulmonary and pleural malignancies |
| Romidepsin in treating patients with relapsed or refractory non-Hodgkin’s Lymphoma |
Data from US National Cancer Institute
Table 3.
In vivo HDACi effects from clinical data
| HDACi (metabolism, half-life, bioavailability) and combinations | MTD, cancer target, DLTs | Biological analyses (source) | Remarks or recommendations | |
|---|---|---|---|---|
| PB converted in vivo to the active metabolite PA, not indicated, 78% at 0.5 mM | A | Phase II, 27 g/day, GBM, common DLTs | Contradictory with P450-inducing anticonvulsants. One complete response for 5 years |
|
| B | 300–410 mg/kg/day, various cancers, CNS | |||
| PB+5-aza | C | AML/MDS, skin reaction (5-aza) | H4 acetylation increased (not correlated with response) | |
| D | 25 mg/m2/d SQ d. 1–14, Several cancers, common DLTs + confusion, hearing loss, triglyceridemia and hyperuricema | Low DNMT activity | ||
| AN-9 Is converted in vivo to the active metabolite BA, half-life <2 minutes |
E | 3.3 g/m2/day for the solubility limits, advanced solid malignancies, common DLTs + visual complaintsa | ||
| F | Phase II refractory NSCLC, common DLTs and dysgeusia | Well tolerated, active alone and usable for NSCLC with chemotherapy, 1-year survival around 30% | ||
| VPA Glucuronylation, glutamination, half-life: 9–18 hours |
G | 60 mg/kg/d, refractory advanced cancer, neurological side effects (Grade 3/4) | H3, H4 acetylation increased, HDAC2 decreased (PMBC) | |
| VPA+ATRA | H | AML, neurologic and cardiovascular toxicity | HDAC2 decreased (PMBC) | |
| I | MDS and relapsed or refractory AML | Bone marrow blast count correlated with response | VPA should be used alone for low risk MDS and with other chemotherapeutics for high risk MDS | |
| J | Recurrent or refractory AML or MDS, neurocortical, severe bone pain (Grade 3/4) | No significant blast count reduction, cytogenetic analysis of patients is described | Platelet transfusion independence should reduce palliative care and improve the quality of life | |
| K | AML | Particular response from patients with AML-M6 | ||
| VPA+13-cis RA or vitamin D3 | L | MDS or CMML | No relation between VPA serum level, H3 acetylation (PMBC, BMMC) or clinical response | Near 50% patients had to end the treatment |
| VPA+5-azaDc | M | Phase I//II leukemia | DNA demethylation decreased, H3 and H4 acetylation increased, p15 reactivation, p21 cip1 not stimulated | Objective responses rate: 22%, complete remissions: 19%, safety and efficacy correlated with reversal of epigenetics marks |
| N | AML, limited non hematologic toxicity (5-azaDc), encephalopathy (VPA) | Correlation with re-expression of ER mRNA and clinical response, p15 promoter methylation decreased DNA methylation decreased, DNMT1 decreased, histone acetylation increased | ||
| VPA + 5-aza | O | Advanced cancers | DNA methylation decreased (not significant), H3 acetylation increased (PMBC) | Safe at doses up to 75 mg/m2 for 5-aza |
| VPA + 5-aza + ATRA | P | 50 mg/kg/d for 7 days, AML and MDS, neurotoxicity | DNA methylation decreased, H3 and H4 deacetylation increased, p21 cip1 and p15 mRNA expression not associated with clinical response | Combination safe with significant clinical activity |
| VPA + epirubicin or 5-FU or cyclophosphamide | Q | Breast cancers | H3 and H4 acetylation increased (PMBC), strong correlation for HDAC2 decreased in MCF-7 cells, no correlation for HDAC6 | Objective responses for 64% (9/15) of the patients |
| VPA + epirubicin | R | Solid tumors, confusion, hallucinations, hearing loss and dizziness (due to VPA half-life) | 48 hours exposure VPA for chromatin decondensation prior to epirubicin exposure. Histone acetylation increased (PBMN) | Responses were obtained for anthracycline-resistant cancers (breast, cervical and NSCLC). |
| VPA + KTN | S | Melanoma, no VPA/KTN synergistic toxicity | H3 and H4 acetylation increased (PMBC, apparent plateau for 60 mg/kg/day VPA) | Potential use in randomized trials where topoisomerase I inhibitors are involved |
| VPA + dazacarbine + interferon-α | T | Advanced inoperable or metastatic melanoma, high doses VPA side effects | Histone acetylation increased (PMBC) with adjusted VPA doses. | Modification of the schedule for further evaluation of VPA with chemo-immunotherapy |
| VPA Mg salt | U | Cervical cancer, depressed level of consciousness | H3 and H4 acetylation increased (PMBC and tumors). | |
| VPA Mg salt + hydralazine | V | Chemotherapy resistant refractory solid tumors, hematologic toxicity | DNA methylation decreased, histone deacetylase activity decreased, promoter methylation decreased for RAR-α and DPK. 1091 genes upregulated, 89 genes downregulated. | Patients from cisplatin, carboplatin, paclitaxel, vinorelbine, gemcitabine, pemetrexed, topotecan, doxorubicin, cyclophosphamide, and anastrozole treatments. Supports epigenetic-driven tumor-cell chemoresistance hypothesis. |
| Vorinostat, glucuronylated, β-oxidized, half-life <2 hours, 43% oral bioavailability | W | Solid tumors and hematological malignancies, leukcopenia, thrombocytopenia, respiratory distress (Grade 3/4) | Histones acetylation increased (v) | |
| X | Mesothelioma, commonb DLTs. | A randomized Phase III study can be proposed for patients already treated unsuccessfully with pemetrexed | ||
| Y | Hematologic malignancies and solid, common DLTs + anorexia | Histone acetylation (PMBC, 200 to 600 mg). | Safe when administered chronically, broad range of antitumor activity. | |
| Z | Advanced leukemias and MDS (AML, CLL, MDS, ALL, CML), common DLTs (Grade 3/4) | Incomplete blood count recovery (AML), histone acetylation increased at all doses | None of the responding patients have a specific mRNA signature for antioxidant genes to be used as a biomarker for further studies. | |
| AA | Recurrent or persistent epithelial ovarian or primary peritoneal carcinoma platinum-resistant/refractory, common Grade 3 DLTs +leucopoenia and neutropenia (Grade 4). | SAHA is well tolerated but had minimal activity as a single agent | ||
| AB | Measurable, relapsed or refractory breast cancer or NSCLC or colorectal cancer, common DLTs (300–400 mg) No DLTs at 200 mg. | The limited patient exposure was not sufficient to assess SAHA efficacy. | ||
| AC | Recurrent and/or metastatic head and neck tumors, thrombocytopenia, anorexia, and dehydration | |||
| AD | GBM, nonhematologic, and hematologic toxicities (Grade 3). | H2B, H4 and H3 acetylation increased. Upregulation of E-cadherin. | Enzyme-inducing anticonvulsants gave lower SAHA concentrations, well tolerated, modest activity. | |
| AE | Metastatic radioiodine-refractory thyroid carcinoma, common DLTs (Grade 3), pneumonia, severe thrombocytopenia | Tg (DTC) and calcitonin (MTC) are not convenient biomarkers. | Lack of therapeutic effect | |
| Vorinostat + carboplatin and paclitaxel | AF | Advanced solid malignancies, common DLTs + emesis (Grade 3), neutropenia (Grade 4) | SAHA metabolite 4-anilino-4-oxobutanoic acid used as a marker to monitor for adherence to SAHA therapy. | Combinations well tolerated and increased SAHA half-life, paclitaxel PKs not altered |
| Belinostat, half-life 1–2 hours | AG | 1000 mg/m2/day, refractory solid tumors, common DLTs + atrial fibrillation | H4 acetylation (PMBC), IL-6 expression levels proposed as a marker for HDACi toxicity209 | 50% of the patients achieve stable disease |
| AH | 1000 mg/m2/day, heavily pre-treated patients with advanced hematological neoplasia, common DLTs | Histone acetylation increased (PMBC) up to 24 hours post injection. | No bone marrow toxicity as a parameter for combination therapies. | |
| AI | Relapsed malignant pleural mesothelioma, common DLTs | No objective responses. One death from cardiac arrhythmia, possibly related to therapy. | ||
| AJ | Resistant micro papillary ovarian tumors (LMP) and epithelial ovarian cancer, common DLTs + thrombosis (Grade 3) | H3 and H4 acetylation increased (PMBC and tumor tissue). Disease. | Diseases with poor prognostic and scarce studies, well tolerated, promising results for LMP | |
| Givinostat | AK | Relapsed/refractory HL, thrombocytopenia and prolongation of QTc interval | QTc interval in some cases prompting drug discontinuation | |
| Givinostat, alone or + dexamethasone | AL | Twice daily 100 mg/4 days/week, 12 weeks, MM, one death, cardiac toxicities (givinostat) | Already treated patients, one death was reported | Unlikely to play a significant role for MM, other combination may be investigated |
| Givinostat + meclorethamine | AM | Relapsed/refractory HL | TARC decrease in serum as an easy-to-detect biomarker predicting response to therapy, five (15%) complete remissions and eight (23%) partial remissions, median survival 28 months, projected 2-year survival 52% | 15% complete remissions, median survival at 28 months, projected 2-year survival of 52%. |
| Panobinostat, half-life 11–16 hours | AN | <11.5 mg/m2, AML, ALL, MDS, common minor DLTs, and cardiac toxicity (Grade 3) | H3 acetylation increased (B-cells (CD19+) and blasts (CD34+)), H2B acetylation increased (CD19+ and CD34+ cells), apoptosis increased for CD34+. | |
| AO | CTCL, classical DLTs at 20 mg + thrombocytopenia, toxic at 30 mg | RNA profiling: 23 genes regulated in all patients. H3 acetylation increased (PMBC and tumors) | Complete remission | |
| Panobinostat + docetaxel | AP | Castration resistant prostate cancer, neutropenia and dyspnea (Grade 3) | Progressive disease despite histone acetylation increased (PMBC) in first regimen, PSA decreased in second regimen | Intravenous administration suggested for further investigations |
| Dacinostat, half-life 9–18 hours | AQ | Advanced solid tumors, common DLTs and transaminitis, fibrillation, raised serum creatinine, and hyperbilirubinemia. | Histones acetylation increased (PMBC), HSP90210 inhibition measured by HSP72 levels increased; H3 and H4 acetylation increased for >24 hours, Hsp70 increased and c-Raf decreased. | Nonhistone-mediated effects requires further study |
| AR | ALL, AML, CLL, CML, MDS, dose dependant DLTs: cerebral bleed secondary to thrombocytopenia (CLL), reversible hyperbilirubinemia | Histone acetylation increased >24 hours, nonlinear PK | Rematologic improvement observed, mean terminal half-lives 9–18 hours (maximum plasma concentrations 1.5 hours after the beginning of infusion). | |
| PCI-24781 half-life 5.9 hours, oral bioavailability 34% | AS | Refractory advanced solid tumors, common and cardiac DLTs | Acetylation levels increased at 1.5 hours post dose sustained ≥24 hours (oral). | |
| Entinostat, half-life 34–50 hours, highly protein bound, apparent linear PKs | AT | 10 mg/m2, advanced solid tumors or lymphoma, common DLTs | HDAC inhibition (PMBC) | More frequent dosing proposed for evaluation from linear PKs data, elimination half-life dose-independent clearance |
| AU | Refractory solid tumors and human lymphoid malignancies, reversible DLTs (Grade 3, hypophosphatemia, hyponatremia, and hypoalbuminemia) | Protein acetylation increased (by multivariable flow cytometry in PMBC (T cells (most robust response), B cells, and monocytes)) | Well tolerated administered weekly with food | |
| AV | 8 mg/m2 weekly for 4 weeks every 6 weeks, AML, infections and neurologic toxicity | Protein and histone H3/H4 acetylation increased (PMBC, BMMC), p21 expression increased, and caspase-3 activation (BMMC) | Detailed cytogenetic analysis performed on patients, inherent resistance to MS-275 for advanced leukemia with complex karyotype | |
| AW | Advanced solid malignancies and lymphomas, hypophosphatemia, and asthenia | High degree of interpatient variations in H3 and H4 acetylation increased (PMBC) | ||
| AX | Metastatic melanoma, toxicity mild to moderate | Patients with pretreated metastatic, melanoma, treatment well tolerated, no objective responses, median time-to-progression was 51–56 days | ||
| Mocetinostat, half-life 6.7–12.2 hours | AY | 45 mg/m2/d, advanced solid tumors, rare common DLTs | H3 acetylation increased (PWBC, measured with the BOC-Lys(ε-Ac)-AMC fluorophore), IL-6 induction | Interpatient variability improved with low pH beverages |
| AZ | 60 mg/m2, AML, MDS, ALL, and CML, common DLTs (Grade 3) | Histone acetylation increased (PWBC, measured with the BOC-Lys(ε-Ac)-AMC fluorophore). | Three complete bone marrow response, cytogenetic analysis of patients not correlated with responses, safe and anti-leukemia activity for advanced leukemia | |
| BA | Advanced leukemias or MDS, common DLTs | HDAC inhibition (PMBC) | Four patients with stable disease | |
| Mocetinostat continued | BB | 85 mg dose exhibited meaningful activity, HL | TARC levels correlated with clinical response | Two complete responses (10%), six partial responses (29%) |
| BC | FL, common Grade 3 DLTs + anorexia, thrombocytopenia, pericardial serious adverse event | No clear relationships with schedules, cardiac diseases, pathologies, and biomarkers such as HDAC activity. | ||
| Tacedinaline | BD | 8 mg/m2/day for 8 weeks, repeated after a 2-week drug-free interval, solid tumors, common DLTs + thrombocytopenia, anemia, mucositis | ||
| Tacedinaline + capecitabine | BE | 6 mg/m2 Tacedinaline and 2000 mg/m2/day capecitabine, for 2 weeks of a 3-week cycle, advanced solid malignancy, DLT was thrombocytopenia | No overlapping toxicity | |
| Tacedinaline + gemcitabine | BF | Advanced pancreatic cancers, neutropenia, and thrombocytopenia | Combination does not improve treatment | |
| Depsipeptide, natural disulfide prodrug, half-life 8 hours | BG | 13.3 mg/m2, incurable cancers, common DLTs + thrombocytopenia, and fatigue. | Histone acetylation increased (MNPB), PC3 cell cycle arrest induction, MDR-1 induced, functional PgP. | 4-hour infusion safe |
| BH | 17.8 mg/m2/4 h, advanced or refractory neoplasms, common DLTs + grade-4 thrombocytopenia and cardiac arrhythmia. | Histone acetylation increased (PMBC), MDR-1 induced. | Continuous cardiac monitoring, one partial response, 472.6 ng/mL mean maximum plasma concentration at MTD | |
| BI | CLL, AML, common DLTs | HDAC inhibition increased, histone and p21 promoter H4 acetylation increased, p21 protein and 1D10 antigen expression increased, acetylation increased for H4 K5, K12, K8, K16, and H3 K9, K14 | ||
| BJ | 17 mg/m2, refractory or recurrent solid tumors, reversible, asymptomatic T-wave inversions, transient asymptomatic sick sinus syndrome, and hypocalcemia | HDAC inhibition increased (PMBC) | Depsipeptide is well tolerated but no objective responses | |
| BK | MDS and AML, common DLTs (Grade 3/4 asymptomatic hypophosphatemia) | Apoptosis increased and changes in myeloid maturation marker expression. No changes in H3 and H4 acetylation, CD34/C13 stimulation. | One complete remission (AML) acceptable toxicity, limited activity in unselected AML/MDS patients | |
| BL | Refractory renal cell cancer, cardiac side effects | One complete response but not active enough in this population, one sudden death | ||
| BM | Metastatic neuroendocrine tumors, common DLTs, serious cardiac adverse events | One sudden death | ||
| BN | Lung cancers | H4 acetylation increased, p21 expression increased. 16 gene expressions stimulated ≥2-fold, >1000 genes repressed ≥2-fold. | Depsipeptide not appropriate but renormalize lung cancer cells to normal bronchial epithelia |
Notes:
Resulted from formaldehyde released after AN-9 metabolism;
Common DLTs are considered to be fatigue, nausea, vomiting.
Abbreviaions: 5-aza, 5-aza-cytidine; 5-azaDc, 5-aza-2′-deoxycytidine; 5-FU, 5-fluorouracil; 13-cis-RA, 13-cis-retinoic acid; ALL, acute lymphoblastic leukemia; AMC, 7-amino-4-methylcoumarin; AML, acute myeloid leukemia; AN-9, pivaloyloxymethyl butyrate 9; ATRA, all-trans retinoic acid; BA, butyric acid; BMMC, bone marrow mononuclear cell; CLL, chronic lymphocytic leukemia; CML, chronic myelocytic leukemia; CMML, chronic myelomonocytic leukemia; CNS, central nervous system; CTCL, cutaneous T-cell lymphoma; DLT, dose-limiting toxicity; DNMT, DNA methyltransferases; DTC, differentiated thyroid carcinoma; ER, estrogen receptor; FL, follicular lymphoma; GBM, glioblastoma multiform; HDAC, histone deacetylase; HDACi, HDAC inhibitor; HL, Hodgkin lymphoma; IL-6, interleukin-6; KTN, karenitecin; LMP, low malignant potential; MDS, myelodysplastic syndrome; MM, multiple myeloma; MTC, medullary thyroid cancer; MTD, maximum tolerated dose; NSCLC, nonsmall cell lung cancer; PA, phenylacetate; PB, phenylbutyrate; PgP, P-glycoprotein; PK, pharmacokinetic; PMBC, peripheral mononuclear blood cell; PSA, prostate specific antigen; PWBC, peripheral white blood cell; QTc, QT interval corrected for heart rate; RAR-α, retinoic acid receptor-α; SAHA, suberoylanilide hydroxamicacid; TARC, thymus and activation regulated chemokine; Tg, thyroglobulin; VPA, valproic acid.
PB or its sodium salt
PB or its corresponding sodium salt (NaPB) is a short chain fatty acid approved by the Food and Drug Administration (FDA) for the treatment of hyperammonemia. It stops the cell cycle in its G1–G0 phase. PB is an efficient HDACi at about 0.5 mM.54,55 PB induces apoptosis – probably via c-jun N-terminal kinase (JNK) – in lung carcinoma cells,56 p21waf1-mediated growth arrest in MCF-7 cells,57 tumor necrosis factor (TNF)-α58 or peroxisome proliferator-activated receptor (PPAR)λ-mediated59 cell differentiation, and is more potent than phenylacetate in prostate cancer cells,60 while increasing MHC class I expression. PB is converted in vivo into the active metabolite phenylacetate (PA) by β-oxidation in the liver and kidney mitochondria.61 Most dose-limiting toxicities (DLTs) are fatigue, nausea, and somnolence. Preliminary studies have been conducted in patients with recurrent glioblastoma multiform (GBM)62 (Table 3, A). Phase I studies have been conducted in patients with hormone refractory prostate cancers,63 refractory solid tumor malignancies64 like colon carcinoma, non small cell lung cancer (NSCLC), anaplastic astrocytoma, GBM, bladder carcinoma, sarcoma, ovarian carcinoma, rectal hemangiopericytoma, and pancreatic carcinoma,65 mainly as intravenous infusions but also in AML and myelodysplastic syndrome (MDS).66 DLTs were neuro-cortical with milder fatigue and nausea/vomiting, light-headedness, short-term memory loss, sedation, confusion, and hypocalcemia. Although central nervous system (CNS) toxicity was observed, infusions were well tolerated (Table 3, B). The active metabolite PA accumulated.
In the AML/MDS study,67 with sequential administration of 5-aza-cytidine (5-aza) (Table 3, C), partial remissions or stable diseases were obtained. Targeting different biological mechanisms is feasible with acceptable toxicity. Phase I trials in combination with several drugs have been reported. Prostate, colorectal, leiomyosarcoma, and esophageal cancers were treated in combination with 5-aza (Table 3, C),68 metastatic colorectal cancer with fluorouracil 5-FU as a 24-hour continuous intravenous infusion (CIV).69 With 5-aza, no re-expression of E-cadherin, endothelin B, and glutathione S transferase (GST) pi was observed, a result explained by the lack of dose effect or by the fact that DNA methylation is an S-phase-dependent process while in-vivo prostatic cells may be in S-phase at any given time. Stable disease was the best response. Combining 5-FU appeared also feasible.
Pivaloyloxymethyl butyrate
Pivaloyloxymethyl butyrate (AN)-9, is an ester prodrug of butyric acid (BA)70 but with a greater potency at inducing malignant cell differentiation and tumor growth inhibition. It showed more favorable toxicological, pharmacological, and pharmaceutical properties than BA in preclinical studies. BA itself induces p16 expression and growth arrest of colon cancer cells,71 and modifies caspase distribution during apoptosis.72 AN-9 down regulates c-jun and c-myc and induces differentiation in leukemia cells.73 It is decomposed by esterases in vivo to yield butyric and pivaloyl acids and a formaldehyde molecule, responsible for toxicity resulting in visual acuity disorders. It has demonstrated a synergistic effect with other anticancer agents by reducing bcl-2 levels.74 Initial study75 with I.V. of AN-9 in advanced solid malignancies (Table 3, E) gave partial responses, and stable diseases as best responses. Later, a multicenter trial of pivaloyloxymethyl butyrate76 in refractory NSCLC (Table 3, F), administered as a continuous I.V. infusion, gave partial responses.
VPA
VPA is a nontoxic short-chain carboxylic acid used for the treatment of epilepsy with a long clinical history and well known pharmacokinetics (PKs) and pharmacodynamics (PDs).77,78 VPA induces chromatin decondensation,79 and differentiation in neural progenitor cells,80 and inhibits HDAC activity81 in the mM range (preferentially HDAC1, 2).82 The antiproliferative activity was associated with aberrant cyclin D3 functionality during the C6 glioma G1 phase.83 Activation of PPARδ was present in F9 cells.84 VPA induces caspase-dependent and -independent apoptosis in leukemia cells,85 and in AML cells expressing P-gp and multidrug resistance protein 1 (MRP1),86 inhibits production of TNF-α and interleukin (IL)-6 and activates nuclear factor kappa B (NF-κB).87 VPA has been evaluated in combination with other anticancer compounds. For AML, increased 5-aza cytotoxicity was associated to cyclin D1 and p27(Kip1) expression,88 while sequential VPA/ATRA treatment reprograms differentiation.89 VPA induces p16INK4A upregulation and apoptosis and sensitizes melanoma cells to chemotherapy.90 Interestingly, most of the clinical trials reported are for combination therapies.
A Phase I was conducted91 for refractory advanced cancer (colorectal, melanoma, NSCLC, and others) (Table 3, G). VPA/ATRA combination was evaluated for several diseases. Poor risk AML92 (Table 3, H), MDS and relapsed or refractory AML93 (Table 3, I) have also been investigated. A 52% response rate was observed in MDS patients. ATRA exerted no additional effect in patients receiving the combination, but could be used to induce a second response in relapsing VPA-treated patients. In recurrent or refractory AML or MDS in a Phase II protocol94 (Table 3, J), ATRA was administered when VPA reached the target serum concentration. The differentiation therapy with VPA was effective in 30% of patients. In 11 elderly patients, de novo AML95 (Table 3, K) was also treated with theophyllin to increase cAMP levels and major cell differentiation.96 Complete marrow response was observed in three patients, including one complete remission. Two additional patients had hematologic improvement. Patients with AML-M6 were found particularly97 responsive, probably due to T-cell acute lymphocytic leukemia 1 (TAL1)98 and GATA199 interactions with HDACi, inducing differentiation in murine erythroleukemia (MEL) cells. Siitonen et al100 reported a negative study trying VPA, in combination with 13-cis-retinoic acid (13-cis-RA)101 and 1,25-dihydroxyvitamin D3, in 19 naive patients with MDS or chronic myelomonocytic leukemia (CMML) (Table 3, L). Combinations with demethylating agents have been reported. Phase I/II102 study with 5-aza-2′-deoxycytidine (5-azaDc) in leukemia (Table 3, M) included gene expression analysis (p57kip2, p15, p73, MDR1 and THBS2). Initial DNMT1 levels were too low to be informative. A Phase I study103 with 5-azaDc in AML (Table 3, N) gave partial to complete remissions, warranting further studies of 5-azaDc alone or with alternative HDACi. A Phase I104 study of epigenetic modulation with 5-aza for advanced cancers (Table 3, O, colon, skin melanoma, breast, other) gave stable diseases. A Phase I/II study105 with 5-aza and ATRA for AML and MDS (Table 3, P) gave 42% positive overall responses.
Other combinations were investigated: a Phase I dose escalation combination trial with epirubicin, 5-FU, and cyclophosphamide106 in breast cancer (Table 3, Q), and a Phase I trial107 with epirubicin for solid tumors (Table 3, R). The rationale for the combination was to facilitate epirubicin access to DNA to potentiate its strand breaks activity as a topoisomerase II inhibitor. Intrinsic epirubicin toxicity was not exacerbated. Reverse combination was found inadequate by the same group. The same group investigated combination with the topoisomerase I inhibitor karenitecin (KTN) (Table 3, S) for treating melanoma with both Phase I/II trials.108 No VPA/KTN synergistic toxicity was observed. The best response was disease stabilization. VPA plus chemoimmunotherapy was investigated in a Phase II study109 for advanced inoperable or metastatic melanoma (Table 3, T), HDACi having been previously found to have a tumorigenic potential in melanoma.110 Some patients then received dacarbazine plus interferon-α with VPA.
The magnesium salt of VPA has been tested in phase I111 for cervical cancer (squamous and in adenocarcinoma) and Phase II112 clinical trials. In the Phase I study (Table 3, U), VPA was given per os, and the authors emphasized the requirement for new endpoint trials based on biomarker analysis113,114 with, in this particular case, H3 and H4 acetylation and in vivo HDAC inhibition detection. The Phase II study was conducted with hydralazine, a demethylating agent,115 (Table 3, V) to overcome chemotherapy resistance in refractory solid tumors (cervix, breast, ovarian, and others). Partial responses and disease stabilization were the best responses.
Vorinostat
Vorinostat (suberoylanilide hydroxamicacid [SAHA], Zolinza®) has probably been the most studied compound in clinical trials on several cancer types. SAHA induces differentiation,116 growth arrest,117 or apoptosis at micromolar concentrations. Vorinostat is an unselective zinc-binding118 hydroxamic-acid-type inhibitor of HDAC1, 2, 3, 6, and 8. In glioma cells, SAHA induced expression of DR5, TNFα, p21Waf1, and p27Kip1 and reduced expression of CDK2, CDK4, cyclin D1, and cyclin D2.119 SAHA can induce thyroid cancer cell death by caspase-mediated pathways,120 and induces G1 and G2-M arrest and apoptosis in several types of breast cancer cell lines,121,122 NSCLC,123 and prostate cancer cells.124 It potentiates the activity of other molecules like Paclitaxel in ovarian cancers.125
Phase I trials have been described for both oral and I.V administrations. Escalating I.V. administration126 in solid tumors and hematological malignancies (Table 3, W) gave hypotension for one schedule. In mesothelioma, with I.V. or oral formulations,127 the best responses were partial (Table 3, X). An oral formulation for hematologic malignancies (Hodgkin’s and others) (Table 3, Y) and solid tumors (mainly mesothelioma, prostate, urothelial, thyroid, and renal)128 gave one complete response while others were incomplete. Oral twice- or thrice-daily administrations in advanced leukemias and MDS (AML, CLL [chronic lymphocytic leukemia], MDS, ALL and CML [chronic myelocytic leukemia]) (Table 3, Z)129 gave two complete responses and two complete responses with incomplete blood-count recovery (all with AML treated at/below maximum tolerated dose [MTD]).
Phase II clinical trials were mainly proposed with oral formulations. A multi-institutional trial130 in women with recurrent or persistent epithelial ovarian (Table 3, AA) or primary peritoneal carcinoma platinum-resistant/refractory gave one partial response. Another multicenter open-label oral trial131 investigated measurable, relapsed, or refractory breast cancer, NSCLC, or colorectal cancer (Table 3, AB). Disease stabilization was observed in eight patients. SAHA is tolerated at 200 mg only, in a daily oral schedule for 14 days–3 weeks. In recurrent and/or metastatic head and neck cancer (400 mg every day) (Table AC)132 no confirmed responses have been observed. In patients with metastatic breast cancer,133 there were no complete or partial responses, and the heterogeneity of the recruited patients did not allow production of significant statistical results. Eight patients were positive for estrogen and/or progesterone receptors, four had amplified CerB-2. Fatigue, nausea, diarrhea, and lymphopenia were the most frequent clinically significant adverse effects. In GBM134 (Table 3 AD), an oral dose of 200 mg followed by a 7-day rest period showed that SAHA monotherapy is well tolerated with modest single-agent activity. Although HDACi were shown to induce cell death and sensitize cells to cytotoxic chemotherapy in thyroid cancer cell lines, Woyach et al135 described the lack of therapeutic effect of SAHA in patients with metastatic radioiodine-refractory thyroid carcinoma in a Phase II study (Table 3, AE). A Phase II oral combination therapy was proposed with carboplatin (I.V.) and Paclitaxel (I.V.) for advanced solid malignancies136 (Table 3, AF). Eleven partial responses occurred and seven disease stabilizations. The regimen requires drug–drug interaction to be determined. Encouraging results were obtained in patients with previously untreated NSCLC.
Belinostat
Belinostat (PXD101) is a recent hydroxamic acid HDACi that has growth-inhibitory and pro-apoptotic activity in several cancer types at submicromolar concentrations,137,138 and that has been investigated in ovarian cancers.139 It down regulates thymidilate synthase, vascular endothelial growth factor (VEGF), aurora kinase, and epidermal growth factor receptor (EGFR), and up regulates cyclin A. It has been used in combination. A gene expression-signature profiling has been reported for Belinostat.140 According to publications141 PKs gave a general 1–2-hour half-life. In early trials, DNA fragmentation increased with a combination of 5-FU in HCT116 colon cancer cells in vitro and in both HT-29 and HCT116 in xenograft models,142 and also a poly(ADP-ribose) polymerase (PARP) cleavage and down regulation of thymidylate synthase expression in HCT116. Improved tumor reduction was obtained in vivo with mouse HT29 and HCT116 xenograft models compared with single compounds, validating a rationale for the clinical schedule.
In Phase I treatment of refractory solid tumors by I.V. administration (Table 3, AG)143 the caspase-dependent cleavage of cytokeratin-18 was determined to measure the level of apoptosis.144 Heavily pre-treated patients with advanced hematological neoplasia145 (Table 3, AH) were also treated. In Phase II trials, investigations of I.V. administration of relapsed malignant pleural mesothelioma146 (Table 3, AI) indicated that combination strategies or alternate dosing schedules might be necessary. In resistant micro-papillary ovarian tumors (low malignant potential [LMP]) and epithelial ovarian cancers (EOC) (Table 3, AJ),147 a Phase II trial with Belinostat gave partial responses or stable diseases for LMP, and stabilized diseases for EOC.
Givinostat
Givinostat (ITF2357) belongs to the hydroxamic acid family of HDACi which is very similar to SAHA. It inhibits IL-6 and VEGF production in stromal cells.148
Two Phase II studies were described for relapsed/refractory Hodgkin lymphoma (HL) (Table 3, AK). A first oral one, gave stable diseases by computed tomography (CT) scan that have been associated with a significant reduction in fluorodeoxyglucose-positron emission tomography scan uptake.149 Galli et al150 developed a Phase II multicenter trial in 19 heavily treated patients that were relapsing from progressive multiple myeloma (MM) (Table 3, AL). The best responses were disease stabilization. This regimen appears as unlikely to play a significant role for advanced MM, and other combinations with currently used drugs should be investigated. A combination with the alkylating agent mechlorethamine151 (Table 3, AM) was investigated in relapsed/refractory HL.
Panobinostat
Panobisnostat (LBH589) is a hydroxamic acid HDACi, which has demonstrated anti-angiogenic and anti-proliferative activities in human prostate carcinoma cell PC-3 xenografts in vivo, inducing H3 and tubulin acetylation152 in human umbilical vascular endothelial cells (HUVEC), which corresponded to G2-M cell cycle arrest and inhibition of HUVEC cell proliferation and viability. Non cytotoxic concentrations of Panobinostat inhibited endothelial tube formation, matrigel invasion, AKT, extracellular signal-regulated kinase 1/2 phosphorylation, and chemokine receptor CXCR4 expression. Association with anti-VEGF therapies should be considered. Prince et al have discussed preclinical data on Panobinostat and emerging data from Phase I and II studies in cancer patients.153
A Phase I study in refractory hematologic malignancies (AML, ALL, and MDS) (Table 3, AN)154 with I.V. administration appeared convenient to obtain anti-leukemic and biological effects. In cutaneous T-cell lymphoma (CTCL)47 with oral formulation (Table 3, AO), the responses ranged from disease stabilization to complete remission, showing the potential of this molecule in CTCL. In combination with Docetaxel155 (Table 3, AP), a microtubule interacting agent for castration-resistant prostate cancer, Panobinostat inhibited LnCAP androgen receptor positive prostate cancer cell proliferation, potentiated by Docetaxel. Single or combined treatments were administered with oral Panobinostat.
Dacinostat
Dacinostat (NVPLAQ824, LAQ) is a hydroxamic acid derivative similar to Panobinostat.156 It showed anti-neoplastic activity and can activate genes that produce cell cycle arrest. It acetylates hsp90, inducing proteosomal degradation of Bcr-Abl and HER-2. Combination of Dacinostat with 5-azaDc157 in human MDA-MB-231 and MCF-7 breast carcinoma cells showed a synergic anti-neoplastic activity for the MDA-MB-231. For the MCF-7 tumor cells, simultaneous 5-azaDc and Dacinostat administration were antagonistic, unseen when used in a sequential schedule (5-azaDc first). This is probably due to interference in the S-phase of Dacinostat since 5-azaDc is a S-phase specific interfering molecule. Dacinostat appeared to be well tolerated in clinical trials. Phase I investigations158 in advanced solid tumors included measure of HSP72 levels and was consistent with HSP90 inhibition (Table 3, AQ). Another group159 reported the same results with increased expression of Hsp70 and decreased c-Raf levels. The biological importance of these non histones mediated effects requires further study. I.V. administration for ALL, AML, CLL, CML160 (Table 3, AR) in blast crisis or advanced MDS gave some stable diseases.
PCI-24781
PCI-24781 is a broad-spectrum hydroxamic acid-based HDACi. PCI-24781 reverses drug resistance in four multidrug resistant sarcoma cell lines and synergizes with chemotherapeutic agents to enhance caspase-3/7 activity.161
In refractory advanced solid tumors162 (Table 3, AS), I.V. administration followed by dose escalation was well tolerated. Electro cardiac monitoring revealed grade ≤1 QTcF (QT interval corrected for heart rate using Fridericia’s formula) prolongation and asymptomatic nonspecific ST and T wave changes leading to discontinuation.
Entinostat
Entinostat (MS-275, SNX-275) is a benzamide HDACi, which promotes expression of genes involved in growth arrest and differentiation, like p21 and the maturation marker: gelsolin,163 inducing caspase-dependant apoptosis in CLL B-cells,164 p21CIP1/WAF1 differentiation or apoptosis in human leukemia cells,165 and also tissue growth factor (TGF)βII receptor expression in human breast cancer.166 Reported half-life in animals is about 1 hour, and species-variable protein binding was reported.167 Half-life in human plasma was higher than in animals, which is supposedly to be linked to protein binding, as Entinostat was found to be 80% bound.167
Phase I study in advanced solid tumors or lymphoma by oral route168 (Table 3, AT) was reasonably well tolerated. In refractory solid tumors and human lymphoid malignancies169 (Table 3, AU), drug exposure increases linearly with dose. In AML170 (Table 3, AV), results showed that Entinostat effectively inhibits HDAC in vivo in patients with AML and should be further tested, preferably in patients with less-advanced disease. Several protocols were designed for patients with advanced solid malignancies and lymphomas171 (Table 3, AW). PKs revealed dose-dependent and dose-proportional increases. Responses were partial remissions and prolonged disease stabilization. In a Phase II study for metastatic melanoma (Table 3, AX) with low efficacy treatment,172 long-term tumor stabilizations have been observed, but no objective responses was assessed.
Mocetinostat
Mocetinostat (MGCD0103)173 is a benzamide selective class I/IV HDACi (1, 2, 3, and 11). It inhibits neoplastic growth in multiple human tumor xenograft models including colon (HCT116, SW48, and Colo205), NSCLC (A549), prostate (DU145), pancreatic (PANC1), and vulval epidermal (A431) cancer models and does not interact with the potassium voltage-gated channel, subfamily H (eag-related), member 2 (HERG) channel. Gene expression induced by Mocetinostat is modest compared with other hydroxamic HDACi.174
In patients with advanced solid tumors175 (Table 3, AY), a phase I study gave disease stabilization as the best response. IL-6 induction related to HDACi activity has been postulated but not confirmed. At the tested doses, Mocetinostat appeared tolerable and exhibited favorable PK and PD profiles, as well as evidence of target inhibition in surrogate tissues. Cytogenetically analyzed patients with AML, MDS, ALL, and CML176 (Table 3, AZ) were treated orally. A total of 18 of the 29 patients had abnormal cytogenetics. PK analyses indicated rapid absorption of Mocetinostat. Several administration schedules have been proposed for advanced leukemias or MDS177 (Table 3, BA). A Phase II study for HL178 (Table 3, BB) demonstrated significant anti-tumor activity in relapsed/refractory post-transplant HL. For 437 patients179 (Table 3, BC), partial responses were obtained. Extended studies are ongoing.
Tacedinaline
The long-known molecule Tacedinaline (CI-994) is a benzamide HDACi similar to MS-275180 with anti-tumor activities in HCT-8 colon carcinoma.181 Following Tacedinaline administration, inhibition of both histone deacetylation and cellular proliferation at the G1 to S transition phase of the cell cycle are observed.
Oral administration with food intake for solid tumors182 (Table 3, BD) did not affect the rate or extent of the drug absorption. Best responses were partial or stable diseases. Advanced solid malignancies (mainly colorectal, pancreatic and mesothelioma) were treated in combination with Capecitabine183 (Table 3, BE), an FDA-approved compound used to treat a variety of cancer. Three treatment protocols were implemented. A combination Phase II study with Gemcitabine184 for advanced pancreatic carcinoma (Table 3, BF) gave no improvement.
Depsipeptide
Depsipeptide (Romidepsin, FK228, FR901228) is a cyclic tetrapeptide isolated from Chromobacterium violaceum which has demonstrated anti tumor activities (A549 lung adenocarcinoma, MCF-7 and ZR-75-1 breast adenocarcinoma, and LOX IMVI melanoma cell lines) and is postulated as a Pg-p substrate.185,186 It is considered as a natural HDACi prodrug, as its disulfide bond is reduced in vivo to give the active species40 and is the only reported sulfur-based HDACi used in clinical trials. It received FDA approval for cutaneous T-cell lymphoma in 2009. Romidepsin induces growth arrest and apoptosis in lung cancer cells.187 Romidepsin induces p21-dependent G1 arrest and p21-independent G2 arrest188 by downregulating cyclin D1 and upregulating cyclin E.189 It inhibits c-Myc and Fas ligand expression,190 modulates p53, ErbB1, HER2, and Raf-1 expression in lung cancer cells,191 increases p21, phosphorylation of Bcl2, and apoptosis in human breast cancer cells,192 increases expression of a NaI symporter in thyroid carcinoma cells for possible resensitization of radio resistant thyroid cancer,193 and activates the caspase 8-mediated apoptosis and down regulates the c-FLIP protein.194 Sequential treatments with 5-azaDc facilitates cancer cell recognition by T lymphocytes specific for cancer/testis antigen 1B (NY-ESO-1) as a possible option for immunotherapy195 or induces tissue factor pathway inhibitor (TFPI)-2 expression in cancer cells.196 Initial cardiac toxicity was resolved by convenient administration schedules but cardiac monitoring is most of the time implemented during clinical investigations. Concentrations studies in CLL and AML have correlated apoptosis induction and HDAC inhibition. Combination of Romidepsin and DNA demethylating agents is potentiated in ETO positive leukemia cells.197 A gene signature specific for Romidepsin sensitivity has been reported.198 Due to the recent approval of Romidepsin in CTCL, all early published clinical trials for this disease are not discussed.199,200 Romidepsin induced MDR-1 gene expression in several cancer cell lines.
A Phase I study in advanced, incurable cancers201 (Table 3, BG) indicated that further clinical trials are warranted. Used for advanced or refractory neoplasms202 (Table 3, BH), elimination half-life was 8.1 hours. In CCL and AML203 (Table 3, BI), intravenous treatment gave no responses. Romidepsin effectively inhibits HDAC in vivo in patients with CLL and AML, but future studies should examine alternative administration routes. In refractory or recurrent solid tumors204 (Table 3, BJ), DLTs were not associated with changes in troponin levels or evidence of ventricular dysfunction, transient asymptomatic sick sinus syndrome and hypocalcemia. For MDS and AML205 (Table 3, BK), intravenous administration gave no significant cardiac toxicity. Romidepsin therapy can be administered with acceptable short-term toxicity. Gastrointestinal symptoms and fatigue seemed to be treatment-limiting after multiple cycles. Phase II performed on refractory renal cell cancer206 (Table 3, BL), I.V., gave classical but serious toxicities. Two patients developed a prolonged QT interval, one patient developed grade 3 atrial fibrillation and tachycardia, and there was one sudden death. In metastatic neuroendocrine tumors207 (Table 3, BM), adverse events were ventricular tachycardia and prolonged QT, possibly resulting in a sudden death, terminating the study prematurely. Romidepsin has serious cardiac adverse events, and risks need to be comprehensively evaluated. In lung cancers (NSCLC and SCLC) (Table 3, BN),208 Romidepsin was not appropriate. This study presented an in depth gene expression profiling.
Conclusion
A number of clinical trials have been completed and many others are ongoing using HDACi as single agents and in combination with radiotherapy and/or chemotherapy for the treatment of various hematological and solid malignancies with some promising early results. Vorinostat is the most established HDACi, and was approved in October 2006 by the FDA for the treatment of advanced forms of cutaneous T-cell lymphoma that have failed multiple other systemic treatment options. Significant single agent activity for Romidepsin has also been demonstrated in peripheral cutaneous T-cell lymphoma, and encouraging results have also been seen in HL with Mocetinostat. From the trials conducted, it is also clear that a major clinical advantage is that HDACi are well tolerated in the majority of patients. The future of HDACi lies in designing rational combination therapies. The sequence of drug administration may be of paramount importance to avoid antagonistic effects. The possibility of drug–drug interactions and enhanced toxicities is to be considered. HDACi are also evaluated in non cancerous pathologies like AIDS, graft versus host diseases, and polycythemia verae. Very soon, SIRT activators could find therapeutic applications in lung interstitial diseases. Like for the kinase inhibitors, more selective third generation HDACi are sought, yet specific tests remain to be designed to screen for bioactivity in vitro and in vivo.211
Acknowledgments
This work was supported in part by the COST action TD0905 Epigenetics: bench to bedside and by the Agence Nationale de la Recherche (France).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicol Appl Pharmacol. 2010;245(3):378–393. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos-Rosa H, Caldas C. Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer. 2005;41(16):2381–2402. doi: 10.1016/j.ejca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Oliver SS, Denu JM. Dynamic interplay between histone H3 modifications and protein interpreters: emerging evidence for a “histone language”. Chembiochem. 2011;12(2):299–307. doi: 10.1002/cbic.201000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zee BM, Levin RS, Dimaggio PA, et al. Global turnover of histone post-translational modifications and variants in human cells. Epigenetics Chromatin. 2010;3:22–33. doi: 10.1186/1756-8935-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dekker F, Haisma HJ. Histone acetyl transferases as emerging drug targets. Drug Discovery Today. 2009;14(19/20):942–948. doi: 10.1016/j.drudis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Lim S, Metzger E, Schüle R, et al. Epigenetic regulation of cancer growth by histone demethylases. Int J Cancer. 2010;127(9):1991–1998. doi: 10.1002/ijc.25538. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson TB, Chen T, Richard S. The physiological and pathophysiological role of PRMT1-mediated protein arginine methylation. Pharmacol Res. 2009;60(6):466–474. doi: 10.1016/j.phrs.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Fackelmayer FO. Protein arginine methyltransferases: guardians of the Arg? Trends Biochem Sci. 2005;30(12):666–671. doi: 10.1016/j.tibs.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Tini M, Naeem H, Torchia J. Biochemical analysis of arginine methylation in transcription. Methods Mol Biol. 2009;523:235–247. doi: 10.1007/978-1-59745-190-1_16. [DOI] [PubMed] [Google Scholar]
- 10.Spannhoff AT, Hauser R, Heinke W, et al. The emerging therapeutic potential of histone methyltransferase and demethylase inhibitors. ChemMedChem. 2009;4(10):1568–1582. doi: 10.1002/cmdc.200900301. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Xu K, Ni M, et al. Nucleosome dynamics define transcriptional enhancers. Nature Genetics. 2010;42(4):343–347. doi: 10.1038/ng.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland RA, Olhava EJ, Scott MP. Targeting epigenetic enzymes for drug discovery. Curr Opin Chem Biol. 2010;14(4):505–510. doi: 10.1016/j.cbpa.2010.06.174. [DOI] [PubMed] [Google Scholar]
- 13.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 14.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in purkinje neurons and the brain. Science. 2009;324(5929):929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Wahab O, Mullally A, Hedvat C, et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood. 2009;114(1):144–147. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabbri M, Calin GA. Epigenetics and miRNAs in human cancer. Adv Genet. 2010;70:87–99. doi: 10.1016/B978-0-12-380866-0.60004-6. [DOI] [PubMed] [Google Scholar]
- 17.Zain J, Kaminetzki D, O’Connor OA. Emerging role of epigenetic therapies in cutaneous T-cell lymphomas. Expert Rev Hematol. 2010;3(2):187–203. doi: 10.1586/ehm.10.9. [DOI] [PubMed] [Google Scholar]
- 18.Graham JS, Kaye SB, Brown R. The promises and pitfalls of epigenetic therapies in solid tumors. Eur J Cancer. 2009;45(7):1129–1136. doi: 10.1016/j.ejca.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5(9):5769–5784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 20.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razidlo DF, Whitney TJ, Casper ME, et al. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS One. 2010;5(7):e11492. doi: 10.1371/journal.pone.0011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskara S, Chyla BJ, Amann JM, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Mol Cell. 2008;30(1):61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Zoeten EF, Wang L, Sai H, et al. Inhibition of HDAC9 increases T regulatory cell function and prevents colitis in mice. Gastroenterology. 2010;138(2):583–594. doi: 10.1053/j.gastro.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotian S, Liyanarachchi S, Zelent A, et al. Histone deacetylases 9 and 10 are required for homologous recombination. J Biol Chem. Epub 2011 Jan 18. [DOI] [PMC free article] [PubMed]
- 25.Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Altern Med Rev. 2010;15(3):245–263. [PubMed] [Google Scholar]
- 26.Zhang Y, Kwon S, Yamaguchi T, et al. Mice lacking histone deacetylase 6 have hyperacetylated tubulin but are viable and develop normally. Mol Cell Biol. 2008;28(5):1688–1701. doi: 10.1128/MCB.01154-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mai A, Altucci L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol. 2009;41(1):199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14(12):1497–1511. doi: 10.1517/13543784.14.12.1497. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand P. Inside HDAC with HDAC inhibitors. Eur J Med Chem. 2010;45(6):2095–2116. doi: 10.1016/j.ejmech.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 30.Yurek-George A, Cecil AR, Mo AH, et al. The first biologically active synthetic analogues of FK228, the depsipeptide histone deacetylase inhibitor. J Med Chem. 2007;50(23):5720–5726. doi: 10.1021/jm0703800. [DOI] [PubMed] [Google Scholar]
- 31.Khan N, Jeffers M, Kumar S, et al. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409(2):581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 32.KrennHrubec K, Marshall BL, Hedglin M, et al. Design and evaluation of ‘linkerless’ hydroxamic acids as selective HDAC8 inhibitors. Bioorg Med Chem Lett. 2007;17(10):2874–2878. doi: 10.1016/j.bmcl.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 33.Estiu G, Greenberg E, Harrison CB, et al. Structural origin of selectivity in class II-selective histone deacetylase inhibitors. J Med Chem. 2008;51(10):2898–2906. doi: 10.1021/jm7015254. [DOI] [PubMed] [Google Scholar]
- 34.Taori K, Paul VJ, Luesch H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J Am Chem Soc. 2008;130(6):1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- 35.Huhtiniemi T, Suuronen T, Lahtela-Kakkonen M, et al. Nε-Modified lysine containing inhibitors for SIRT1 and SIRT2. Bioorg Med Chem. 2010;18(15):5616–5625. doi: 10.1016/j.bmc.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Huhtiniemi T, Wittekindt C, Laitinen T, et al. Comparative and pharmacophore model for deacetylase SIRT1. J Comput Aided Mol Des. 2006;20(9):589–599. doi: 10.1007/s10822-006-9084-9. [DOI] [PubMed] [Google Scholar]
- 37.Elaut G, Török G, Vinken M, et al. Major phase I biotransformation pathways of Trichostatin A in rat hepatocytes and in rat and human liver microsomes. Drug Metab Dispos. 2002;30(12):1320–1328. doi: 10.1124/dmd.30.12.1320. [DOI] [PubMed] [Google Scholar]
- 38.Ebbel EN, Leymarie N, Schiavo S, et al. Identification of phenylbutyrate-generated metabolites in Huntington disease patients using parallel liquid chromatography/electrochemical array/mass spectrometry and off-line tandem mass spectrometry. Analytical Biochem. 2010;399(2):152–161. doi: 10.1016/j.ab.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parise RA, Holleran JL, Beuner JH, et al. A liquid chromatography–electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chrom B. 2006;840(2):108–115. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 40.Du L, Musson DG, Wang AQ. Stability studies of vorinostat and its two metabolites in human plasma, serum and urine. J Pharm Biomed Anal. 2006;42(5):556–564. doi: 10.1016/j.jpba.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Furumai R, Matsuyama A, Kobashi N, et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62(17):4916–4921. [PubMed] [Google Scholar]
- 42.Xiao JJ, Byrd J, Marcucci G, et al. Identification of thiols and glutathione conjugates of depsipeptide FK228 (FR901228), a novel histone protein deacetylase inhibitor, in the blood. Rapid Commun Mass Spectrom. 2003;17(8):757–766. doi: 10.1002/rcm.976. [DOI] [PubMed] [Google Scholar]
- 43.Shiraga T, Tozuka Z, Ishimura R, et al. Identification of cytochrome P450 enzymes involved in the metabolism of FK228, a potent histone deacetylase inhibitor, in human liver microsomes. Biol Pharm Bull. 2005;28(1):124–129. doi: 10.1248/bpb.28.124. [DOI] [PubMed] [Google Scholar]
- 44.Kim HM, Oh SJ, Park SK, et al. In vitro metabolism of KBH-A40, a novel delta-lactam-based histone deacetylase (HDAC) inhibitor, in human liver microsomes and serum. Xenobiotica. 2008;38(3):281–293. doi: 10.1080/00498250701813222. [DOI] [PubMed] [Google Scholar]
- 45.Fonsi M, Fiore F, Jones P, et al. Metabolism-related liabilities of a potent histone deacetylase (HDAC) inhibitor and relevance of the route of administration on its metabolic fate. Xenobiotica. 2009;39(10):722–737. doi: 10.1080/00498250903082279. [DOI] [PubMed] [Google Scholar]
- 46.Peart MJ, Smyth GK, van Laar RK, et al. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. PNAS. 2005;102(10):3697–3702. doi: 10.1073/pnas.0500369102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis L, Pan Y, Smyth GK, et al. Histone deacetylase inhibitor panobinostat induces clinical responses with associated alterations in gene expression profiles in cutaneous T-cell lymphoma. Clin Cancer Res. 2008;14(14):4500–4510. doi: 10.1158/1078-0432.CCR-07-4262. [DOI] [PubMed] [Google Scholar]
- 48.Kato N, Tanaka J, Sugita J, et al. Regulation of the expression of MHC class I-related chain A, B (MICA, MICB) via chromatin remodeling and its impact on the susceptibility of leukemic cells to the cytotoxicity of NKG2D-expressing cells. Leukemia. 2007;21(10):2103–2108. doi: 10.1038/sj.leu.2404862. [DOI] [PubMed] [Google Scholar]
- 49.Vo DD, Prins RM, Begley JL, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69(22):8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cameron EE, Bachman KE, Myöhänen S, et al. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21(1):103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 51.Appelbaum FR, Baer MR, Carabasi MH, et al. NCCN Practice guidelines for acute myelogenous leukemia. Oncology. 2000;14(11A):53–61. [PubMed] [Google Scholar]
- 52.Snykers S, Vinken M, Rogiers V, et al. Differential role of epigenetic modulators in malignant and normal stem cells: a novel tool in preclinical in vitro toxicology and clinical therapy. Arch Toxicol. 2007;81(8):533–544. doi: 10.1007/s00204-007-0195-4. [DOI] [PubMed] [Google Scholar]
- 53.Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3(4):344–351. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 54.DiGiuseppe JA, Weng LJ, Yu KH, et al. Phenylbutyrate-induced G1 arrest and apoptosis in myeloid leukemia cells: structure-function analysis. Leukemia. 1999;13(8):1243–1253. doi: 10.1038/sj.leu.2401471. [DOI] [PubMed] [Google Scholar]
- 55.Carducci MA, Nelson JB, Chan-Tack KM, et al. Phenylbutyrate induces apoptosis in human prostate cancer and is more potent than phenylacetate. Clin Cancer Res. 1996;2(2):379–387. [PubMed] [Google Scholar]
- 56.Zhang X, Wei L, Yang Y, Yu Q. Sodium 4-phenylbutyrate induces apoptosis of human lung carcinoma cells through activating JNK pathway. J Cell Biochem. 2004;93(4):819–829. doi: 10.1002/jcb.20173. [DOI] [PubMed] [Google Scholar]
- 57.Gorospe M, Shack S, Guyton KZ, et al. Up-regulation and functional role of p21Waf1/Cip1 during growth arrest of human breast carcinoma MCF-7 cells by phenylacetate. Cell Growth Differ. 1996;7(12):1609–1615. [PubMed] [Google Scholar]
- 58.Liu L, Hudgins WR, Miller AC, et al. Transcriptional upregulation of TGF-α by phenylacetate and phenylbuytrate is associated with differentiation of human melanoma cells. Cytokine. 1995;7(5):449–456. doi: 10.1006/cyto.1995.0061. [DOI] [PubMed] [Google Scholar]
- 59.Han S, Wada RK, Sidell N. Differentiation of human neuroblastoma by phenylacetate is mediated by peroxisome proliferatoractivated receptor gamma. Cancer Res. 2001;61(10):3998–4002. [PubMed] [Google Scholar]
- 60.Carducci MA, Nelson JB, Chan-Tack KM, et al. Phenylbutyrate induces apoptosis in human prostate cancer and is more potent than phenylacetate. Clin Cancer Res. 1996;2(2):379–387. [PubMed] [Google Scholar]
- 61.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res. 1991;29(2):147–150. doi: 10.1203/00006450-199102000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Phuphanich S, Baker SD, Grossman SA, et al. Oral sodium phenylbutyrate in patients with recurrent malignant gliomas: a dose escalation and pharmacologic study. Neuro Oncol. 2005;7(2):177–182. doi: 10.1215/S1152851704000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carducci MA, Gilbert J, Bowling MK, et al. A phase I clinical and pharmacological evaluation of sodium phenylbutyrate on an 120-h infusion schedule. Clin Cancer Res. 2001;7(10):3047–3055. [PubMed] [Google Scholar]
- 64.Gilbert J, Baker SD, Bowling MK, et al. A phase I dose escalation and bioavailability study of oral sodium phenylbutyrate in patients with refractory solid tumor malignancies. Clin Cancer Res. 2001;7(8):2292–2300. [PubMed] [Google Scholar]
- 65.Camacho LH, Olson J, Tong WP, et al. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Invest New Drugs. 2007;25(2):131–138. doi: 10.1007/s10637-006-9017-4. [DOI] [PubMed] [Google Scholar]
- 66.Gore SD, Weng LJ, Zhai S, et al. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res. 2002;8(4):963–970. [PubMed] [Google Scholar]
- 67.Maslak P, Chanel S, Camacho LH, et al. Pilot study of combination transcriptional modulation therapy with sodium phenylbutyrate and 5-azacytidine in patients with acute myeloid leukemia or myelodysplastic syndrome. Leukemia. 2006;20(2):212–217. doi: 10.1038/sj.leu.2404050. [DOI] [PubMed] [Google Scholar]
- 68.Gilbert J, Baker SD, Donehower R, et al. Methytransferase (MT) activity and gene expression in tumor biopsies from patients enrolled in a Phase I study of the MT inhibitor, 5-azacytidine (5AC), and the histone deacetylase inhibitor, phenylbutyrate (PB), in refractory solid tumors. Proc Am Soc Clin Onc. 2001:87a. [Google Scholar]
- 69.Sung MW, Waxman S. Combination of cytotoxicdifferentiation therapy with 5-fluorouracil and phenylbutyrate in patients with advanced colorectal cancer. Anticancer Res. 2007;27(2):995–1001. [PubMed] [Google Scholar]
- 70.Rephaeli A, Rabizadeh E, Aviram A, et al. Derivatives of butyric acid as potential anti-neoplastic agents. Int J Cancer. 1991;49(1):66–72. doi: 10.1002/ijc.2910490113. [DOI] [PubMed] [Google Scholar]
- 71.Schwartz B, Avivi-Green C, Polak-Charcon S. Sodium butyrate induces retinoblastoma protein dephosphorylation, p16 expression and growth arrest of colon cancer cells. Mol Cell Biochem. 1998;188(1–2):21–30. [PubMed] [Google Scholar]
- 72.Mandal M, Adam L, Kumar R. Redistribution of activated caspase-3 to the nucleus during butyric acid-induced apoptosis. Biochem Biophys Res Commun. 1999;260(3):775–780. doi: 10.1006/bbrc.1999.0966. [DOI] [PubMed] [Google Scholar]
- 73.Rabizadeh E, Shaklai M, Nudelman A, et al. Rapid alteration of c-myc and c-jun expression in leukemic cells induced to differentiate by a butyric acid prodrug. FEBS Lett. 1993;328(3):225–229. doi: 10.1016/0014-5793(93)80932-k. [DOI] [PubMed] [Google Scholar]
- 74.Rabizadeh E, Bairey O, Aviram A, et al. Doxorubicin and a butyric acid derivative effectively reduce levels of Bcl-2 protein in the cells of chronic lymphocytic leukemia patient. Eur J Haematol. 2001;66(4):263–271. doi: 10.1034/j.1600-0609.2001.066004263.x. [DOI] [PubMed] [Google Scholar]
- 75.Patnaik A, Rowinsky EK, Villalona MA, et al. A phase I study of pivaloyloxymethyl butyrate, a prodrug of the differentiating agent butyric acid, in patients with advanced solid malignancies. Clin Cancer Res. 2002;8(7):2142–2148. [PubMed] [Google Scholar]
- 76.Reid T, Valone F, Lipera W, et al. Phase II trial of the histone deacetylase inhibitor pivaloyloxymethyl butyrate (Pivanex, AN-9) in advanced non-small cell lung cancer. Lung Cancer. 2004;45(3):381–386. doi: 10.1016/j.lungcan.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Loscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669–694. doi: 10.2165/00023210-200216100-00003. [DOI] [PubMed] [Google Scholar]
- 78.Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16(10):695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- 79.Marchion DC, Bicaku E, Daud AI, et al. Valproic acid alters chromatin structure by regulation of chromatin modulation proteins. Cancer Res. 2005;65(9):3815–3822. doi: 10.1158/0008-5472.CAN-04-2478. [DOI] [PubMed] [Google Scholar]
- 80.Jung GA, Yoon JY, Moon BS, et al. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BMC Cell Biol. 2008;9:66–78. doi: 10.1186/1471-2121-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gottlicher M, Minucci S, Zhu J, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20(24):6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gurvich N, Tsygankova OM, Meinkoth JL, et al. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 2004;64(3):1079–1086. doi: 10.1158/0008-5472.can-03-0799. [DOI] [PubMed] [Google Scholar]
- 83.Bacon CL, Gallagher HC, Haughey JC, et al. Antiproliferative action of valproate is associated with aberrant expression and nuclear translocation of cyclin D3 during the C6 glioma G1 phase. J Neurochem. 2002;83(1):12–19. doi: 10.1046/j.1471-4159.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 84.Werling U, Siehler S, Litfin M, et al. Induction of differentiation in F9 cells and activation of peroxisome proliferator-activated receptor delta by valproic acid and its teratogenic derivatives. Mol Pharmacol. 2001;59(5):1269–1276. doi: 10.1124/mol.59.5.1269. [DOI] [PubMed] [Google Scholar]
- 85.Kawagoe R, Kawagoe H, Sano K. Valproic acid induces apoptosis in human leukemia cells by stimulating both caspase dependent and -independent apoptotic signaling pathways. Leuk Res. 2002;26(5):495–502. doi: 10.1016/s0145-2126(01)00151-5. [DOI] [PubMed] [Google Scholar]
- 86.Tang R, Faussat AM, Majdak P, et al. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia. 2004;18(7):1246–1251. doi: 10.1038/sj.leu.2403390. [DOI] [PubMed] [Google Scholar]
- 87.Ichiyama T, Okada K, Lipton JM, et al. Sodium valproate inhibits production of TNF-α and IL-6 and activation of NF-κB. Brain Res. 2000;857(1–2):246–251. doi: 10.1016/s0006-8993(99)02439-7. [DOI] [PubMed] [Google Scholar]
- 88.Siitonen T, Koistinen P, Savolainen ER. Increase in Ara-C cytotoxicity in the presence of valproate, a histone deacetylase inhibitor, is associated with the concurrent expression of cyclin D1 and p27(Kip 1) in acute myeloblastic leukemia cells. Leuk Res. 2005;29(11):1335–1342. doi: 10.1016/j.leukres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Cimino G, Lo-Coco F, Fenu S, et al. Sequential valproic acid/Alltrans retinoic acid treatment reprograms differentiation in refractory and highrisk acute myeloid leukemia. Cancer Res. 2006;66(17):8903–8911. doi: 10.1158/0008-5472.CAN-05-2726. [DOI] [PubMed] [Google Scholar]
- 90.Valentini A, Gravina P, Federici G, et al. Valproic acid induces apoptosis, p16INK4A upregulation and sensitization to chemotherapy in human melanoma cells. Cancer Biol Ther. 2007;6(2):185–191. doi: 10.4161/cbt.6.2.3578. [DOI] [PubMed] [Google Scholar]
- 91.Atmaca A, Al-Batran SE, Maurer A, et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating Phase I clinical trial. British J Cancer. 2007;97(2):177–182. doi: 10.1038/sj.bjc.6603851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bug G, Ritter M, Wassmann B, et al. Clinical trial of valproic acid and all-trans retinoic acid in patients with poor-risk acute myeloid leukemia. Cancer. 2005;104(12):2717–2725. doi: 10.1002/cncr.21589. [DOI] [PubMed] [Google Scholar]
- 93.Kuendgen A, Knipp S, Fox F, et al. Results of a Phase 2 study of valproic acid alone or in combination with alltrans retinoic acid in 75 patients with myelodysplastic syndrome and relapsed or refractory acute myeloid leukemia. Ann Hematol. 2005;84(Suppl 1):61–66. doi: 10.1007/s00277-005-0026-8. [DOI] [PubMed] [Google Scholar]
- 94.Pilatrino C, Cilloni D, Messa E, et al. Increase in platelet count in older, poor-risk patients with acute myeloid leukemia or myelodysplastic syndrome treated with valproic acid and all-trans retinoic acid. Cancer. 2005;104(1):101–109. doi: 10.1002/cncr.21132. [DOI] [PubMed] [Google Scholar]
- 95.Raffoux E, Chaibi P, Dombret H, et al. Valproic acid and all-trans retinoic acid for the treatment of elderly patients with acute myeloid leukemia. Haematologica. 2005;90(7):986–988. [PubMed] [Google Scholar]
- 96.Duprez E, Lillehaug JR, Naoe T, et al. cAMP signalling is decisive for recovery of nuclear bodies (PODs) during maturation of RA-resistant t(15;17) promyelocytic leukemia NB4 cells expressing PML-RAR alpha. Oncogene. 1996;12(11):2451–2459. [PubMed] [Google Scholar]
- 97.Kuendgen A, Schmid M, Schlenk R, et al. The histone deacetylase (HDAC) inhibitor valproic acid as monotherapy or in combination with all-trans retinoic acid in patients with acute myeloid leukemia. Cancer. 2006;106(1):112–119. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 98.Huang S, Brandt SJ. mSin3A regulates murine erythroleukemia cell differentiation through association with the TAL1 (or SCL) transcription factor. Mol Cell Biol. 2000;20(6):2248–2259. doi: 10.1128/mcb.20.6.2248-2259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watamoto K, Towatari M, Ozawa Y, et al. Altered interaction of HDAC5 with GATA-1 during MEL cell differentiation. Oncogene. 2003;22(57):9176–9184. doi: 10.1038/sj.onc.1206902. [DOI] [PubMed] [Google Scholar]
- 100.Siitonen T, Timonen T, Juvonen E, et al. Valproic acid combined with 13-cis retinoic acid and 1,25-dihydroxyvitamin D3 in the treatment of patients with myelodysplastic syndromes. Haematologica. 2007;92(8):1119–1122. doi: 10.3324/haematol.11262. [DOI] [PubMed] [Google Scholar]
- 101.Santini V, Ferrini PR. Differentiation therapy of myelodysplastic syndromes: fact or fiction? Br J Heamatol. 1998;102(5):1124–1138. doi: 10.1046/j.1365-2141.1998.00881.x. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, et al. Phase 1/2 study of the combination of 5-aza-2′-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–3279. doi: 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blum W, Klisovic RB, Hackanson B, et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J Clin Oncol. 2007;25(25):3884–3891. doi: 10.1200/JCO.2006.09.4169. [DOI] [PubMed] [Google Scholar]
- 104.Braiteh F, Soriano AO, Garcia-Manero G, et al. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res. 2008;14(19):6296–6301. doi: 10.1158/1078-0432.CCR-08-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soriano AO, Yang H, Faderl S, et al. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–2308. doi: 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 106.Münster P, Marchion D, Bicaku E, et al. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: Phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res. 2009;15(7):2488–2496. doi: 10.1158/1078-0432.CCR-08-1930. [DOI] [PubMed] [Google Scholar]
- 107.Münster P, Marchion D, Bicaku E, et al. Phase I trial of histone deacetylase inhibition by valproic acid followed by the topoisomerase II inhibitor epirubicin in advanced solid tumors: a clinical and translational study. J Clin Oncology. 2007;25(15):1979–1985. doi: 10.1200/JCO.2006.08.6165. [DOI] [PubMed] [Google Scholar]
- 108.Daud AI, Dawson J, DeConti RC, et al. Potentiation of a topoisomerase I inhibitor, karenitecin, by the histone deacetylase inhibitor valproic acid in melanoma: translational and Phase I/II clinical trial. Clin Cancer Res. 2009;15(7):2479–2489. doi: 10.1158/1078-0432.CCR-08-1931. [DOI] [PubMed] [Google Scholar]
- 109.Rocca A, Minucci S, Tosti G, et al. A Phase I–II study of the histone deacetylase inhibitor valproic acid plus chemoimmunotherapy in patients with advanced melanoma. British J Cancer. 2009;100(1):28–36. doi: 10.1038/sj.bjc.6604817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fachetti F, Previdi S, Ballarini M, et al. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis. 2004;9(5):573–582. doi: 10.1023/B:APPT.0000038036.31271.50. [DOI] [PubMed] [Google Scholar]
- 111.Chavez-Blanco A, Segura-Pacheco B, Perez-Cardenas E, et al. Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer. A Phase I study. Mol Cancer. 2005;4(1):22. doi: 10.1186/1476-4598-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Candelaria M, Gallardo-Rincon D, Arce C, et al. A Phase II study of epigenetic therapy with hydralazine and magnesium valproate to overcome chemotherapy resistance in refractory solid tumors. Ann Oncol. 2007;18(9):1529–1538. doi: 10.1093/annonc/mdm204. [DOI] [PubMed] [Google Scholar]
- 113.Parulekar WR, Einsenhauer EA. Novel endpoints and design of early clinical trials. Annal Oncol. 2002;13(Suppl 4):139–143. doi: 10.1093/annonc/mdf651. [DOI] [PubMed] [Google Scholar]
- 114.Hunsberger S, Rubinstein LV, Dancey J, et al. Dose escalation trial designs based on a molecularly targeted endpoint. Stat Med. 2005;24(14):2171–2181. doi: 10.1002/sim.2102. [DOI] [PubMed] [Google Scholar]
- 115.Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, et al. Global DNA hypermethylation-associated cancer chemotherapy resistance and its reversion with the demethylating agent hydralazine. J Transl Med. 2006;4:32. doi: 10.1186/1479-5876-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Richon VM, Webb Y, Merger R, et al. Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci U S A. 1996;93(12):5705–5708. doi: 10.1073/pnas.93.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Butler LM, Agus DB, Scher HL, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60(78):5165–5170. [PubMed] [Google Scholar]
- 118.Finnin MS, Donigian JR, Cohen A, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401(6749):188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 119.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 120.Mitsiades CS, Poulaki V, McMullan C, et al. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11(10):3958–3965. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- 121.Munster PN, Troso-Sandoval T, Rosen N, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001;61(23):8492–8497. [PubMed] [Google Scholar]
- 122.Bali P, Pranpat M, Swaby R, et al. Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005;11(17):6382–6389. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 123.Komatsu N, Kawamata N, Takeuchi S, et al. SAHA, a HDAC inhibitor, has profound anti-growth activity against non-small cell lung cancer cells. Oncol Rep. 2006;15(1):187–191. [PubMed] [Google Scholar]
- 124.Butler LM, Agus DB, Scher HI, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60(18):5165–5170. [PubMed] [Google Scholar]
- 125.Dietrich C, Greenberg VL, Desimone CP, et al. Suberoylanilide hydroxamic acid potentiates paclitaxel-induced apoptosis in ovarian cancer cell lines. Gynecol Oncol. 2006;116(1):126–130. doi: 10.1016/j.ygyno.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 126.Kelly WK, Richon VM, O’Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9(10 Pt 1):3578–3588. [PubMed] [Google Scholar]
- 127.Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7(4):257–261. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 128.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garcia-Manero G, Yangn H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias andmyelodysplastic syndromes. Blood. 2008;111(3):1060–1066. doi: 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 130.Modesitt SC, Sill M, Hoffman JS, et al. A Phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;109(2):182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Vansteenkiste J, Van Cutsem E, Dumez H, et al. Early Phase II trial of oral vorinostat in relapsed or refractory breast, colorectal, or nonsmall cell lung cancer. Invest New Drugs. 2008;26(5):483–488. doi: 10.1007/s10637-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 132.Blumenschein GR, Jr, Kies MS, Papadimitrakopoulou VA, et al. Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs. 2008;26(1):81–87. doi: 10.1007/s10637-007-9075-2. [DOI] [PubMed] [Google Scholar]
- 133.Luu TH, Morgan RJ, Leong L, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res. 2008;14(21):7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woyach JA, Kloos RT, Ringel MD, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009;94(1):164–170. doi: 10.1210/jc.2008-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramalingam SS, Parise RA, Ramanathan RK, et al. Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res. 2007;13(12):3605–3610. doi: 10.1158/1078-0432.CCR-07-0162. [DOI] [PubMed] [Google Scholar]
- 137.Plumb JA, Finn PW, Williams RJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003;2(8):721–728. [PubMed] [Google Scholar]
- 138.Gimsing P. Belinostat: a new broad acting antineoplastic histone deacetylase inhibitor. Expert Opin Investig Drugs. 2009;18(4):501–508. doi: 10.1517/13543780902852560. [DOI] [PubMed] [Google Scholar]
- 139.Gian X, LaRochelle WJ, Ara G, et al. Activity of PXD101, a histone deacetylase inhibitor, in preclinical ovarian cancer studies. Mol Cancer Ther. 2006;5(8):2086–2095. doi: 10.1158/1535-7163.MCT-06-0111. [DOI] [PubMed] [Google Scholar]
- 140.Monks A, Hose CD, Pezzoli P, et al. Gene expression-signature of belinostat in cell lines is specific for histone deacetylase inhibitor treatment, with a corresponding signature in xenografts. Anticancer Drugs. 2009;20(8):682–692. doi: 10.1097/CAD.0b013e32832e14e1. [DOI] [PubMed] [Google Scholar]
- 141.Warren KE, McCully C, Dvinge H, et al. Plasma and cerebrospinal fluid pharmacokinetics of the histone deacetylase inhibitor, belinostat (PXD101), in non-human primates. Cancer Chemother Pharmacol. 2008;62(3):433–437. doi: 10.1007/s00280-007-0622-5. [DOI] [PubMed] [Google Scholar]
- 142.Tumber A, Collins LS, Petersen K, et al. The histone deacetylase inhibitor PXD101 synergises with 5-Xuorouracil to inhibit colon cancer cell growth in vitro and in vivo. Cancer Chemother Pharmacol. 2007;60:275–283. doi: 10.1007/s00280-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 143.Steele NL, Plumb JA, Vidal L, et al. A Phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14(3):804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 144.Takada M, Kataoka A, Toi M, et al. A close association between alteration in growth kinetics by neoadjuvant chemotherapy and survival outcome in primary breast cancer. Int J Oncol. 2004;25(2):397–405. [PubMed] [Google Scholar]
- 145.Gimsing P, Hansen M, Knudsen LM, et al. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Hematol. 2008;81(3):170–176. doi: 10.1111/j.1600-0609.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 146.Ramalingam SS, Belani CP, Ruel C, et al. Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol. 2009;4(1):97–101. doi: 10.1097/JTO.0b013e318191520c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mackay HJ, Hirte H, Colgan T, et al. Phase II trial of the histone deacetylase inhibitor belinostat in women with platinum resistant epithelial ovarian cancer and micropapillary (LMP) ovarian tumours. Eur J Cancer. 2010;46(9):1573–1579. doi: 10.1016/j.ejca.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Golay J, Cuppini L, Leoni F, et al. The histone deacetylase inhibitor ITF2357 has anti-leukemic activity in vitro and in vivo and inhibits IL-6 and VEGF production by stromal cells. Leukemia. 2007;21(9):1892–1900. doi: 10.1038/sj.leu.2404860. [DOI] [PubMed] [Google Scholar]
- 149.Viviani S, Bonfante V, Fasola C, et al. Phase II study of the histonedeacetylase inhibitor ITF2357 in relapsed/refractory Hodgkin’s lymphoma patients. J Clin Oncol. 2008;26 abstract 8532. [Google Scholar]
- 150.Galli M, Salmoiraghi S, Golay J, et al. A Phase II multiple dose clinical trial of histone deacetylase inhibitor ITF2357 in patients with relapsed or progressive multiple myeloma. Ann Hematol. 2010;89(2):185–190. doi: 10.1007/s00277-009-0793-8. [DOI] [PubMed] [Google Scholar]
- 151.Carlo-Stella C, Guidetti A, Viviani S, et al. Safety and clinical activity of the histone deacetylase inhibitor givinostat in combination with meclorethamine in relapsed/refractory Hodgkin lymphoma (HL) J Clin Oncol. 2010;28 abstract 3068. [Google Scholar]
- 152.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12(2):634–642. doi: 10.1158/1078-0432.CCR-05-1132. [DOI] [PubMed] [Google Scholar]
- 153.Prince HM, Bishton MJ, Johnstone RW. Panobinostat (LBH589): a potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009;5(5):601–612. doi: 10.2217/fon.09.36. [DOI] [PubMed] [Google Scholar]
- 154.Giles F, Fischer T, Cortes J, et al. Phase I Study of Intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12(15):4628–4635. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- 155.Rathkopf D, Wong BY, Ross RW, et al. A Phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66(1):181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 156.Remiszewski SW. The discovery of NVPLAQ824: from concept to clinic. Curr Med Chem. 2003;10(22):2393–2402. doi: 10.2174/0929867033456675. [DOI] [PubMed] [Google Scholar]
- 157.Hurtubise A, Momparler RL. Effect of histone deacetylase inhibitor LAQ824 on antineoplastic action of 5-Aza-2-deoxycytidine (decitabine) on human breast carcinoma cells. Cancer Chemother Pharmacol. 2006;58(5):618–625. doi: 10.1007/s00280-006-0225-6. [DOI] [PubMed] [Google Scholar]
- 158.De Bono JS, Kristeleit R, Tolcher A, et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin Cancer Res. 2008;14(20):6663–6673. doi: 10.1158/1078-0432.CCR-08-0376. [DOI] [PubMed] [Google Scholar]
- 159.Kristeleit RS, Tandy D, Atadja P, et al. Effects of the histone deacetylase inhibitor (HDACI) LAQ824 on histone acetylation, Hsp70 and c-Raf in peripheral blood lymphocytes from patients with advanced solid tumours enrolled in a phase I clinical trial. J Clin Oncol. 2004;22 abstract 3023. [Google Scholar]
- 160.Ottmann OG, Deangelo DJ, Stone RM, et al. A Phase I, pharmacokinetic (PK) and pharmacodynamic (PD) study of a novel histone deacetylase inhibitor LAQ824 in patients with hematologic malignancies. J Clin Oncol. 2004;22 abstract 3024. [Google Scholar]
- 161.Choy E, Cao Y, Hornicek F, et al. Effect of histone deacetylase inhibitor (HDACI) PCI-24781 on chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. J Clin Oncol. 2010;28 abstract 10089. [PMC free article] [PubMed] [Google Scholar]
- 162.Undevia SD, Janisch L, Schilsky RL, et al. Phase I study of the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of the histone deacetylase inhibitor (HDACi) PCI-24781. J Clin Oncol. 2008;26 abstract 14514. [Google Scholar]
- 163.Jaboin J, Wild J, Hamidi H, et al. MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res. 2002;62(21):6108–6115. [PubMed] [Google Scholar]
- 164.Lucas DM, Davis ME, Parthun MR, et al. The histone deacetylase inhibitor MS-275 induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia cells. Leukemia. 2004;18(7):1207–1214. doi: 10.1038/sj.leu.2403388. [DOI] [PubMed] [Google Scholar]
- 165.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63(13):3637–3645. [PubMed] [Google Scholar]
- 166.Lee BI, Park SH, Kim JW, et al. MS-275, a histone deacetylase inhibitor, selectively induces transforming growth factor beta type II receptor expression in human breast cancer cells. Cancer Res. 2001;61(3):931–934. [PubMed] [Google Scholar]
- 167.Acharya MR, Sparreboom A, Sausville EA, et al. Interspecies differences in plasma protein binding of MS-275, a novel histone deacetylase inhibitor. Cancer Chemother Pharmacol. 2006;57(3):275–281. doi: 10.1007/s00280-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 168.Ryan QC, Headlee D, Acharya M, et al. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23(17):3912–3922. doi: 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 169.Kummar S, Gutierrez M, Gardner ER, et al. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res. 2007;13(18 Pt 1):5411–5417. doi: 10.1158/1078-0432.CCR-07-0791. [DOI] [PubMed] [Google Scholar]
- 170.Gojo I, Jiemjit A, Trepel JB, et al. Phase 1 and pharmacologic study of MS-275, a histone deacetylase inhibitor, in adults with refractory and relapsed acute leukemias. Blood. 2007;109(7):2781–2790. doi: 10.1182/blood-2006-05-021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gore L, Rothenberg ML, O’Bryant CL, et al. A phase I and pharmacokinetic study of the oral histone deacetylase inhibitor, MS-275, in patients with refractory solid tumors and lymphomas. Clin Cancer Res. 2008;14(14):4517–4525. doi: 10.1158/1078-0432.CCR-07-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hauschild A, Trefzer U, Garbe C, et al. Multicenter phase II trial of the histone deacetylase inhibitor pyridylmethyl-N-{4-[(2-aminophenyl)-carbamoyl]-benzyl}-carbamate in pretreated metastatic melanoma. Melanoma Res. 2008;18(4):274–278. doi: 10.1097/CMR.0b013e328307c248. [DOI] [PubMed] [Google Scholar]
- 173.Zhou N, Moradei O, Raeppel S, et al. Discovery of N-(2-aminophenyl)-4-[(4-pyridin-3-ylpyrimidin-2-ylamino)methyl]benzamide (MGCD0103), an orally active histone deacethylase inhibitor. J Med Chem. 2008;51(14):4072–4075. doi: 10.1021/jm800251w. [DOI] [PubMed] [Google Scholar]
- 174.Fournel M, Bonfils C, Hou Y, et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Mol Cancer Ther. 2008;7(4):759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 175.Siu LL, Pili R, Duran I, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol. 2008;26(12):1940–1947. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garcia-Manero G, Assouline S, Cortes J, et al. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112(4):981–989. doi: 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lancet JE, Nichols G, Assouline S, et al. A Phase I study of MGCD0103 given as a twice weekly oral dose in patients with advanced leukemias or myelodysplastic syndromes (MDS) J Clin Oncol. 2007;25 abstract 2516. [Google Scholar]
- 178.Bociek RG, Kuruvilla GJ, Pro B, et al. Isotype-selective histone deacetylase (HDAC) inhibitor MGCD0103 demonstrates clinical activity and safety in patients with relapsed/refractory classical Hodgkin Lymphoma (HL) J Clin Oncol. 2008;26 abstract 8507. [Google Scholar]
- 179.Martell RE, Younes A, Assouline SE, et al. Phase II study of MGCD0103 in patients with relapsed follicular lymphoma (FL): Study reinitiation and update of clinical efficacy and safety. J Clin Oncol. 2010;28 abstract 8086. [Google Scholar]
- 180.Berger MR, Bischoff H, Fritschi E, et al. Synthesis, toxicity, and therapeutic efficacy of 4-amino-N-(2-aminophenyl) benzamide: a new compound preferentially active in slowly growing tumors. Cancer Treat Rep. 1985;69(12):1415–1424. [PubMed] [Google Scholar]
- 181.Kraker AJ, Mizzen CA, Hartl BG, et al. Modulation of histone acetylation by [4-(acetylamino)-N-(2-amino-phenyl) benzamide] in HCT-8 colon carcinoma. Mol Cancer Ther. 2003;2(4):401–408. [PubMed] [Google Scholar]
- 182.Prakash S, Foster BJ, Meyer M, et al. Chronic oral administration of CI-994: a Phase 1 study. Invest New Drugs. 2001;19(1):1–11. doi: 10.1023/a:1006489328324. [DOI] [PubMed] [Google Scholar]
- 183.Undevia SD, Kindler HL, Janisch L, et al. A Phase I study of the oral combination of CI-994, a putative histone deacetylase inhibitor, and capecitabine. Ann Oncol. 2004;15(11):1705–1711. doi: 10.1093/annonc/mdh438. [DOI] [PubMed] [Google Scholar]
- 184.Richards DA, Boehm KA, Waterhouse DM, et al. Gemcitabine plus CI-994 offers no advantage over gemcitabine alone in the treatment of patients with advanced pancreatic cancer: results of a phase II randomized, double-blind, placebo-controlled, multicenter study. Ann Oncol. 2006;17(7):1096–1102. doi: 10.1093/annonc/mdl081. [DOI] [PubMed] [Google Scholar]
- 185.Ueda H, Manda T, Matsumoto S, et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968, III: antitumor activities on experimental tumors in mice. J Antibiot. 1994;47(3):315–323. doi: 10.7164/antibiotics.47.315. [DOI] [PubMed] [Google Scholar]
- 186.Ueda H, Nakajima H, Hori Y, et al. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physicochemical and biological properties, and antitumor activity. J Antibiot. 1994;47(3):301–310. doi: 10.7164/antibiotics.47.301. [DOI] [PubMed] [Google Scholar]
- 187.Yu XD, Wang SY, Chen GA, et al. Apoptosis induced by depsipeptide FK228 coincides with inhibition of survival signaling in lung cancer cells. Cancer J. 2007;13(2):105–113. doi: 10.1097/PPO.0b013e318046eedc. [DOI] [PubMed] [Google Scholar]
- 188.Sandor V, Robbins AR, Robey R, et al. FR901228 causes mitotic arrest but does not alter microtubule polymerization. Anticancer Drugs. 2000;11(11):445–454. doi: 10.1097/00001813-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 189.Sandor V, Senderowicz A, Mertins S, et al. P21-dependent G(1) arrest with downregulation of cyclin D1 and upregulation of cyclin E by the histone deacetylase inhibitor FR901228. Br J Cancer. 2000;83(6):817–825. doi: 10.1054/bjoc.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang R, Brunner T, Zhang L, et al. Fungal metabolite FR901228 inhibits c-Myc and Fas ligand expression. Oncogene. 1998;17(12):1503–1508. doi: 10.1038/sj.onc.1202059. [DOI] [PubMed] [Google Scholar]
- 191.Yu X, Guo ZS, Marcu MG, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94(7):504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 192.Rajgolikar G, Chan KK, Wang HC. Effects of a novel antitumor depsipeptide, FR901228, on human breast cancer cells. Breast Cancer Res Treat. 1998;51(1):29–38. doi: 10.1023/a:1006091014092. [DOI] [PubMed] [Google Scholar]
- 193.Kitazono M, Robey R, Zhan Z, et al. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na/I symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86(7):3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 194.Aron JL, Parthun MR, Marcucci G, et al. Depsipeptide (FR901228) induces histone acetylation and inhibition of histone deacetylase in chronic lymphocytic leukemia cells concurrent with activation of caspase 8-mediated apoptosis and down-regulation of c-FLIP protein. Blood. 2003;102(2):652–658. doi: 10.1182/blood-2002-12-3794. [DOI] [PubMed] [Google Scholar]
- 195.Weiser TS, Guo ZS, Ohnmacht GA, et al. Sequential 5-aza-2 deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother. 2001;24(2):151–161. doi: 10.1097/00002371-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 196.Steiner FA, Hong JA, Fischette MR, et al. Sequential 5-Aza 2-deoxycytidine/depsipeptide FK228 treatment induces tissue factor pathway inhibitor 2 (TFPI-2) expression in cancer cells. Oncogene. 2005;24(14):2386–2397. doi: 10.1038/sj.onc.1208376. [DOI] [PubMed] [Google Scholar]
- 197.Klisovic MI, Maghraby EA, Parthun MR, et al. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia. 2003;17(2):350–358. doi: 10.1038/sj.leu.2402776. [DOI] [PubMed] [Google Scholar]
- 198.Sasakawa Y, Naoe Y, Sogo N, et al. Marker genes to predict sensitivityto FK228, a histone deacetylase inhibitor. Biochem Pharmacol. 2005;69(4):603–616. doi: 10.1016/j.bcp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 199.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27(32):5410–5417. doi: 10.1200/JCO.2008.21.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Woo S, Gardner ER, Chen X, et al. Population pharmacokinetics of romidepsin in patients with cutaneous T-cell lymphoma and relapsed peripheral T-cell lymphoma. Clin Cancer Res. 2009;15(4):1496–1503. doi: 10.1158/1078-0432.CCR-08-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Marshall JL, Rizvi N, Kauh J, et al. A Phase I trial of depsipeptide (FR901228) in patients with advanced cancer. J Exp Ther Oncol. 2002;2(6):325–332. doi: 10.1046/j.1359-4117.2002.01039.x. [DOI] [PubMed] [Google Scholar]
- 202.Sandor V, Bakkes S, Robey RW, et al. Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin Cancer Res. 2002;8(3):718–728. [PubMed] [Google Scholar]
- 203.Byrd JC, Marcucci G, Parthun MR, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105(3):959–967. doi: 10.1182/blood-2004-05-1693. [DOI] [PubMed] [Google Scholar]
- 204.Fouladi M, Furman WL, Chin T, et al. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children’s Oncology Group report. J Clin Oncol. 2006;24(22):3678–3685. doi: 10.1200/JCO.2006.06.4964. [DOI] [PubMed] [Google Scholar]
- 205.Klimek VM, Fircanis S, Maslak P, et al. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin Cancer Res. 2008;14(3):826–832. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 206.Stadler WM, Margolin K, Ferber S, et al. A Phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin Genitourin Cancer. 2006;5(1):57–60. doi: 10.3816/CGC.2006.n.018. [DOI] [PubMed] [Google Scholar]
- 207.Shah MH, Binkley P, Chan K, et al. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12(13):3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 208.Schrump DS, Fischette MR, Nguyen DM, et al. Clinical and molecular responses in lung cancer patients receiving Romidepsin. Clin Cancer Res. 2008;14(1):188–198. doi: 10.1158/1078-0432.CCR-07-0135. [DOI] [PubMed] [Google Scholar]
- 209.Sosman JA, Aronson FR, Sznol M, et al. Concurrent phase I trials of intravenous interleukin 6 in solid tumor patients: reversible dose-limiting neurological toxicity. Clin Cancer Res. 1997;3(1):39–46. [PubMed] [Google Scholar]
- 210.Maloney A, Workman P. HSP90 as a new therapeutic target for cancer therapy: the story unfolds. Expert Opin Biol Ther. 2002;2(1):3–24. doi: 10.1517/14712598.2.1.3. [DOI] [PubMed] [Google Scholar]
- 211.Bantscheff M, Hopf C, Savitski MM, et al. Chemoproteomics profiling of HDAC inhibitors reveals selective targeting of HDAC complexes. Nat Biotechnol. 2011. In press. [DOI] [PubMed]