Abstract
We describe a six step synthesis to water soluble doxorubicin (DOX)-loaded biodegradable PEGylated star-comb polymers with favorable pharmaceutical properties by atom transfer radical polymerization (ATRP) starting with a commercially available tripentaerythritol carrying eight reactive sites. The low polydispersity polymers degrade in a stepwise manner into lower molecular weight (MW) fragments by 15 days at 37 °C at either pH 5.0 or pH 7.4. The half-life of the star-comb polymers in blood is dependent upon the molecular weight; the 44 kDa star-comb has a t1/2, β of 30.5 ± 2.1 h, which is not significantly changed (28.6 ±2.7 h) when 6.6 wt% of DOX is attached to it via a pH-sensitive hydrazone linker. The star-comb polymers have low accumulation in organs but a high accumulation in C26 flank tumors implanted in Balb/C mice. The hydrodynamic diameter of polymer-DOX conjugates measured by dynamic light scattering increases from 8 to 35 to 41 nm as the loading is increased from 6.6 to 8.4 to 10.2 wt%. Although there is no significant difference in the t1/2, β or in the accumulation of polymer-DOX in C-26 tumors, the uptake of polymer in the spleen is significantly higher for polymers with DOX loadings greater than 6.6 wt%. Polymer accumulation in other vital organs is independent of the DOX loading. The facile synthesis, biodegradability, long circulation time and high tumor accumulation of the attached drug suggests that the water-soluble star-comb polymers have promise in therapeutic applications.
INTRODUCTION
In the past three decades, there has been an extensive exploration of a variety of polymers as drug carriers, a concept introduced by Kopecek, Ringsdorf and Duncan.1 In a recent exploration, PEGylated star polymers have been demonstrated to be well-suited for drug delivery applications due to their high solubility, biocompatibility, low toxicity, low organ accumulation and high accumulation in tumor sites.2–5 In contrast to traditional linear polymers, star polymers possess a very narrow polydispersity index and a defined number of functional groups; this enables a consistent drug loading and promotes reproducible pharmacokinetic behavior.2,6 Polyester linkages provide the additional advantage of biodegradability,7 and PEGylated polyester star polymers of high molecular weight show excellent promise as drug carriers.2,6,8 The doxorubicin (DOX) conjugated “bow-tie” structure with a fourth generation of 2, 2-bis(hydroxymethyl)propionic acid (bis HMPA) and eight 5 kDa PEG chains showed anti-tumor activity comparable to the FDA approved liposome DOX (DOXIL™).2
PEGylated star polymers are commonly prepared by coupling activated PEG chains to dendrimer cores, which are prepared by a multi-step synthetic procedure.2,4,9–12 Specialized PEG reagents with reactive functionalities and long reaction times are required to transform the dendritic cores into high molecular weight, branched polymers. PEGylated star polymers have also been prepared by living polymerization, such as atom transfer radical polymerization (ATRP) of poly(ethylene glycol) methylether methacrylate (PEGMA) using dendritic cores as initiators, which allows for the rapid build-up of polymers while also providing for control over molecular weight and polydispersity.10,13,14 However, the core initiators of these polymers either have a core that is difficult to synthesize13 or they are not biodegradable.10 In addition, these PEGylated star polymers prepared by living polymerization have neither been characterized in vivo nor used as drug carriers.14
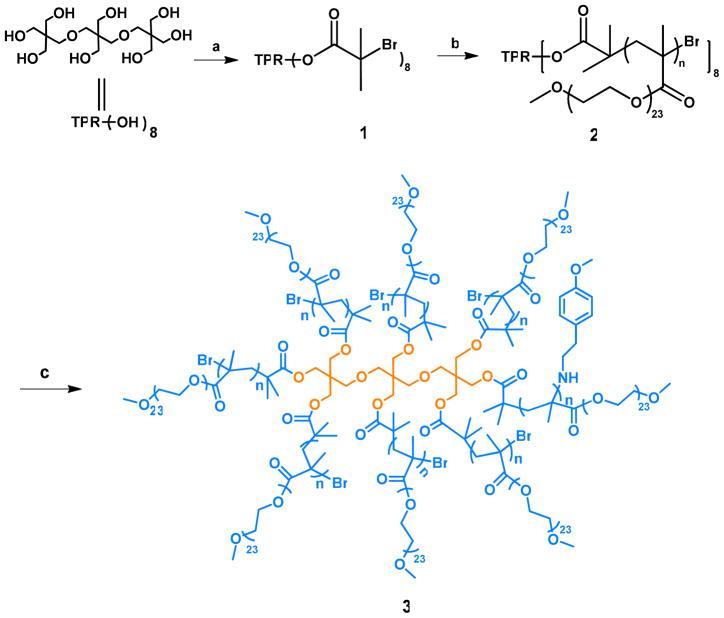
Herein we describe a facile approach to biodegradable PEGylated star-comb polymers (Scheme 1) by using a commercially available “tripentaerythritol” with eight reactive sites. Due to the large amount of PEGMA incorporated into the carrier by grafting from the tripentaerythritol core, this structure should be inherently water-soluble and exist as a unimolecular species,15,16 as opposed to non-covalent aggregate structures such as the micelles prepared from amphiphilic block copolymers17–19 or other systems such as shell cross-linked knedel-like (SCK) nanoparticles.20,21 We evaluated the pharmacokinetics of the star-comb polymers with various molecular weights in mice. Following these initial studies, the polymer with a molecular weight of 45 kDa was selected to attach DOX via a pH sensitive acyl hydrazone bond (Scheme 2).2,22 We studied the biodistribution of this polymer-DOX conjugate to explore the potential of this star-comb polymer as an antitumor-modality.
Scheme 1.
Synthesis of star-comb polymer with one methoxyphenol groupi
ia. DCM, pyridine, 2-bromoisobutyrl bromide; b. PEGMA, CuBr, PMDETA, anisole;
c. TEA, MPEA, DMF
Scheme 2.
Synthesis of star-comb polymer (45 kDa) - DOX conjugatei
ia. PEGMA, CuBr, PMDETA, t-butyl methacrylate, anisole; b. TFA, DCM; c. HBTU, HOBt, DiPEA, t-butyl carbazate, DMF; d. DOX, acetic acid, pyridine, methanol
MATERIALS AND METHODS
General Procedures and Materials
2-(4-Methoxyphenyl) ethylamine (MPEA) was purchased from ARCO Chemical Company. All other chemicals of the highest grade available, including solvents, were purchased from Sigma-Aldrich and used without further purification. Poly(ethylene glycol) methyl ether methacrylate (PEGMA) with molecular weights of 1100 and 2080 Daltons were purchased from Sigma-Aldrich. High performance flash chromatography (HPFC) was carried out on a Biotage (Charlottesville, VA) Horizon system with prepacked silica gel columns. 1H NMR spectra were recorded on a Bruker Avance 300 MHz ultrashield NMR. NMR chemical shifts are reported in ppm and calibrated against solvent signals. MALDI-TOF MS data was collected on a PerSeptive Biosystems Voyager-DE instrument in positive ion mode using a 2,5-dihydroxybenzoic acid (DHB) matrix and calibration against bovine insulin standards. Gel Permeation Chromatography (GPC) consisted of a Viscotek 1122 pump, a Viscotek 270 dual detector and a Viscotek 3500 differential refractive index (RI) detector. GPC was performed at 1.0 mL/min in two Resipore (3 μm) columns (Polymer Laboratories, 300 × 7.5 mm) thermostated to 50 °C using THF as the mobile phase and poly(methyl methacrylate) as the calibration standards. Refractive index increment (dn/dc) values are determined by plotting the refractive index values of polymer solutions versus varying polymer concentrations (2.5, 5.0, 10.0 and 15.0 mg/mL). The number average diameters of the polymer-DOX conjugates were determined by using dynamic light scattering. Experiments were performed three times at 25 °C in distilled water using filtered 1.0 mg/mL samples with a Zetasizer Nano ZS (Malvern Instruments, Malvern, U.K.) equipped with a 4-mW He-Ne laser at 633 nm.
ATRP Initiator (1)
Tripentaerythritol (1.0 g, 2.7 mmol) was suspended in dry dichloromethane (25.0 mL), followed by the addition of pyridine (10.0 mL). The solution was then cooled by ice and 2-bromoisobutyryl bromide (9.7 g, 42.0 mmol) dissolved in 20.0 mL of dichloromethane was added dropwise over a period of 30 min. The reaction continued 2 days at room temperature. The mixture was washed with 0.3 M NaHCO3 (3 × 50.0 mL), 0.3 M NaHSO4 (3 × 50.0 mL) and water (2 × 50.0 mL). The organic layer was dried over Na2SO4 and filtered. The solution was concentrated by rotary evaporator and purified by HPFC to provide initiator 1 as a white solid (2.5 g, 61%). 1H NMR (300 MHz, CDCl3): δ 1.94 (m, 48H), 3.60 (d, 8H), 4.29 (d, 16H) MALDI-TOF MS. [M + H]+ Calcd: m/z = 1563.81. Found: m/z = 1565.15
Synthesis of Polymer 2
In a typical experiment, initiator 1 (50.0 mg, 32.0 μmol) and PEGMA (1.1 kDa) (563.0 mg, 512.0 μmol) were added in anisole (0.5 mL) followed by the addition under argon of CuBr (55.5 mg, 384.0 μmol) and N,N,N′,N′,N″-pentamethyldiethylenetriamine (PMDETA) in a round bottom flask equipped with a magnetic stir bar. The solution was deoxygenated by three freeze-pump-thaw cycles. The polymerization was carried out at 90 °C under argon and stopped after the appropriate time by being exposed to air and diluted with dichloromethane. The solution was passed through an alumina column to remove the copper catalyst. The excess solvent was removed on a rotary evaporator under reduced pressure. The unreacted PEG monomers were removed by precipitation of the polymer into diethyl ether. The molecular weight of polymers was determined by 1H NMR and GPC (Table 1).
Table 1.
The Synthesis Conditions and Characterization of Star-Comb Polymers
| [M]/[I] | Time (min) | Conversion % a | Mn (kDa) a | Mn (kDa) b | PDI b |
|---|---|---|---|---|---|
| 10 | 30 | 95 | 12 | 12 | 1.10 |
| 20 | 30 | 95 | 23 | 19 | 1.09 |
| 30 | 35 | 95 | 33 | 32 | 1.08 |
| 50 | 60 | 80 | 46 | 44 | 1.13 |
| 70 | 60 | 80 | 63 | 64 | 1.06 |
| 150 | 90 | 60 | 101 | 97 | 1.07 |
Determined by 1H NMR
Determined by GPC
Synthesis of Polymer 3
Polymer 2 (0.1 g) was dissolved in 2.0 mL of anhydrous DMF, and 10 μL (12.0 equiv) of triethylamine was added, followed by MPEA (12.0 mg, 6.0 equiv per polymer hydroxyl). The reaction mixture was stirred at room temperature for 2 days and dialyzed against methanol to remove the unreacted MPEA. The degree of substitution on the polymer was quantified by 1H NMR spectroscopy and ranged from 1.0 to 3.5/polymer (Table 2).
Table 2.
Pharmacokinetic Data for the Evaluated Polymers
| Polymer Mw (kDa) | av. no. of MPEA per polymer | t1/2, α (h)* | t1/2, β (h)* | AUC0→∞ (% dose. h/g)* |
|---|---|---|---|---|
| 12 | 3.5 | 0.10 ± 0.04 | 6.7 ± 0.8 | 30 ± 10 |
| 19 | 3.5 | 0.24 ± 0.06 | 8.7 ± 1.8 | 110 ± 40 |
| 32 | 3 | 0.61 ± 0.12 | 24.5 ± 4.6 | 1110 ± 140 |
| 44 | 2 | 0.89 ± 0.24 | 30.5 ± 2.1 | 1310 ± 130 |
| 64 | 2.5 | 0.95 ± 0.45 | 33.2 ± 3.4 | 1540 ± 170 |
| 97 | 1 | 0.96 ± 0.31 | 36.9 ± 6.3 | 1800 ± 290 |
Values are the mean ± S.D., n=3.
General Deprotection by Trifluoroacetic Acid
Polymer 4 or 5 (0.1 g) was dissolved in a 1:1 mixture of trifluoroacetic acid (TFA) and CH2Cl2. After stirring overnight, the solvents were removed under reduced pressure, and the products were precipitated into diethyl ether twice. 1H NMR spectroscopy was used to verify the absence of residual t-butyloxycarbonyl protecting groups.
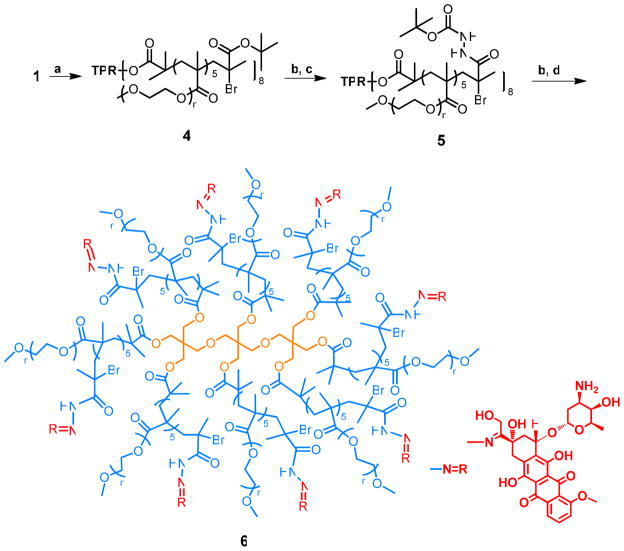
Synthesis of Polymer 4
Initiator 1 (50.0 mg, 32.0 μmol) and PEGMA (1.1 kDa) (1.7g, 1.6 mmol) were added in anisole (1.7 mL) followed by the addition under argon of CuBr (55.5 mg, 0.4 mmol) and PMDETA (155 μL) in a round-bottom flask equipped with a magnetic stir bar. The solution was deoxygenated by three freeze-pump-thaw cycles. The polymerization was carried out at 90 °C, and degassed t-butyl methacrylate (420 μL, 2.9 mmol) was added after 50 min. After 2.5 h, the reaction was exposed to air and diluted with dichloromethane. The solution was passed through an alumina column to remove the copper catalyst. The excess solvent was removed on a rotary evaporator under reduced pressure. The unreacted PEG monomers and t-butyl methacrylate were removed by precipitation of the polymer into diethyl ether. The molecular weight of polymers was determined by GPC (Mn = 45 kDa, PDI =1.13). About five molecules of PEGMA and one molecule of t-butyl methacrylate (tBMA) were added to each initiator site by 1H NMR.
Synthesis of Polymer 5
The deprotected polymer 4 (820.0 mg, ~ 150.0 μmol of acid) was dissolved in 5.0 mL of DMF, and then 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU, 170.0 mg, 450.0 μmol) and hydroxybenzotriazole (HOBt, 61.0 mg, 450.0 μmol) were added. Next, t-butyl carbazate (59.0 mg, 450.0 μmol) and N,N-diisopropylethylamine (DiPEA, 270 μL) were added and the reaction mixture was stirred at room temperature for one day. The product was then precipitated into diethyl ether three times. The average number of t-butyl carbazate moieties attached to each polymer was about 8.2 by 1HNMR. The average molecular weight (Mn) is about 46 kDa by GPC (PDI = 1.13).
Synthesis of the Acyl Hydrazone-Linked Polymer-DOX Conjugate (6)
The deprotected polymer 5 (35.0 mg, 76.0 μmol) was dissolved in 0.4 mL methanol with 40 μL acetic acid and 40 μL pyridine. DOX HCl (3.3 mg, 5.7 mmol, 8.0 equiv) was added, and the reaction mixture was heated at 60 °C overnight in the dark. The reaction mixture was then purified by preparative SEC (Sephadex LH20, GE Healthcare, Piscataway, NJ) in methanol to separate polymer-bound DOX from free DOX. The high molecular weight fractions were evaporated, dissolved in water, and purified further by preparative SEC (Sephadex G25, GE Healthcare) in water. The high-molecular-weight fraction was lyophilized to provide the product as a red powder. The amount of DOX conjugated to the polymer was determined by measuring the absorbance at 485 nm of the polymer solution in water (Table 3).
Table 3.
Characterization and Pharmacokinetic Data for the Evaluated Polymer-DOX Conjugates.
| Polymer-DOX conjugate | PEG monomer Mw (kDa) | Dox Loading (wt %) | Hydrodynamic diameter (nm) | t1/2,β (h)* |
|---|---|---|---|---|
| 1 | 1 | 6.6 | 8±2.3 | 28.6±2.7 |
| 2 | 1 | 8.4 | 35±6.4 | 31.2±3.4 |
| 3 | 1 | 10.2 | 41±7.5 | 31.7±4.2 |
| 4 | 2 | 8.4 | 56±10.8 | 25.7±5.3 |
Values are the mean ± S.D., n=3.
Polymer Degradation Study
Polymer 2 (40 kDa) was dissolved at a concentration of 1.0 mg/mL in a solution containing either 30.0 mM acetate buffer (pH 5.0) with 70.0 mM NaNO3 or 30.0 mM phosphate buffer (pH 7.4) with 70.0 mM NaNO3. The samples were incubated at 37 °C, and a 0.3 mL aliquot was removed at intervals, lyophilized and re-dissolved in THF, then measured by GPC.
Radioiodination of Methoxyphenol Functionalized Polymers
2-(4-Methoxyphenyl) ethylamine functionalized polymers were iodinated based upon the method described by Krummeich et al.23 In brief, 3.0 mg of polymer 3 was dissolved in 250 μL of trifluoroacetic acid (TFA), and 10 μL of an aqueous solution of 125I was added containing 1.0 mCi 125I. Chloramine T (540.0 μg) dissolved in 50 μL of TFA was added to the mixture, and the reaction was heated at 60 °C for 5 min. The reaction was stopped by the addition of sodium metabisulfite (1.0 mg in 10 μL of water), and the TFA was evaporated under a stream of nitrogen that was vented through a trap containing activated carbon (Dupont-New England Nuclear, MA). The residue was dissolved in 10 μL of 100.0 mM HEPES buffer (pH 7.4) and chromatographed on a column containing 1.8 mL bed volume of Dowex 2 anion-exchange resin in the chloride form. The radiolabeled polymer was eluted in the first 4.0 mL using water as the mobile phase. Polymers were separated from low molecular weight radioactive contaminants by SEC on Bio-Rad 10DG desalting columns equilibrated with phosphate buffered saline (PBS). The initial, high molecular weight fractions were collected and pooled. A suitable quantity of radiolabeled polymer was mixed with cold polymer in sterile PBS to create a solution with 5.0 mg/mL polymer and specific activities ranging from 3.0 to 25.0 μCi/mL.
Animal Experiments
All animal experiments were performed in compliance with the National Institutes of Health guidelines for animal research under a protocol approved by the Committee on Animal Research at University of California (San Francisco, CA) (UCSF). C26 colon carcinoma cells obtained from the UCSF cell culture facility were cultured in RPMI medium 1640 containing 10% FBS. Female BALB/c mice were obtained from Simonsen Laboratories, Inc. (Gilroy, CA).
Biodistribution Studies
Polymer solutions (200 μL) were administered intravenously via the tail vein to 6- to 8-week-old BALB/c female mice. The mice were sacrificed at seven different time points (three mice per group) ranging from 5 min to 48 h postinjection for biodistribution analyses. The blood (collected by heart puncture), heart, lungs, liver, stomach, spleen, intestines, kidney, body, tail and head were weighed, and the amount of radioactivity present in each organ was quantified. Periodically, blood was also collected from the retroorbital sinus at intermediate time points to determine the dose of polymer in the blood. For the 24 h time points, mice were housed in metabolic cages to allow for the collection of urine and feces. The % injected dose per gram (% ID/g) of blood versus time curve was analyzed using a two-compartment model.24
Biodistribution Studies of Radiolabeled Polymer in Tumored Mice
The 6- to 8-week-old female BALB/c mice were injected with 3.0 × 105 C26 cells colon carcinoma cells on the right rear flank by subcutaneous administration. The mice (three per group) were injected intravenously via the tail vein with 200 μL of radiolabeled polymer solution prepared as described previously. The mice were sacrificed at 24 h and 48 h postinjection, their blood, liver, muscle and tumor were collected and weighed, and the radioactivity in each was quantified.
Biodistribution Studies of Polymer-DOX Conjugates in Tumored Mice
The 6- to 8-week-old female BALB/c mice were injected with 3.0 × 105 C26 colon carcinoma cells on the right rear flank by subcutaneous administration. The mice (three per group) with tumor size about 100.0 – 250.0 mg were injected intravenously via the tail vein with 200 μL of polymer-DOX solution (8.0 mg Dox equiv/kg) in PBS. Two control mice were also injected with 200 μL of PBS. The mice were sacrificed 48 h postinjection. The blood (collected by heart puncture), heart, liver, spleen, kidney, muscle, and tumor were collected for analyses by following the procedure described before.9 Briefly, each organ was weighed and 200–300 mg of the collected organs were homogenized with zirconium beads and 1.0 mL acidified isopropyl alcohol (75.0 mM HCl, 90% IPA). The samples then incubated at 4 °C for 24 h. Serum was collected using Microtainer serum separator vials and processed in the same manner as the organs. The samples were frozen in a −80 °C freezer until measurements could be made. At measurement time, samples were thawed, briefly vortexed, and centrifuged for 5 min at 14,000 rpm. Then, 80 μL of supernatant was combined with 920 μL of acidified IPA for fluorescence measurements. Dox fluorescence (excitation 485 nm; emission 595 nm) was measured on a Spex Fluorolog fluorimeter. Calibration curves were made from organ samples collected from an untreated mouse.
RESULTS AND DISCUSSION
A straightforward two-step synthesis was used to create the desired eight arm star-comb architecture (Scheme 1). The polymerization of PEGMA (1100 Da) was initiated by a-bromoisobutyryl functionalized tripentaerythritol. This novel dendritic core has eight sites that may be used to initiate polymerization. The molecular weights of the star-combs were adjusted by controlling the feed ratio and using appropriate reaction times (Table 1). The star-combs are soluble in a variety of common organic solvents and water. Precipitation in diethyl ether or dialysis using 50,000 molecular weight cut-off regenerated cellulose membranes against methanol was used to remove unreacted monomers, generally producing isolated yields of polymer greater than 60%. In the synthesis of polymers with molecular weight less than 32 kDa, the monomers react completely so that no further purification is required. The polydispersity index of all the eight arm star-combs was less than 1.15, and their molecular weights were in good agreement with the theoretical molecular weight values. Furthermore, the eight reactive bromide functionalities that remain on each star-comb can easily be used to generate sites for drug attachment.25,26 A drug such as DOX can be attached to the star-comb in six rapid and easily scalable synthetic steps, which is fewer than that typically used in the preparation of drug-loaded star polymers.2 The starting materials, including tripentaerythritol and PEGMA, are inexpensive and commercially available.
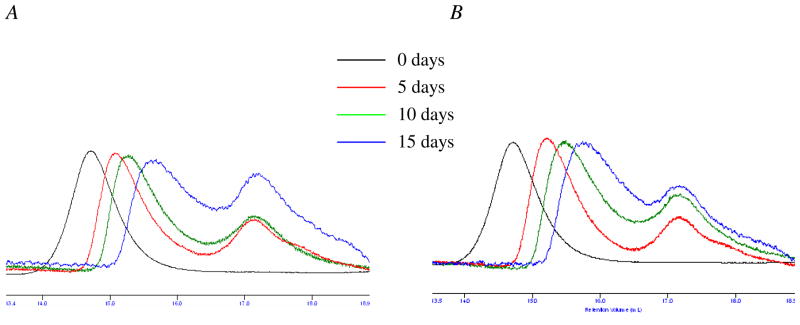
PEG is attached to the backbone of the branches by ester bonds while the eight branches are themselves attached via ester bonds to the dendritic scaffold, and both ester linkages are susceptible to hydrolysis.2 Complete ester hydrolysis should release PEG chains (1 kDa) as well as the dendritic core. To confirm that the polymeric carriers degrade under physiological conditions, a representative polymer (40 kDa) was incubated in pH 5.0 and pH 7.4 buffers at 37 °C, and the molecular weight distribution was monitored over time by SEC. At pH 5.0 and over a period of 5 days, there is an increase in the retention time of the peak corresponding to the PEG - dendrimer hybrid (Figure 1A), indicating a decrease in molecular weight due to polymer degradation. In addition, a new peak corresponding to the molecular weight of the 1 kDa PEG monomer appears in the chromatographic trace. Almost complete degradation was evidenced by the presence of a high proportion of low molecular weight material after 15 days. At pH 7.4 (Figure 1B), a similar decrease in the polymer molecular weight was observed with the appearance of a new peak with a retention time corresponding to a molecular weight of 1 kDa.
Figure 1.
Time-dependent GPC profiles of the polymer (40 kDa) upon incubation in (A) pH 5.0 and (B) pH 7.4 buffer at 37 °C
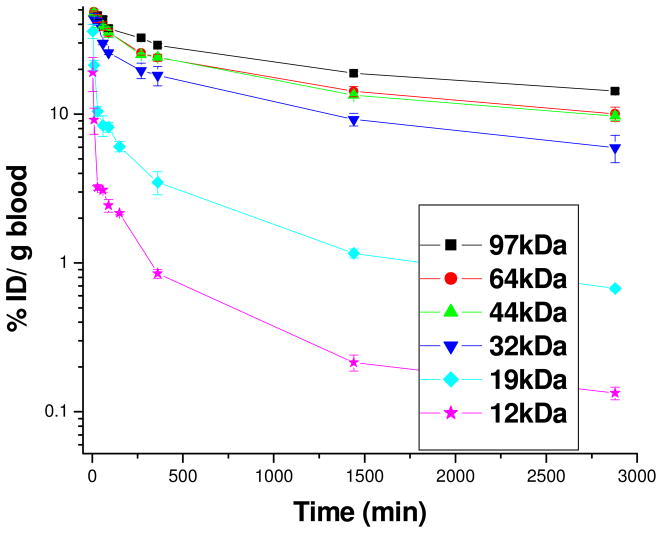
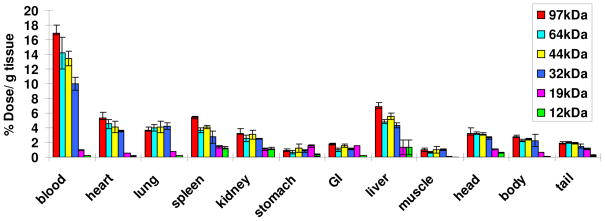
We also determined the time-dependent biodistribution profiles to examine the effect of molecular weight on bioaccumulation. In order to track the polymers in vivo, a number of terminal bromide groups of each star-comb polymer were substituted by 2-(4-Methoxyphenyl) ethylamine (MPEA) as shown in Scheme 1. The number of MPEA molecules on the polymers were subsequently quantified by 1H NMR spectroscopy (Table 2), and the polymers were then radiolabeled with 125I using the electrophilic chloramine T method as previously described23 before being administered to mice. Elimination half-lives (t1/2) and areas under the % dose/g versus time curve from 0 to infinity (AUC0→∞) were calculated using the two-compartment model24 and are shown in Table 2. As expected, increasing the molecular weight of these star-comb polymers from 12 to 97 kDa, leads to the expected increases in both the t1/2, β and AUC0→∞ values (Figure 2). Interestingly, there is a sharp decrease of t1/2, β when the molecular weight was decreased from 32 to 19 kDa, which indicates that the threshold molecular weight for renal filtration is within this range. This value is lower than the molecular weight cutoff for renal filtration of 30 to 40 kDa previously described for linear PEG.27 Indeed, these star-comb polymers showed much longer circulation time than linear PEG. The t1/2, β values reported for linear 50 kDa PEG is 16.5 h and the corresponding AUC0→∞ value is 600 % dose.h/g. In this study, the star-combs with molecular weights above 32 kDa had long blood circulation times. As molecular weights increases from 32, to 44, to 64, and to 97 kDa, t1/2, β increased from 24.5, to 30.5, 33.2 and 36.9 h, while the corresponding AUC0→∞ values increased from 1110, 1310, 1540 to 1800 % dose.h/g, respectively. We observed that the 44 kDa star-comb had similar t1/2, β and AUC0→∞ values to a “bow-tie” dendritic polymer2 with a molecular weight of 45 kDa and greater values than those of a PEGylated 42 kDa dendrimer with an aspartic acid modified bis-2,2-hydroxymethyl propanoic acid (bisHMPA) based core.9 In contrast to polymers with higher molecular weights, the 19 and 12 kDa polymers are cleared rapidly from circulation. Significant amounts of polymer were found in urine (36% for 12 kDa and 29% for 19 kDa) and feces (15% for 12 kDa and 26% for 19 kDa), while only small amount of polymers with molecular weights above 32 kDa were eliminated in urine (around 10%) and feces (<5%) at 24 h. No specific organ accumulation was observed for these macromolecules (Figure 3). The results indicate that the hydrolysis of the star-comb polymers larger than 32 kDa can lead to fast elimination, thus preventing their long-term accumulation.
Figure 2.
Polymer concentration in the blood over time as a function of time after intravenous administration (mean ± S.D., n=3).
Figure 3.
Biodistribution of drug-free polymers in non-tumored mice at 48 hours as a function of molecular weight reported as % dose/g tissue (mean ± S.D., n=3).
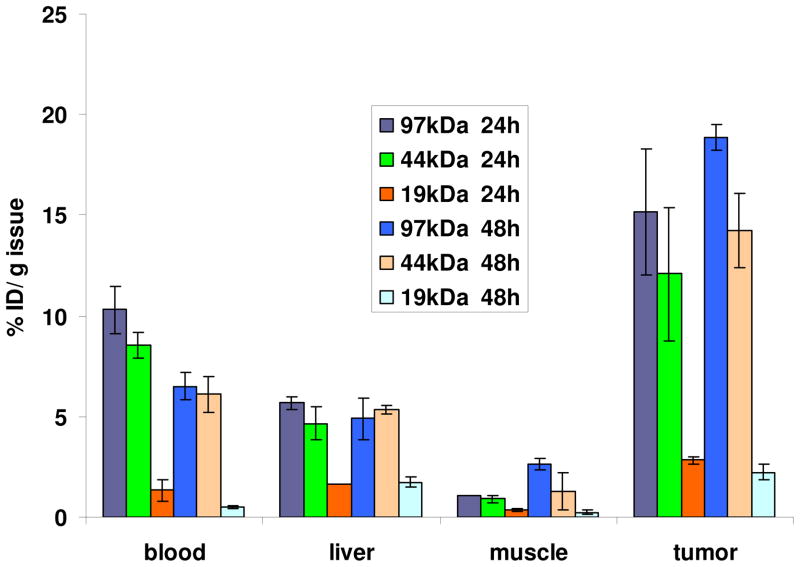
A biodistribution study in BALB/c mice tumored with the C26 colon carcinoma in the flank was performed to evaluate the tumor accumulation of star-comb polymers with different molecular weights. Polymers with molecular weights of 97, 44 and 19 kDa were selected in order to span a range of circulation life-times. The concentration of polymer found in tumor tissue was directly related to molecular weight. Polymers having molecular weights of 19, 44 to 97 kDa had drug tumor levels of 2.8 ± 0.17, 12.1 ± 3.3 and 15.2 ± 3.1 % dose/g, respectively after 24 h and 2.2 ± 0.36, 14.2 ± 1.9 and 18.9 ± 0.62 % dose/g, respectively after 48 h (Figure 4). The 19 kDa star-comb had a maximum tumor accumulation at 24 h. The tumor concentration of star-combs with molecular weights of 44 and 97 kDa increased from 24 h to 48 h. This can be ascribed to the EPR effect.2,28,29 These values compare favorably with the tumor accumulations reported for other polymers such as PEGylated “bow-tie” dendrimer, HPMA and PEO.2,30,31 The tumor/muscles accumulation ratios for the 44 kDa polymer are 16 at 24 h and 11 at 48 h, and the ratios of the 97 kDa polymer are 11 at 24 h and 7 at 48 h on a % dose/g basis. The tumor/muscle ratios decrease from 24 h to 48 h due to the increased accumulation in muscles for both polymers at 48 h. Such high levels of tumor accumulation should provide star-combs with the capability to effectively deliver drugs to tumor sites.
Figure 4.
Biodistribution of drug-free polymers in mice bearing subcutaneous C26 tumors at 24 and 48 h based on % dose/g tissue (mean ± S.D., n=3).
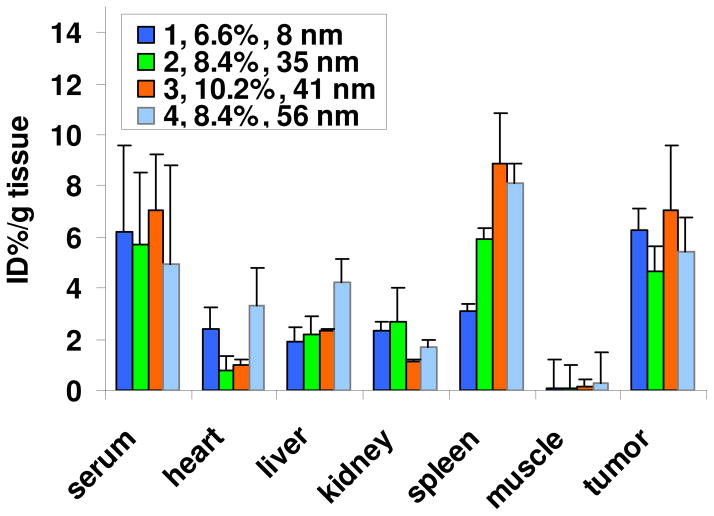
To further evaluate the potential of this polymer to be a drug carrier, DOX was conjugated to the 45 kDa star-comb through a hydrazone bond2,22 to form a stealth PEG corona-drug core structure (Scheme 2). The molecular weight of 45 kDa was chosen because it provides a good balance between the theoretical drug loading and the circulation half-life based on the previous studies.2,8,9 It is also easy to compare this polymer with other the DOX loaded polymers, such as PEGylated ester-amide dendrimer and polylysine dendrimer.8,9 The hydrazone linkage can release DOX in its biologically active form after endocytosis but is stable at the physiological pH of 7.4. About one t-butyl methacrylate was introduced to each of the eight branches for attachment of t-butylcarbazate. Deprotection of the hydrazide Boc group followed by hydrazone formation with DOX in methanol afforded polymer containing DOX (Scheme 2). The loading of DOX is controlled by the molar ratio of DOX and hydrazide (Table 3). Interestingly, the size of the particles is dependent on the loading of DOX. As the loading increased from 6.6 to 8.4 and 10.2 wt%, the polymer-DOX conjugates formed aggregates and the particle size increased from 8 nm to 35 nm to 41 nm (Table 3). Even when 2 kDa PEGMA was used instead of the corresponding 1 kDa as monomer and the molecular weight of the polymer was kept at 45 kDa, aggregates were still observed (56 nm) at a loading of 8.4 wt%. Tumored mice were injected with polymer-DOX conjugates (1–4) at a dose of 8.0 mg Dox/kg. All the polymer-DOX conjugates, similar to the radiolabeled 44 kDa polymer, have t1/2, β values of about 30 h (Table 3). Therefore, their blood circulation time was not significantly changed by the addition of the functional moieties, the attachment of a drug payload, or the diameter of the polymer-DOX conjugates.
Furthermore, the accumulation of DOX loaded polymers in the tumor (~ 6 % ID/g tissue) are comparable to the levels of accumulation we have previously observed for related macromolecules.2,8,9 The tumor accumulation of DOX for the polymer-DOX conjugates was also similar to what is observed for the PEGylated liposome formulation known as DOXIL™ (Figure 5).9 Interestingly, the polymer-DOX conjugate with a diameter of 8 nm showed a lower accumulation in the spleen than the other three polymer-DOX conjugates with larger diameters or DOXIL™ with a diameter of 100 nm. All of the DOX-loaded polymers accumulated at less than 5 % ID/g tissue in the other vital organs. Therefore, the polymer-DOX conjugate with a diameter of 8 nm has a more favorable tissue distribution compared to the other polymers, as it maintains a high drug concentration in the tumor while accumulating less in the organs. Because of this carrier’s low toxicity and high tumor accumulation, it could provide an alternative solution to DOXIL™ for reducing the side effects caused by DOX.
Figure 5.
Biodistribution of polymer-DOX conjugates in mice bearing subcutaneous C26 tumors at 48 h based on % dose/g tissue (mean ± S.D., n=3). 1–4 are corresponding to the samples in Table 3.
In conclusion, a biodegradable polyester star-comb polymer-PEG hybrid was synthesized by ATRP using an inexpensive dendritic core that is accessible in a rapid, high yielding and reproducible synthetic scheme. The polymer was also shown to deliver large dose percentages of DOX to C26 tumors when injected into mice.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-EB 002047.
Footnotes
Supporting Information Available: 1H NMR spectra for target compounds. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Duncan R. Development of HPMA copolymer-anticancer conjugates: Clinical experience and lessons learnt. Adv Drug Deliver Rev. 2009;61:1131–1148. doi: 10.1016/j.addr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Frechet JM, Dy EE, Szoka FC. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103:16649–16654. doi: 10.1073/pnas.0607705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson SM, Godbey WT. The role of macromolecular architecture in passively targeted polymeric carriers for drug and gene delivery. Journal of drug targeting. 2008;16:329–356. doi: 10.1080/10611860801969616. [DOI] [PubMed] [Google Scholar]
- 4.Lim J, Chouai A, Lo ST, Liu W, Sun X, Simanek EE. Design, synthesis, characterization, and biological evaluation of triazine dendrimers bearing paclitaxel using ester and ester/disulfide linkages. Bioconjug Chem. 2009;20:2154–2161. doi: 10.1021/bc900324z. [DOI] [PubMed] [Google Scholar]
- 5.Lo ST, Stern S, Clogston JD, Zheng J, Adiseshaiah PP, Dobrovolskaia M, Lim J, Patri AK, Sun X, Simanek E. E. Biological assessment of triazine dendrimer: toxicological profiles, solution behavior, biodistribution, drug release and efficacy in a PEGylated, paclitaxel construct. Mol Pharm. 2010;7:993–1006. doi: 10.1021/mp100104x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox ME, Szoka FC, Frechet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dailey LA, Wittmar M, Kissel T. The role of branched polyesters and their modifications in the development of modern drug delivery vehicles. J Control Release. 2005;101:137–149. doi: 10.1016/j.jconrel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Frechet JM. Design, synthesis, and biological evaluation of a robust, biodegradable dendrimer. Bioconjug Chem. 2010;21:764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillaudeu SJ, Fox ME, Haidar YM, Dy EE, Szoka FC, Frechet JM. PEGylated dendrimers with core functionality for biological applications. Bioconjug Chem. 2008;19:461–469. doi: 10.1021/bc700264g. [DOI] [PubMed] [Google Scholar]
- 10.Chen HT, Neerman MF, Parrish AR, Simanek EE. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J Am Chem Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 11.Kaminskas LM, Boyd BJ, Karellas P, Krippner GY, Lessene R, Kelly B, Porter CJ. The impact of molecular weight and PEG chain length on the systemic pharmacokinetics of PEGylated poly l-lysine dendrimers. Mol Pharm. 2008;5:449–463. doi: 10.1021/mp7001208. [DOI] [PubMed] [Google Scholar]
- 12.Kim Y, Hechler B, Gao ZG, Gachet C, Jacobson KA. PEGylated Dendritic Unimolecular Micelles as Versatile Carriers for Ligands of G Protein-Coupled Receptors. Bioconjug Chem. 2009;20:1988–1998. doi: 10.1021/bc9001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick JL, Trollsas M, Hawker CJ, Atthoff B, Claesson H, Heise A, Miller RD, Mecerreyes D, Jerome R, Dubois P. Dendrimer-like Star Block and Amphiphilic Copolymers by Combination of Ring Opening and Atom Transfer Radical Polymerization. Macromolecules. 1998;31:8691–8705. [Google Scholar]
- 14.Schramm OG, Pavlov GM, van Erp HP, Meier MAR, Hoogenboom R, Schubert US. A Versatile Approach to Unimolecular Water-Soluble Carriers: ATRP of PEGMA with Hydrophobic Star-Shaped Polymeric Core Molecules as an Alternative for PEGylation. Macromolecules. 2009;42:1808–1816. [Google Scholar]
- 15.Jones MC, Ranger M, Leroux JC. pH-sensitive unimolecular polymeric micelles: synthesis of a novel drug carrier. Bioconjug Chem. 2003;14:774–781. doi: 10.1021/bc020041f. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Bronich TK, Kabanov AV, Rauh RD, Roovers J. Synthesis and characterization of star poly(epsilon-caprolactone)-b-poly(ethylene glycol) and poly(L-lactide)-b-poly(ethylene glycol) copolymers: evaluation as drug delivery carriers. Bioconjug Chem. 2008;19:1423–1429. doi: 10.1021/bc7004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasongkla N, Shuai X, Ai H, Weinberg BD, Pink J, Boothman DA, Gao J. cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem Int Ed Engl. 2004;43:6323–6327. doi: 10.1002/anie.200460800. [DOI] [PubMed] [Google Scholar]
- 18.Torchilin VP. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv Drug Deliv Rev. 2002;54:235–252. doi: 10.1016/s0169-409x(02)00019-4. [DOI] [PubMed] [Google Scholar]
- 19.Kabanov AV, Slepnev VI, Kuznetsova LE, Batrakova EV, Alakhov V, Melik-Nubarov NS, Sveshnikov PG, Kabanov VA. Pluronic micelles as a tool for low-molecular compound vector delivery into a cell: effect of Staphylococcus aureus enterotoxin B on cell loading with micelle incorporated fluorescent dye. Biochem Int. 1992;26:1035–1042. [PubMed] [Google Scholar]
- 20.Sun X, Rossin R, Turner JL, Becker ML, Joralemon MJ, Welch MJ, Wooley KL. An assessment of the effects of shell cross-linked nanoparticle size, core composition, and surface PEGylation on in vivo biodistribution. Biomacromolecules. 2005;6:2541–2554. doi: 10.1021/bm050260e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley KL, Welch MJ, Hawker CJ. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 22.Lee CC, Cramer AT, Szoka FC, Frechet JMJ. An intramolecular cyclization reaction is responsible for the in vivo inefficacy and apparent pH insensitive hydrolysis kinetics of hydrazone carboxylate derivatives of doxorubicin. Bioconjug Chem. 2006;17:1364–1368. doi: 10.1021/bc060117y. [DOI] [PubMed] [Google Scholar]
- 23.Krummeich C, Holschbach M, Stocklin G. Direct n.c.a. electrophilic radioiodination of tyrosine analogues; their in vivo stability and brain-uptake in mice. Appl Radiat Isot. 1994;45:929–935. doi: 10.1016/0969-8043(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 24.Welling PG. Pharmacokinetics. Americal Chemical Society; Washington: 1986. [Google Scholar]
- 25.Van Renterghem LM, Lammens M, Dervaux B, Viville P, Lazzaroni R, Du Prez FE. Design and use of organic nanoparticles prepared from star-shaped polymers with reactive end groups. J Am Chem Soc. 2008;130:10802–10811. doi: 10.1021/ja801055f. [DOI] [PubMed] [Google Scholar]
- 26.Coessens V, Pintauer T, Matyjaszewski K. Functional polymers by atom transfer radical polymerization. Progress in Polymer Science. 2001;26:337–377. [Google Scholar]
- 27.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 28.Turk H, Shukla A, Alves Rodrigues PC, Rehage H, Haag R. Water-soluble dendritic core-shell-type architectures based on polyglycerol for solubilization of hydrophobic drugs. Chemistry. 2007;13:4187–4196. doi: 10.1002/chem.200601337. [DOI] [PubMed] [Google Scholar]
- 29.Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 30.Seymour LW, Miyamoto Y, Maeda H, Brereton M, Strohalm J, Ulbrich K, Duncan R. Influence of molecular weight on passive tumour accumulation of a soluble macromolecular drug carrier. Eur J Cancer. 1995;31A:766–770. doi: 10.1016/0959-8049(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 31.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn J Cancer Res. 1997;88:1108–1116. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.