Abstract
Gonadotropin-releasing hormone (GnRH)-secreting neurons are the final output of the central nervous system driving fertility in all mammals. Although it has been known for decades that the efficiency of communication between the hypothalamus and the pituitary depends on the pulsatile profile of GnRH secretion, how GnRH neuronal activity is patterned to generate pulses at the median eminence is unknown. To date, the scattered distribution of the GnRH cell bodies remains the main limitation to assess the cellular events which could lead to pulsatile GnRH secretion. Taking advantage of the unique developmental feature of GnRH neurons, the nasal explant model allows primary GnRH neurons to be maintained within a micro-network where pulsatile secretion is preserved and where individual cellular activity can be monitored simultaneously across the cell population. This article summarizes data from work using this in vitro model, and brings some insights into GnRH cellular physiology.
Introduction
Reproductive physiology and fertility rely on a critical dialogue between neurons secreting gonadotropin-releasing hormone-1 (GnRH), gonadotrophs and the gonads, together forming the hypothalamic-pituitary-gonadal (HPG) axis. GnRH axons which extend to the external zone of the median eminence (1–3), release GnRH peptide close to the fenestrated capillary bed of the hypophyseal portal blood, allowing GnRH to be transported to the pituitary gland. The GnRH signal is subsequently amplified by gonadotrophs whose secretion reaches the gonads via the systemic circulation (4).
Breeding, unlike hunger and thirst, is not an acute physiological cue that needs a fast hormonal response but rather is a long process that can span over months. The ultimate function of the reproductive neuroendocrine system is to ensure survival of the species by preserving the integrity of an organism and its offspring. Therefore, GnRH neurons are the final output of an upstream network that conveys a wide range of signals such as metabolic balance, stress, hormonal status, developmental and environmental cues (5, 6). A tremendous amount of in vivo experiments supports this broad integration. Perturbations of surrounding neurotransmission pharmacologically [I.V. injection (7–10), I.C.V. infusion (7, 9, 11, 12), central infusion (13–15)], or surgically [deafferentation/disconnection (16, 17), lesions (18–20)] alter the GnRH pulse generator or GnRH/LH secretion. However, the activity of the GnRH pulse generator is the result of highly integrated animal physiology. In such experiments, drugs diffuse quite unpredictably, disconnections or lesions severeentire cellular paths or cell populations and the precise cell-to-cell communication altered during these challenges is unknown.
For understanding the etiology of infertility, it is important to keep in mind a global view of the GnRH neuronal network. However, despite the subtle equilibrium between cues leading to an operative GnRH output, the reproductive neuroendocrine system is a conserved and robust physiological system, with most physiological cues being disruptive rather than permissive. In mammals, with the exception of seasonal breeders (21) and alterations inherent to aging (22, 23), once triggered at puberty and under optimal breeding conditions, the reproductive axis remains active, displaying cyclicity in females (24). GnRH neurons indirectly control both the maturation of oocytes and ovulation by triggering secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) respectively (4). These two processes are driven by two specific secretory modes – tonic and phasic – interlaced to form a profile of GnRH release that fluctuates over days (25). It is critical for GnRH to be released in a pulsatile pattern since constant stimulation of gonadotropic cells with GnRH suppresses LH secretion (26). The preovulatory surge may occur via a sudden and massive release of GnRH rather than the summation of high frequency GnRH pulses (27, 28) but both secretory modes (tonic and phasic) are achieved by fine-tuning GnRH pulse activity over the cycle. It is known that the switch between secretory modes is dependent on hormonal feedback, negative or positive, from the gonads (29). Interestingly, released from gonadal steroidal feedback by ovariectomy, the GnRH network settles down and its basal activity is exposed. GnRH release remains pulsatile, occurring every 30 (in mice, rats) to 60 (in sheep, monkey) minutes depending on the species (30–33). Although the etiology of polycystic ovary syndrome (PCOS) is uncertain, PCOS is a clinical example where both steroid environment and pulsatility are altered in humans (34). Thus, delineating the mechanisms underlying GnRH pulsatility remains one of the most important issues to understand infertility as well as comprehensive therapeutic strategies. This article will give an overview about the rhythmicity of GnRH neurons and its modulation, focusing on data collected from one particular in vitro model, nasal explants.
The GnRH challenge
Although it has been known for over 30 years that GnRH has to be released in a pulsatile fashion to keep the reproductive axis operative (24, 26), the mechanisms underlying these pulses are unknown. The main hindrance to the study of these mechanisms is anatomical. GnRH-secreting neurons are a small population of neurons (~800 in mouse; (22)), scattered from the rostral pre-optic area (rPOA) to the caudal hypothalamus, forming an “inverted Y”-shaped continuum pattern, whose junction sits around the organum vasculosum of the lamina terminalis (35). Therefore, progress in the understanding of GnRH cellular physiology has relied on scientific ingenuity to conquer these anatomical difficulties.
Three main approaches have been particularly successful in studying the cellular physiology of GnRH neurons. The first one uses brain slices from animals that have cell specific genetically encoded tags or probes (green fluorescent protein (36, 37), pericam (38)) that allow the GnRH neurons to be identified in situ and thereby avoid the fastidious process of post-hoc identification (39, 40). The second one is based on cell specific targeted oncogenesis that produced immortalized GnRH neuronal cell lines, GT1 (41) and GN cells (42). Finally, the third approach is based on the fact that GnRH cells first differentiate outside the central nervous system, in the nasal area (43) (Figure 1A) and therefore, organotypic cultures of nasal explants are used to obtain large numbers of primary GnRH cells (44–47).
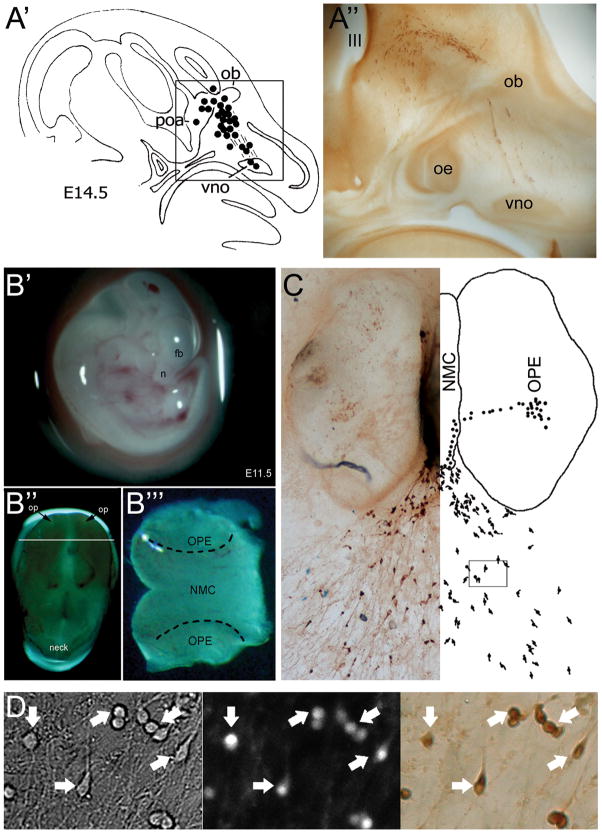
Figure 1. GnRH neuron migration is maintained in nasal explants, allowing cellular physiological studies on native GnRH neurons.
A′. Diagram showing the migratory track of GnRH neurons in a E14.5 mouse, from the vomeronasal organ (vno) to the rostral preoptic area (poa), entering the brain with the olfactory fibers at the level of the olfactory bulbs (ob) (49); A″. Photomicrograph boxed area in A Section has been immunocytochemically stained for GnRH (oe, olfactory epithelium). B. Preparation of nasal explants at E11.5 (B′, n: nose, fb: forebrain); B″, Isolated head sitting on its top, displaying the nasal placodes (op); B‴, The tip of the nose sectioned where the nasal midline cartilage (NMC) and the olfactory placode epithelium (OPE) are delimited. C. Representative picture of a nasal explant after 7 days in culture and the mirror corresponding diagram showing the differentiated GnRH-expressing cells in the OPE, migrating towards the NMC and emerging outside the tissue mass as they migration. D. Representative picture of GnRH neurons used for calcium imaging in an area corresponding to the box delimited in (C). Left panel, bright field; Middle panel, calcium dye-loaded cells; Right panel, post hoc identification by immunocytochemistry for GnRH. Arrows indicate the identical cells over the procedure. [Adapted with permission from (49) for A & (43) for A′, (63) for D; pictures for B were generously provided by Dr. P. Giacobini].
The use of nasal explants is based on the spatiotemporal development of the GnRH neuronal population. At embryonic day 10.5 (E10.5) in the mouse, GnRH neurons originate in the nasal placode. At E11.5, they initiate their journey within the nasal area. They migrate along olfactory sensory axons to the cribriform plate (E13.5), enter the forebrain and turn caudal to reach their final forebrain destinations (E16.5) from where they extend their axons towards the median eminence (48, 49). By dissecting out the nasal area at E11.5 and placing it in culture (Figure 1B), both olfactory neurons and midline cartilage still differentiate, allowing the migration of GnRH neurons to be preserved (45) (Figure 1C). Thus, many post mitotic GnRH neurons present in the olfactory placode can be maintained in vitro and are accessible for studies (Figure 1D).
GnRH neuronal migration occurs in vitro (45) as in vivo (50, 51); therefore, nasal explants were initially used to study cell migration [(44, 45); reviewed in (43)]. In addition, GnRH neurons in nasal explant exhibit pulsatile GnRH secretion in all species studied to date (monkey (52); sheep (53); rat (54); mouse (55))(Figure 2A). Notably, the frequency of GnRH pulse varies among species but is consistent with the frequencies of GnRH/LH observed in vivo in gonadectomized animals (~50 min in monkey/sheep (24, 31); ~30 min in rat/mouse (33, 56)).
Figure 2. GnRH cells in nasal explant display spontaneous rhythmic activity.
A. Example of a secretion profile illustrating in vitro release of GnRH with pulsatile pattern (~ 1 pulse/20min). B. Simultaneous recording of electrical activity and intracellular calcium level in a single GnRH neuron revealed spontaneous bursts of activity time-correlated with transient oscillations in [Ca2+]i. C. Simultaneous recordings of a cohort of GnRH neurons illustrating the occurrence of [Ca2+]i oscillations (white ticks) being independent in each cell (one line/cell) (upper panel) but synchronized across the GnRH cells every ~ 20min (lower panel). [Adapted with permission from (55) for A, (63) for B–C].
GnRH neurons from nasal explants have never entered the brain, are devoid of their physiological central inputs and, like in vivo, the mechanisms responsible for episodic release of GnRH are unknown. Thus, thorough studies of the GnRH neurons in this model will likely provide important clues about GnRH pulsatility in vivo.
Cell phenotypes within nasal explants
Despite its ‘simple’ concept, dissecting out the developing nasal region did not conserve only GnRH neurons but rather unraveled the numerous cell types that develop within the nasal placode, both non-neuronal and neuronal types, whose list may not be exhaustive. Some non-neuronal cell populations have been immunocytochemically characterized. Fibronectin/laminin positive cells are most likely fibroblasts and mesenchymal cells (45). Epithelial cells (57) and p75 nerve growth factor (p75NGF) receptor positive cells may be olfactory ensheathing cells (58). Among the neuronal population, a NCAM- and/or peripherin- positive olfactory axonal complex has been described, in agreement with in vivo observations (45, 50). In addition, the GABAergic neuronal population that arises from the olfactory pit (59) is maintained in nasal explants (59–65). Finally, cholecystokininergic (66–68) and glutamatergic (68) neuronal populations are also reported both in nasal explants and in vivo (66, 69).
Assessing GnRH neuronal physiology
As neuroendocrine cells, GnRH secretion is probably the most relevant output to assess GnRH physiology in nasal explants. However, even in vitro, the level of GnRH release is low, often at the limit of radioimmunoassay whose sensitivity determines the time resolution of the assay. Moreover, such measurements assess the dynamics of the entire cell population rather than individual cellular physiology. Thus, researchers have taken advantage of the fact that neurosecretion requires depolarization of the membrane (70). Hence tracking the electrical activity of the GnRH neurons is an excellent way to correlate cellular activity with secretory events. Although patch clamp techniques are highly sensitive to changes in electrical activity, it is usually performed one cell at a time. This represents an important limitation to assess GnRH pulsatility because the number of cells contributing to a single GnRH pulse is unknown, possibly as low as 12–34% of the total population in mouse (71). In addition, experiments with Fluorogold, an retrograde neuronal tracer used to identify GnRH neurons crossing the blood brain barrier, i.e. actively secreting GnRH, indicate that only ~60% of GnRH cells are back-labeled by Fluorogold during a 5-day survival period in mouse i.e. tonic GnRH secretion plus surge (72). Finally, experiments using c-Fos immunolabeling as an immediate marker of cell activation, show that only ~40% of GnRH neurons display immunoreactivity during the ovulatory surge in rat (73). Together, these data give an index of the complexity of the GnRH neuronal network and illustrate the difficulty in interpreting an electrical event recorded in one GnRH cell or trying to record from a GnRH cell involved in a specific event.
Since secretion is a calcium-dependent mechanism, calcium imaging is an alternative method to investigate GnRH cellular activity. The advantage of this technique is that it allows assessing neuronal activity at the level of single cells but importantly also simultaneously across the cell population (62, 74) (Figure 1D).
Although secretion is likely to occur at the terminals, intracellular calcium concentration ([Ca2+]i) recorded at the cell bodies reveal baseline [Ca2+]i oscillations (65, 74, 75). In agreement with recent finding in adult GnRH neurons (76), there is a clear relationship between soma [Ca2+]i oscillations and electrical activity of GnRH neurons in nasal explants (63). Thus, extrapolating frequency of [Ca2+]i oscillations to GnRH neuronal activity becomes possible (Figure 2B). Surprisingly, while the [Ca2+]i oscillations are primarily driven by ionotropic receptor-dependent changes in membrane potential, most [Ca2+]i oscillations are insensitive to blockers of voltage-gated calcium channels (68). In contrast with data from adult GnRH neurons (76), blockers of intracellular calcium channels, such as ryanodine receptors and inositol 1,3,4- trisphosphate receptors (68) fail to block these oscillations, as does depletion of endoplasmic reticulum calcium stores [(68); reviewed in (77)]. Although the mechanisms by which calcium rises in the cytoplasm of GnRH cells in nasal explant remain obscure, the most fascinating characteristic of these oscillations is that while they occur apparently randomly within individual cells, they synchronize periodically, with a frequency similar to episodic secretion (62, 74) (Figure 2C). This feature prompted investigators to use them as a potential indicator of secretory events. The link between synchronized calcium events and episodic secretion was assumed until a recent study showed that synchronized [Ca2+]i oscillations occurred prior to a GnRH pulse (55). One could argue that [Ca2+]i oscillations observed in GnRH neurons in nasal explants are simply characteristic of developing neurons (78–80), but [Ca2+]i oscillations were recently observed in adult GnRH cells maintained in acute brain slices expressing the calcium dye, Pericam (38). Unfortunately, the low level of fluorescence emitted by Pericam-GnRH neurons (76, 77) and the scattered distribution of the cells make simultaneous recordings virtually impossible in adult brain slices and to date, synchronized events can only be observed in culture models (GT1 cell lines (81–83) or nasal explants (62, 74)). Despite the fact that the [Ca2+]i oscillations seem to result from different mechanisms of intracellular signal amplification (38, 84), the similarities between GnRH cells in nasal explants and adult mice, suggest that [Ca2+]i oscillations might be a precocious ability of GnRH neurons rather than an immature feature (reviewed in (77)), providing a rationale for studying them more extensively.
Voltage-gated channels in GnRH neurons from nasal explants
While [Ca2+]i oscillations are a great parameter to assess GnRH neuronal activity and the dynamics of the cell population, GnRH neurons are part of a cellular network that arises in nasal explants. Therefore, similar to acute brain slices, pharmacological tools may have multiple targets. Although action potentials and [Ca2+]i oscillations occur spontaneously in GnRH neurons maintained in nasal explants (60, 62, 74, 85) or from acute brain slices (36–38), they may not be intrinsic to GnRH neurons but rather the consequence of unidentified afferent inputs. Therefore when a drug is superfused during recording of either electrical activity or [Ca2+]i oscillations, it can act on the inputs, the GnRH neurons or both. Thus, whole cell patch clamp remains the best technique to identify, with certainty voltage-gated channels in GnRH neurons. In agreement with calcium imaging, TTX-sensitive voltage-gated sodium channels (60, 63), low- and high-voltage-activated calcium channels (60) as well as potassium currents composed mainly of a transient outward current and a delayed rectifier (60) are detected in GnRH neurons. Single cell RT-PCR provided additional information about high-voltage calcium current identifying transcripts for N- and L-type voltage-activated calcium channels (86). Since the creation of GFP-tagged GnRH neurons, a decade of electrophysiological recording in acute adult brain slices confirmed this basic combination (TTX-sensitive spiking (40); voltage-gated calcium channel subtypes (87, 88); transient outward (89) and delayed rectifier potassium channels (89, 90)). Although the identity of some channels remains uncertain, a mosaic of ionic currents recorded from GFP-tagged GnRH neurons in acute slices extends this initial combination of channels. This includes TTX-sensitive slow afterdepolarizing potentials (90), hyperpolarization-gated potassium current (91), ATP-sensitive potassium current (92), apamin-sensitive afterhyperpolarizing potentials (93) and UCL-sensitive slow afterhyperpolarizing potentials (76) (reviewed in (94, 95)).
Receptor-mediated regulation of GnRH neuronal activity
Physiologically, the GnRH pulsatile secretory profile is characterized by changes in frequency and constant modulation by hormonal cues. In addition to this fine-tuning, GnRH neurons are also able to respond to a large range of inputs as shown by the wide array of receptors found in adult GnRH neurons (96) as well as GnRH neurons in nasal explants (65, 66, 97–99), many of which are G protein-coupled (GPCR).
G protein-coupled receptors and downstream pathways
Since nearly 70% of GPCR modulate intracellular cyclic adenosine monophosphate (cAMP)(100), recent studies using nasal explants were performed to investigate the effect of cAMP on GnRH neuronal activity (Figure 3A). Depleting the intracellular cAMP content ([cAMP]i) did not alter the frequency of [Ca2+]i oscillations (63), indicating that basal GnRH neuronal activity is not driven by cAMP-dependent mechanisms. However, increasing the synthesis of cAMP dramatically stimulated GnRH neuronal activity (63, 64). This excitatory effect was mediated by protein kinase A (PKA) -dependent phosphorylation, but independent of cyclic nucleotide-gated channels (63) and hyperpolarization-activated cyclic nucleotide-modulated (HCN) channels (64) (Figure 3B). Although the target of the phosphorylation was not investigated, these data indicated that GnRH neurons are able to integrate the signaling cascade downstream of activation of Gs protein-coupled receptors. Recent work on acute brain slices identified PKA-dependent phosphorylation of ATP-sensitive potassium channels (92) as a molecular target for the excitatory action of estradiol directly on GnRH neurons. Since HCN channels or currents are detected in adult GnRH neurons (91, 94), a role of cAMP in modulating their activity cannot be excluded. Work in nasal explants focusing on the signaling pathway in GnRH neurons activated by neuropeptide Y found that inhibition subsequent to the activation of Gi protein-coupled receptors was indeed mediated directly by the activation of Gi protein-coupled inwardly-rectifying potassium channels (99) (Figures 3C–D), consistent with the NPY-induced hyperpolarization observed in adult GnRH neurons (101).
Figure 3. Three different downstream pathways of G-protein coupled receptors can modulate GnRH neuronal activity.
A. Forskolin-evoked increase in intracellular cAMP, modeling the activation of Gs pathway, stimulates GnRH neuronal activity. B. Stimulation can be directly mimicked with Sp-cAMPS, a selective activator of protein kinase A. C–D. Neuropeptide Y inhibits GnRH neuronal activity. TPN-Q prevents the NPY-evoked inhibition, indicating a functional Gi pathway coupled to G-inward rectifier potassium channels (GIRK). E. Kisspeptin-10 induces a potent stimulation. F. A similar response can be induced directly with the phorbol ester PMA, an activator of PKC. [Adapted with permission from - (64) for A–B, (99) for C–D, (65) for E–F]. [TTX=tetrodotoxin 1 μM, voltage-gated sodium channel blocker; BIC=bicuculline 20 μM, A-subtype GABAergic receptor antagonist; CNQX=6-cyano-7-nitroquinoxaline-2,3-dione 10 μM, AMPA-subtype glutamatergic receptor antagonist; PRG=proglumide 100 nM, cholecystokininergic receptor antagonist].
Finally, evidence that GnRH neurons can respond to all three subfamilies of GPCR (i.e. Gs, Gi and Gq- coupled receptors) was provided by a study in nasal explants that investigated kisspeptin activation of GnRH neuronal activity through the Gq-dependent signaling pathway (Figure 3E). This study showed that the synthesis of diacylglycerol and IP3 by phospholipase C, subsequent to the activation of the GPR54, initiated two synergetic pathways, one involving protein kinase C (PKC) -dependent phosphorylation (Figure 3F) and one dependent on the activation of nonselective cationic channels, both stimulating the GnRH neuronal calcium oscillator (65). ATP-sensitive potassium channels have recently been identified as a molecular target of PKC and able to modulate the excitability of adult GnRH neurons (92). Notably, while kisspeptin is associated with different phenomena in juvenile/adult such as puberty (102–104), LH surge and pulses (105, 106) or seasonal fertility (107), the unexpected response of embryonic GnRH neurons in nasal explants was in agreement with that described in adult brain slices (108, 109). The proportion of each downstream component activated by kisspeptin may be slightly different between embryonic (65) and adult (108) GnRH neurons, but the pathways are similar.
Similarly to kisspeptin, CCK is also a ligand whose receptor couples to Gq-protein and is present endogenously in nasal explants. Data show that CCK receptor is constitutively active in GnRH neurons in nasal explants (68) and may explain the stimulatory effect of cholecystokinin upon GnRH secretion in vivo (12, 110, 111).
Taken together, these data indicate that each of the three major signaling cascades activated by GPCRs are functional in GnRH neurons in nasal explants and that the downstream effectors of these receptors can modulate GnRH neuronal activity.
Modulation by amino acids and ionotropic receptors
Gamma-aminobutyric acid (GABA) is known to modulate GnRH neuronal activity in nasal explants (62) and the GABAergic neuronal input to GnRH neurons is well documented. While functional GABAB receptors were previously described as being able to influence GnRH neuronal activity (62), they have also been recently identified pre-synaptically on GABAergic inputs (68). In contrast, electrophysiological data have shown that GnRH neurons in nasal explants exhibit synaptic activity driven by GABA mediated by GABAA receptors (59, 60). Moreover, calcium imaging data have shown that the frequency of [Ca2+]i oscillations is both regulated and triggered by endogenous GABAergic inputs (62–65, 68, 99). The existence of a predominant constitutive GABAergic input onto GnRH neurons in acute brain slices is well described (40). This input is part of an important network integrating signals upstream of GnRH neurons. Indeed it has been demonstrated that estradiol, one of the major hormonal signals GnRH neurons integrate during the estrous cycle, increases the frequency of GABAergic mIPSCs in GnRH neurons and therefore modulate their excitability (112).
In nasal explants, acute application of muscimol, a GABAA receptor selective agonist, indicated a biphasic calcium response to GABA in GnRH neurons. The first transient phase, insensitive to TTX, was followed by a longer lasting second phase that was sensitive to TTX (75). These findings implicate calcium stores and voltage-gated calcium channels in the GABA-evoked response, respectively. Despite the finding that [Ca2+]i oscillations are not sensitive to thapsigargin, a blocker of the calcium pumps that deplete calcium internal stores (68), the observation by Moore et al. (62) is in accordance with the finding that GnRH cells can exhibit TTX-insensitive [Ca2+]i oscillations (Figure 4A), triggered by GABA and blocked by GABAA receptor antagonist (68) (Figure 4B). Recent data using calcium imaging on adult GnRH neurons indicate that the calcium response subsequent to activation of GABAA receptors in the presence of TTX occurs by internal calcium release (84). It is important to note that the electrical response of neurons to GABA, depolarizing or hyperpolarizing, is determined by the chloride gradient, i.e whether membrane transporters accumulate or extrude chloride, respectively. It is known that in neurons, expression of the cation/chloride cotransporters is developmentally regulated (113), leading to a switch of the GABA response from depolarizing towards hyperpolarizing as neurons mature (114). Thus, the depolarizing effect of GABA on GnRH neurons in nasal explants may be interpreted as a common feature of immature neurons (59, 60, 62). Although some data from GnRH neurons in acute brain slices support the idea of a developmental switch occurring at puberty (115), most data indicate that GnRH neurons continue to be depolarized by GABA throughout adult life (84, 116, 117). Depolarization by GABA, is thought to be maintained in GnRH neurons due to continued expression of the embryonic concentrating form of the chloride transporter, NKCC-1 (116), rather than switching to the extruding form of the chloride transporter, KCC-2 (118). Together, data collected on the effect of GABA on GnRH neurons in nasal explants and adult acute brain slices share features, independent of the models used.
Figure 4. The rhythmic activity of GnRH cells in nasal explants arises from different cell populations.

A. Recording of [Ca2+]i in a single GnRH neuron illustrating spontaneous oscillations can persist in presence of TTX. The [Ca2+]i oscillations are partially blocked by bicuculline (B), CNQX (C) and proglumide (D) revealing GABAergic, glutamatergic and cholecystokininergic inputs, respectively. Together, constitutively active endogenous inputs are responsible for the rhythmic activity displayed by GnRH neurons (E). [Adapted with permission from (68)]. [FSK=forskolin 1 μM, adenylyl cyclase activator; Sp-cAMPS=Sp-adenosine-3,5-cyclic monophosphorothioate triethylammonium 100 nM, protein kinase A activator; NPY=neuropeptide-Y 1 nM, NPY receptor agonist; TPN-Q=tertiapin-Q 100 nM, Gi-activated inward rectifier potassium channel blocker; KP-10=kisspeptin-10 10 nM, GPR54 receptor agonist; BIC=bicuculline 20 μM, A-subtype GABAergic receptor antagonist; PMA=phorbol 12-myristate 13-acetate 50 nM, protein kinase C activator].
Electrophysiological recordings described an electrical response of GnRH neurons in nasal explants to glutamate (60). A recent study revealed that endogenous glutamate, released in a TTX-insensitive manner, triggers [Ca2+]i oscillations, via activation of AMPA/kainate glutamatergic receptors (68) (Figure 4C). This observation is consistent with data from adult mouse showing that GnRH neurons in acute brain slices receive glutamate inputs [(119); reviewed in (120)] and that GnRH neurons are able to generate a calcium response to activation of AMPA receptors in the presence of TTX (84).
It is found that both GABAergic and glutamatergic inputs triggered similar numbers of [Ca2+]i oscillations in GnRH neurons over development but that a TTX resistance occured between one and three weeks in vitro (68). Whether inputs developed a TTX-independent release mechanism or the calcium oscillator was able to operate in the presence of TTX after maturation, was not determined. Estradiol-induced oscillations in the presence of TTX have also been described after one week in vitro in favor of the second hypothesis (98, 121). Whether changes in the subunit composition of GABAA receptors modulate this response (97) requires further investigation.
Is pulsatility an intrinsic characteristic of GnRH neurons?
The original concept of an intrinsic ability of GnRH neurons to release their peptide in an episodic manner arose from in vitro observations documenting pulsatile GnRH secretion from isolated hypothalami (122, 123). Experimental support for an endogenous cellular pulse oscillator in GnRH neurons came from GT1 cell lines that are able to generate GnRH pulses (124–126). This concept was reinforced by the pulsatile secretory pattern observed in nasal explants (52–55), where GnRH neurons are devoid of inputs from the central nervous system.
In GT1 cells, gap junctions are known to contribute to synchronized events (127, 128). However, adult (129) GnRH neurons or those maintained in nasal explants do not exhibit dye coupling (60) but rather are part of a rich network (55, 66, 68). There is also evidence for autocrine communication between GnRH neurons in GT1 cell lines (130, 131). Such communication has also been proposed for adult mouse GnRH neurons (96, 132, 133) and GnRH neurons maintained in nasal explants. In nasal explant, GnRH neurons express GnRH receptors, exhibit cell apposition and respond to GnRH itself (134). Certainly, interpreting the effects of exogenous application of GnRH on GnRH neurons whose surrounding levels of GnRH are unknown is problematic. However, samples collected in static incubation (53–55) or during continuous superfusion (52) reveal pulsatile GnRH release in nasal explants and the pulse frequency is not altered by perfusion speed (52). Thus, it seems reasonable to keep GnRH as a modulator rather than as a trigger for GnRH pulses. In agreement with this, recent data from brain slice show a moderate effect of GnRH on adult GnRH neurons (133).
Together, the hypothesis of an “intrinsic capacity” of GnRH neurons should be revisited. The idea arose from pulsatile GnRH secretion (1) by terminals in the basal hypothalamus that was shown to lack GnRH cell bodies (135), (2) by a “pure” GT1 cell culture where dye-coupling is common (136) in contrast with GnRH neurons from nasal explants (60) or adult acute brain slices (129, 137) where either dye- and/or electrically- coupled are never observed, and finally (3) in nasal explant model in which the last decade revealed a great cellular complexity.
The concept of “intrinsic” ability to secrete in a pulsatile profile is based only on the fact that spontaneous release was observed without the intervention of the experimenter. Despite an intrinsic ability of the models to exhibit pulsatile release, no data provide absolute evidence that the pulse generator is actually located within the GnRH neurons. Thus, a broader understanding of the cell-to-cell communication within a simplified, but functional network such as in nasal explant model, is critical to identify the cellular mechanisms that may trigger GnRH release across the GnRH neuronal population.
Could pulsatility be an intrinsic characteristic of the GnRH neuronal network?
Nasal explants show the importance of cell-to-cell communication for GnRH function. While non-neuronal cells may contribute to the synchronization of the GnRH neuronal network (57), possibly via purinergic receptors (138), neuronal interactions endogenously regulating GnRH neuronal activity in nasal explants are now well characterized. GABA, though GABAA receptors, is probably the major endogenous neurotransmitter modulator of GnRH neurons in nasal explants (59, 60, 62–65, 68). Other endogenous neurotransmitters may also play a role. For example, glutamate (68) acting through AMPA receptors (Figure 4C) and CCK (67, 68), acting though CCK-1 receptors (Figure 4D) regulate the spontaneous activity of GnRH neurons in nasal explants. The silence revealed by cocktails of antagonists (Figure 4E) seems to indicate that the spontaneous activity of GnRH neurons could in fact result from synergetic cell-to-cell interactions rather than being an intrinsic ability of isolated GnRH neurons.
Relevance with adult physiology
One would argue that using an embryonic model to study the reproductive system that remains quiescent until puberty is irrelevant. The meaning of adult-type receptors and adult-like responses in embryonic neurons seems questionable. However this bias may revolve around the idea that the same neurotransmitter drives the same information to GnRH neurons throughout life. One example against this postulate can be found in amino acid signaling whose function evolved during development despite a conserved signaling pathway. Indeed, whereas GABA and glutamate are the two major neurotransmitters encoding information in adult brain, they control proliferation, migration, and differentiation prior to synapse formation (139). They do so by determining the expression of membrane channels (140) and promoting synaptogenesis (141). Seeking the mechanisms underlying the responsiveness of embryonic GnRH neurons to adult cues is a hard quest. However, the functionality of GnRH neurons during development may not be surprising. It is well known that physiologically, GnRH neurons are active during the late gestation (142) or around the time of birth (143), depending on the species. Pathologically, precocious puberty can occur shortly after birth (144). Moreover, some neuronal circuits develop during early life and irreversibly condition the adult brain (145, 146). In addition, one cannot ignore the fact that olfactory placode transplantations restore reproductive cycles in lesioned female monkey (20) and fertility in hypogonadal mice (147). These data clearly indicate that, despite their putative immaturity, embryonic GnRH neurons are able to integrate adult signals and transduce them into an adult-like output
Data accumulated from GnRH neurons isolated from nasal placode (summarized in Figure 5) and adult brain show striking similarities in their response to signaling molecules. Whether GnRH neurons remain immature throughout life or are precociously mature depends upon the point of reference used, i.e. compared to other neurons or within their own development, respectively. GnRH neurons in nasal explants may not express the full range of receptors found in adult neurons (96) and might have developmental specificities. However, literature now provides enough evidence to assume that if the receptor is expressed during early development, the signaling pathway may be developed as well, as shown with GABA (75), estradiol (98) or kisspeptin-10 (65).
Figure 5. Schematic of the endogenous inputs modulating GnRH neurons and their signaling pathways.
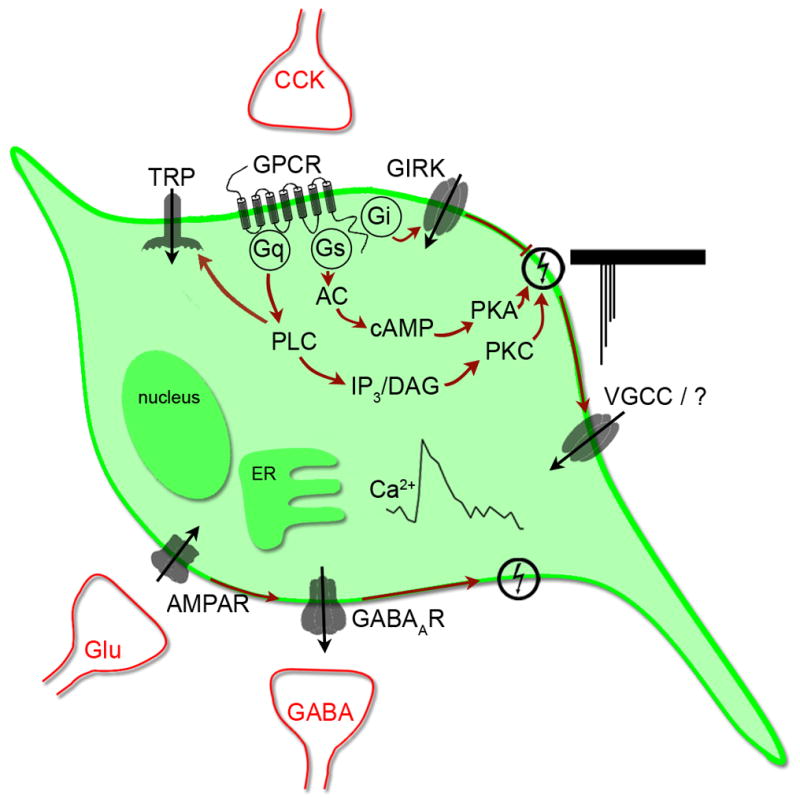
Action potentials induce the activity of the calcium oscillator, resulting in a transient increase in [Ca2+]i via voltage-gated calcium channels and yet to be identified calcium source. GABAergic, glutamatergic and cholecystokininergic inputs, the major inputs present in vitro, are able to trigger the calcium oscillator via the activation of GABAA, AMPA and CCK1 receptors, respectively. Gi-coupled receptors can reduce the activity of the oscillator via a direct coupling to GIRK channels while Gs-coupled receptors can stimulate GnRH neurons by increasing the activity of protein kinase A upon the calcium oscillator. Gq-coupled receptors can stimulate GnRH neuronal calcium oscillator by at least two mechanisms initiated by the activity of phospholipase C: protein kinase C and transient receptor potential channels.
Conclusions
The pulsatile release of GnRH is an essential property of the GnRH neuronal network. However, decades of research only reveal how broad and intricate the GnRH neuronal network is. Genetically tagged GnRH neurons have turned a ‘light’ on the neuronal activity of individual adult GnRH neurons in situ during different physiological states, such as lactation, metabolic balance, estrous cycle, and stress. However, the scattered distribution of GnRH cell bodies across the forebrain and the distal site of GnRH release still present obstacles to understanding the physiological cellular mechanisms that trigger and regulate the GnRH pulsatile release.
Although the nasal explant model carries with it potential GnRH cell immaturity, genetic engineering does not seem to make this model obsolete. To date, the nasal explant model remains the only in vitro model with a restricted but functional cellular network in which native GnRH neurons take part, and that secrete GnRH in a pulsatile manner. While cellular events can be recorded from single cells, the large number of neurons maintained in these explants allows one to also assess global dynamics of the cell population, i.e. cell synchronization, simultaneously with the physiological output of the network, GnRH secretion. Furthermore, work over recent years has revealed striking resemblances between embryonic and adult GnRH neurons, from the display of GnRH neuronal activity to its neuromodulation.
It is important to select the appropriate model for addressing specific questions and to acknowledge the strengths and weaknesses of the approaches. However, future studies combining the use of transgenic mouse line with the use of this in vitro model still seems to offer a wide range of experimental options to tackle the question of how various cell signaling pathways regulate GnRH neurons.
Acknowledgments
I am very grateful to Doctor Susan Wray for her review of the manuscript and to Professor Allan Herbison and Doctor Sandra Petersen for their valuable comments.
This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke and by the New Zealand Research Council.
References
- 1.King JC, Tobet SA, Snavely FL, Arimura AA. LHRH immunopositive cells and their projections to the median eminence and organum vasculosum of the lamina terminalis. J Comp Neurol. 1982;209(3):287–300. doi: 10.1002/cne.902090307. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman GE, Phelps CJ, Khachaturian H, Sladek JR., Jr . Neuroendocrine Projections to the Median Eminence. In: Ganten D, Pfaff D, editors. Morphology of Hypothalamus and Its Connections. Berlin - Heidelberg - New York - London -Paris - Tokyo: Academic Press; 1986. pp. 161–96. [Google Scholar]
- 3.Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci. 1987;7(8):2312–9. [PMC free article] [PubMed] [Google Scholar]
- 4.McCann SM, Ramirez VD. The Neuroendocrine Regulation of Hypophyseal Luteinizing Hormone Secretion. Recent Prog Horm Res. 1964:20131–81. [PubMed] [Google Scholar]
- 5.Spergel DJ, Kruth U, Shimshek DR, Sprengel R, Seeburg PH. Using reporter genes to label selected neuronal populations in transgenic mice for gene promoter, anatomical, and physiological studies. Prog Neurobiol. 2001;63(6):673–86. doi: 10.1016/s0301-0082(00)00038-1. [DOI] [PubMed] [Google Scholar]
- 6.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 7.DePaolo LV, King RA, Carrillo AJ. In vivo and in vitro examination of an autoregulatory mechanism for luteinizing hormone-releasing hormone. Endocrinology. 1987;120(1):272–9. doi: 10.1210/endo-120-1-272. [DOI] [PubMed] [Google Scholar]
- 8.Lamberts R, Vijayan E, Graf M, Mansky T, Wuttke W. Involvement of preoptic-anterior hypothalamic GABA neurons in the regulation of pituitary LH and prolactin release. Exp Brain Res. 1983;52(3):356–62. doi: 10.1007/BF00238029. [DOI] [PubMed] [Google Scholar]
- 9.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16(10):850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 10.Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol. 1989;123(3):375–82. doi: 10.1677/joe.0.1230375. [DOI] [PubMed] [Google Scholar]
- 11.McShane TM, May T, Miner JL, Keisler DH. Central actions of neuropeptide-Y may provide a neuromodulatory link between nutrition and reproduction. Biol Reprod. 1992;46(6):1151–7. doi: 10.1095/biolreprod46.6.1151. [DOI] [PubMed] [Google Scholar]
- 12.Ichimaru T, Matsuyama S, Ohkura S, Mori Y, Okamura H. Central cholecystokinin-octapeptide accelerates the activity of the hypothalamic gonadotropin-releasing hormone pulse generator in goats. J Neuroendocrinol. 2003;15(1):80–6. doi: 10.1046/j.1365-2826.2003.00965.x. [DOI] [PubMed] [Google Scholar]
- 13.Terasawa E, Krook C, Hei DL, Gearing M, Schultz NJ, Davis GA. Norepinephrine is a possible neurotransmitter stimulating pulsatile release of luteinizing hormone-releasing hormone in the rhesus monkey. Endocrinology. 1988;123(4):1808–16. doi: 10.1210/endo-123-4-1808. [DOI] [PubMed] [Google Scholar]
- 14.Woller MJ, Terasawa E. Infusion of neuropeptide Y into the stalk-median eminence stimulates in vivo release of luteinizing hormone-release hormone in gonadectomized rhesus monkeys. Endocrinology. 1991;128(2):1144–50. doi: 10.1210/endo-128-2-1144. [DOI] [PubMed] [Google Scholar]
- 15.Mitsushima D, Marzban F, Luchansky LL, Burich AJ, Keen KL, Durning M, Golos TG, Terasawa E. Role of glutamic acid decarboxylase in the prepubertal inhibition of the luteinizing hormone releasing hormone release in female rhesus monkeys. J Neurosci. 1996;16(8):2563–73. doi: 10.1523/JNEUROSCI.16-08-02563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96(5):1073–87. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 17.Maeda K, Tsukamura H, Ohkura S, Kawakami S, Nagabukuro H, Yokoyama A. The LHRH pulse generator: a mediobasal hypothalamic location. Neurosci Biobehav Rev. 1995;19(3):427–37. doi: 10.1016/0149-7634(94)00069-d. [DOI] [PubMed] [Google Scholar]
- 18.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta) Endocrinology. 1978;102(1):52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 19.Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- 20.Saitoh Y, Luchansky LL, Claude P, Terasawa E. Transplantation of the fetal olfactory placode restores reproductive cycles in female rhesus monkeys (Mucaca mulatta) bearing lesions in the medial basal hypothalamus. Endocrinology. 1995;136(6):2760–9. doi: 10.1210/endo.136.6.7750501. [DOI] [PubMed] [Google Scholar]
- 21.Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144(8):3663–76. doi: 10.1210/en.2002-0188. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman GE, Finch CE. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986;7(1):45–8. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131(3):403–14. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]
- 24.Knobil E. On the control of gonadotropin secretion in the rhesus monkey. Recent Prog Horm Res. 1974;30(0):1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- 25.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129(3):1175–82. doi: 10.1210/endo-129-3-1175. [DOI] [PubMed] [Google Scholar]
- 26.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–3. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 27.Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology. 1992;130(5):2978–84. doi: 10.1210/endo.130.5.1572305. [DOI] [PubMed] [Google Scholar]
- 28.Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology. 1993;133(4):1624–32. doi: 10.1210/endo.133.4.8404603. [DOI] [PubMed] [Google Scholar]
- 29.Herbison AE. Physiology of the Gonadotropin-Releasing Hormone Neuronal Network. In: Knobil E, Neill J, editors. Knobil and Neill's Physiology of Reproduction. 3. San Diego: Academic Press; 2006. pp. 1415–82. [Google Scholar]
- 30.Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107(6):1782–90. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- 31.Caraty A, Orgeur P, Thiery JC. Demonstration of the pulsatile secretion of LH-RH into hypophysial portal blood of ewes using an original technic for multiple samples. C R Seances Acad Sci III. 1982;295(2):103–6. [PubMed] [Google Scholar]
- 32.Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21(1):117–21. doi: 10.1016/0361-9230(88)90126-8. [DOI] [PubMed] [Google Scholar]
- 33.Kokoris GJ, Lam NY, Ferin M, Silverman AJ, Gibson MJ. Transplanted gonadotropin-releasing hormone neurons promote pulsatile luteinizing hormone secretion in congenitally hypogonadal (hpg) male mice. Neuroendocrinology. 1988;48 (1):45–52. doi: 10.1159/000124988. [DOI] [PubMed] [Google Scholar]
- 34.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update. 2006;12 (4):351–61. doi: 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 35.Sternberger LA, Hoffman GE. Immunocytology of luteinizing hormone-releasing hormone. Neuroendocrinology. 1978;25(2):111–28. doi: 10.1159/000122734. [DOI] [PubMed] [Google Scholar]
- 36.Spergel DJ, Kruth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19(6):2037–50. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141(1):412–9. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- 38.Jasoni CL, Todman MG, Strumia MM, Herbison AE. Cell type-specific expression of a genetically encoded calcium indicator reveals intrinsic calcium oscillations in adult gonadotropin-releasing hormone neurons. J Neurosci. 2007;27(4):860–7. doi: 10.1523/JNEUROSCI.3579-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12(4):399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 40.Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21(3):1067–75. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron. 1990;5(1):1–10. doi: 10.1016/0896-6273(90)90028-e. [DOI] [PubMed] [Google Scholar]
- 42.Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler GB, Jr, Wondisford FE. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci U S A. 1991;88(8):3402–6. doi: 10.1073/pnas.88.8.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. J Neuroendocrinol. 2010;22(7):743–53. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology. 1993;133(5):2379–90. doi: 10.1210/endo.133.5.8404690. [DOI] [PubMed] [Google Scholar]
- 45.Fueshko S, Wray S. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol. 1994;166(1):331–48. doi: 10.1006/dbio.1994.1319. [DOI] [PubMed] [Google Scholar]
- 46.Duittoz AH, Batailler M, Caldani M. Primary cell culture of LHRH neurones from embryonic olfactory placode in the sheep (Ovis aries) J Neuroendocrinol. 1997;9 (9):669–75. doi: 10.1046/j.1365-2826.1997.00627.x. [DOI] [PubMed] [Google Scholar]
- 47.Daikoku S, Koide I, Chikamori-Aoyama M, Shimomura Y. Migration of LHRH neurons derived from the olfactory placode in rats. Arch Histol Cytol. 1993;56(4):353–70. doi: 10.1679/aohc.56.353. [DOI] [PubMed] [Google Scholar]
- 48.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86(20):8132–6. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338(6211):161–4. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 50.Wray S, Key S, Qualls R, Fueshko SM. A subset of peripherin positive olfactory axons delineates the luteinizing hormone releasing hormone neuronal migratory pathway in developing mouse. Dev Biol. 1994;166(1):349–54. doi: 10.1006/dbio.1994.1320. [DOI] [PubMed] [Google Scholar]
- 51.Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147(3):1159–65. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 52.Terasawa E, Keen KL, Mogi K, Claude P. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology. 1999;140(3):1432–41. doi: 10.1210/endo.140.3.6559. [DOI] [PubMed] [Google Scholar]
- 53.Duittoz AH, Batailler M. Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil. 2000;120(2):391–6. [PubMed] [Google Scholar]
- 54.Funabashi T, Daikoku S, Shinohara K, Kimura F. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology. 2000;71(2):138–44. doi: 10.1159/000054529. [DOI] [PubMed] [Google Scholar]
- 55.Constantin S, Caraty A, Wray S, Duittoz AH. Development of Gonadotropin-Releasing Hormone-1 Secretion in Mouse Nasal Explants. Endocrinology. 2009;150(7):3221–7. doi: 10.1210/en.2008-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steiner RA, Bremner WJ, Clifton DK. Regulation of luteinizing hormone pulse frequency and amplitude by testosterone in the adult male rat. Endocrinology. 1982;111(6):2055–61. doi: 10.1210/endo-111-6-2055. [DOI] [PubMed] [Google Scholar]
- 57.Richter TA, Keen KL, Terasawa E. Synchronization of Ca(2+) Oscillations Among Primate LHRH Neurons and Nonneuronal Cells In Vitro. J Neurophysiol. 2002;88(3):1559–67. doi: 10.1152/jn.2002.88.3.1559. [DOI] [PubMed] [Google Scholar]
- 58.Raucci F, Wray S. Low affinity p75 Nerve Growth Factor Receptor modulates survival of GnRH-1 neurons during development. Neuroscience 37th Annual Meeting; San Diego, CA. 2007. [Google Scholar]
- 59.Wray S, Fueshko SM, Kusano K, Gainer H. GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol. 1996;180(2):631–45. doi: 10.1006/dbio.1996.0334. [DOI] [PubMed] [Google Scholar]
- 60.Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A. 1995;92(9):3918–22. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fueshko SM, Key S, Wray S. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J Neurosci. 1998;18 (7):2560–9. doi: 10.1523/JNEUROSCI.18-07-02560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore JP, Jr, Shang E, Wray S. In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci. 2002;22(20):8932–41. doi: 10.1523/JNEUROSCI.22-20-08932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology. 2008;149(1):279–90. doi: 10.1210/en.2007-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Constantin S, Wray S. Gonadotropin-releasing hormone-1 neuronal activity is independent of hyperpolarization-activated cyclic nucleotide-modulated channels but is sensitive to protein kinase a-dependent phosphorylation. Endocrinology. 2008;149(7):3500–11. doi: 10.1210/en.2007-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in GnRH-1 neurons. Endocrinology. 2009;150(3):1400–12. doi: 10.1210/en.2008-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24(20):4737–48. doi: 10.1523/JNEUROSCI.0649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giacobini P, Wray S. Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology. 2007;148(1):63–71. doi: 10.1210/en.2006-0758. [DOI] [PubMed] [Google Scholar]
- 68.Constantin S, Klenke U, Wray S. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology. 2010;151(8):3863–73. doi: 10.1210/en.2010-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Honma S, Kawano M, Hayashi S, Kawano H, Hisano S. Expression and immunohistochemical localization of vesicular glutamate transporter 2 in the migratory pathway from the rat olfactory placode. Eur J Neurosci. 2004;20(4):923–36. doi: 10.1111/j.1460-9568.2004.03544.x. [DOI] [PubMed] [Google Scholar]
- 70.Gainer H, Chin H. Molecular diversity in neurosecretion: reflections on the hypothalamo-neurohypophysial system. Cell Mol Neurobiol. 1998;18(2):211–30. doi: 10.1023/A:1022568904002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149(2):597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silverman AJ, Witkin JW, Silverman RC, Gibson MJ. Modulation of gonadotropin-releasing hormone neuronal activity as evidenced by uptake of fluorogold from the vasculature. Synapse. 1990;6(2):154–60. doi: 10.1002/syn.890060206. [DOI] [PubMed] [Google Scholar]
- 73.Lee WS, Smith MS, Hoffman GE. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990;87(13):5163–7. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terasawa E, Schanhofer WK, Keen KL, Luchansky L. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci. 1999;19(14):5898–909. doi: 10.1523/JNEUROSCI.19-14-05898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore JP, Jr, Wray S. Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology. 2000;141(12):4486–95. doi: 10.1210/endo.141.12.7814. [DOI] [PubMed] [Google Scholar]
- 76.Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30(18):6214–24. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jasoni C, Romano N, Constantin S, Lee K, Herbison A. Calcium dynamics in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2010:31259–69. doi: 10.1016/j.yfrne.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Chang LW, Spitzer NC. Spontaneous calcium spike activity in embryonic spinal neurons is regulated by developmental expression of the Na+, K+-ATPase beta3 subunit. J Neurosci. 2009;29(24):7877–85. doi: 10.1523/JNEUROSCI.4264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca(2+) spikes. Nature. 2002;418(6893):93–6. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- 80.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375(6534):784–7. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 81.Charles AC, Hales TG. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. J Neurophysiol. 1995;73(1):56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- 82.Charles AC, Kodali SK, Tyndale RF. Intercellular calcium waves in neurons. Mol Cell Neurosci. 1996;7(5):337–53. doi: 10.1006/mcne.1996.0025. [DOI] [PubMed] [Google Scholar]
- 83.Costantin JL, Charles AC. Spontaneous action potentials initiate rhythmic intercellular calcium waves in immortalized hypothalamic (GT1-1) neurons. J Neurophysiol. 1999;82(1):429–35. doi: 10.1152/jn.1999.82.1.429. [DOI] [PubMed] [Google Scholar]
- 84.Constantin S, Jasoni CL, Wadas B, Herbison AE. Gamma-Aminobutyric Acid and Glutamate Differentially Regulate Intracellular Calcium Concentrations in Mouse Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2010;151(1):262–70. doi: 10.1210/en.2009-0817. [DOI] [PubMed] [Google Scholar]
- 85.Abe H, Terasawa E. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology. 2005;146(10):4312–20. doi: 10.1210/en.2005-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Toba Y, Pakiam JG, Wray S. Voltage-gated calcium channels in developing GnRH-1 neuronal system in the mouse. Eur J Neurosci. 2005;22(1):79–92. doi: 10.1111/j.1460-9568.2005.04194.x. [DOI] [PubMed] [Google Scholar]
- 87.Nunemaker CS, DeFazio RA, Moenter SM. Calcium Current Subtypes in Gonadotropin-Releasing Hormone Neurons. Biol Reprod. 2003:691914–22. doi: 10.1095/biolreprod.103.019265. [DOI] [PubMed] [Google Scholar]
- 88.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144(11):5118–25. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 89.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16 (10):2255–65. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 90.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26(46):11961–73. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373–83. doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151(9):4477–84. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu X, Herbison AE. Small-conductance calcium-activated potassium (SK) channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149(7):3598–604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13(10):409–10. doi: 10.1016/s1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- 95.Moenter SM. GnRH neuron electrophysiology: A decade of study. Brain Res. 2010:136410–24. doi: 10.1016/j.brainres.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132(3):703–12. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 97.Temple JL, Wray S. Developmental Changes in GABA Receptor Subunit Composition Within the Gonadotrophin-Releasing Hormone-1 Neuronal System. J Neuroendocrinol. 2005;17(9):591–9. doi: 10.1111/j.1365-2826.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 98.Temple JL, Laing E, Sunder A, Wray S. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci. 2004;24(28):6326–33. doi: 10.1523/JNEUROSCI.1006-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klenke U, Constantin S, Wray S. Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology. 2010;151(6):2736–46. doi: 10.1210/en.2009-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang Y, Li X, He J, Lu J, Diwu Z. Real-time and high throughput monitoring of cAMP in live cells using a fluorescent membrane potential-sensitive dye. Assay Drug Dev Technol. 2006;4(4):461–71. doi: 10.1089/adt.2006.4.461. [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS. Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology. 2009;150(1):333–40. doi: 10.1210/en.2008-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 103.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uenoyama Y, Tsukamura H, Maeda KI. Kisspeptin/metastin: a key molecule controlling two modes of gonadotrophin-releasing hormone/luteinising hormone release in female rats. J Neuroendocrinol. 2009;21(4):299–304. doi: 10.1111/j.1365-2826.2009.01853.x. [DOI] [PubMed] [Google Scholar]
- 107.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 mRNA Expression in the Hypothalamus of the Ewe is Regulated by Sex Steroids and Season. Endocrinology. 2007:1481150–7. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 108.Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149(9):4605–14. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. Kisspeptin Depolarizes Gonadotropin-Releasing Hormone Neurons through Activation of TRPC-Like Cationic Channels. J Neurosci. 2008;28(17):4423–34. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsukahara S, Yamanouchi K. Distribution of glutamic acid decarboxylase, neurotensin, enkephalin, neuropeptide Y, and cholecystokinin neurons in the septo-preoptic region of male rats. J Reprod Dev. 2003;49(1):67–77. doi: 10.1262/jrd.49.67. [DOI] [PubMed] [Google Scholar]
- 111.Perera AD, Verbalis JG, Mikuma N, Majumdar SS, Plant TM. Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta) Endocrinology. 1993;132(4):1723–8. doi: 10.1210/endo.132.4.8462472. [DOI] [PubMed] [Google Scholar]
- 112.Romano N, Lee K, Abraham IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149(11):5335–44. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 114.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 115.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143(4):1459–66. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 116.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-Type gamma-Aminobutyric Acid Receptors Excites Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol. 2002;16(12):2872–91. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 117.Watanabe M, Sakuma Y, Kato M. GABAA Receptors Mediate Excitation in Adult Rat GnRH Neurons. Biol Reprod. 2009:81327–32. doi: 10.1095/biolreprod.108.074583. [DOI] [PubMed] [Google Scholar]
- 118.Leupen SM, Tobet SA, Crowley WF, Jr, Kaila K. Heterogeneous Expression of the Potassium-Chloride Cotransporter KCC2 in Gonadotropin-Releasing Hormone Neurons of the Adult Mouse. Endocrinology. 2003;144(7):3031–6. doi: 10.1210/en.2002-220995. [DOI] [PubMed] [Google Scholar]
- 119.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102(43):15682–7. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iremonger K, Constantin S, Liu X, Herbison A. Glutamate regulation of GnRH neuron excitability. Brain Res. 2010:136435–43. doi: 10.1016/j.brainres.2010.08.071. [DOI] [PubMed] [Google Scholar]
- 121.Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149(3):1155–62. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bourguignon JP, Gerard A, Debougnoux G, Rose J, Franchimont P. Pulsatile release of gonadotropin-releasing hormone (GnRH) from the rat hypothalamus in vitro: calcium and glucose dependency and inhibition by superactive GnRH analogs. Endocrinology. 1987;121(3):993–9. doi: 10.1210/endo-121-3-993. [DOI] [PubMed] [Google Scholar]
- 123.Rasmussen DD, Gambacciani M, Swartz W, Tueros VS, Yen SS. Pulsatile gonadotropin-releasing hormone release from the human mediobasal hypothalamus in vitro: opiate receptor-mediated suppression. Neuroendocrinology. 1989;49(2):150–6. doi: 10.1159/000125107. [DOI] [PubMed] [Google Scholar]
- 124.Krsmanovic LZ, Stojilkovic SS, Merelli F, Dufour SM, Virmani MA, Catt KJ. Calcium signaling and episodic secretion of gonadotropin-releasing hormone in hypothalamic neurons. Proc Natl Acad Sci U S A. 1992;89(18):8462–6. doi: 10.1073/pnas.89.18.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martinez de la Escalera G, Choi AL, Weiner RI. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc Natl Acad Sci U S A. 1992;89(5):1852–5. doi: 10.1073/pnas.89.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wetsel WC, Valenca MM, Merchenthaler I, Liposits Z, Lopez FJ, Weiner RI, Mellon PL, Negro-Vilar A. Intrinsic pulsatile secretory activity of immortalized luteinizing hormone-releasing hormone-secreting neurons. Proc Natl Acad Sci U S A. 1992;89 (9):4149–53. doi: 10.1073/pnas.89.9.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vazquez-Martinez R, Shorte SL, Boockfor FR, Frawley LS. Synchronized exocytotic bursts from gonadotropin-releasing hormone-expressing cells: dual control by intrinsic cellular pulsatility and gap junctional communication. Endocrinology. 2001;142(5):2095–101. doi: 10.1210/endo.142.5.8123. [DOI] [PubMed] [Google Scholar]
- 128.Funabashi T, Suyama K, Uemura T, Hirose M, Hirahara F, Kimura F. Immortalized gonadotropin-releasing hormone neurons (GT1–7 cells) exhibit synchronous bursts of action potentials. Neuroendocrinology. 2001;73(3):157–65. doi: 10.1159/000054632. [DOI] [PubMed] [Google Scholar]
- 129.Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A. 2009;106(26):10835–40. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Goor F, Krsmanovic LZ, Catt KJ, Stojilkovic SS. Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons. Biochem Cell Biol. 2000;78(3):359–70. [PubMed] [Google Scholar]
- 131.Krsmanovic LZ, Martinez-Fuentes AJ, Arora KK, Mores N, Navarro CE, Chen HC, Stojilkovic SS, Catt KJ. Autocrine regulation of gonadotropin-releasing hormone secretion in cultured hypothalamic neurons. Endocrinology. 1999;140(3):1423–31. doi: 10.1210/endo.140.3.6588. [DOI] [PubMed] [Google Scholar]
- 132.Xu C, Xu XZ, Nunemaker CS, Moenter SM. Dose-dependent switch in response of gonadotropin-releasing hormone (GnRH) neurons to GnRH mediated through the type I GnRH receptor. Endocrinology. 2004;145(2):728–35. doi: 10.1210/en.2003-0562. [DOI] [PubMed] [Google Scholar]
- 133.Han SK, Lee K, Bhattarai JP, Herbison AE. Gonadotropin-releasing hormone (GnRH) exerts stimulatory effects on GnRH neurons in intact adult male and female mice. J Neuroendocrinol. 2010:22188–95. doi: 10.1111/j.1365-2826.2009.01950.x. [DOI] [PubMed] [Google Scholar]
- 134.Duittoz A, Constantin S, Skinner DC, Wray S. GnRH-1 regulates GnRH-1 neurons. Neuroscience 35th Annual Meeting; Washington DC. 2005. [Google Scholar]
- 135.Purnelle G, Gerard A, Czajkowski V, Bourguignon JP. Pulsatile secretion of gonadotropin-releasing hormone by rat hypothalamic explants without cell bodies of GnRH neurons. Neuroendocrinology. 1997;66(5):305–12. doi: 10.1159/000127253. [DOI] [PubMed] [Google Scholar]
- 136.Hu L, Olson AJ, Weiner RI, Goldsmith PC. Connexin 26 expression and extensive gap junctional coupling in cultures of GT1–7 cells secreting gonadotropin-releasing hormone. Neuroendocrinology. 1999;70(4):221–7. doi: 10.1159/000054480. [DOI] [PubMed] [Google Scholar]
- 137.Campbell RE, Ducret E, Porteous R, Liu X, Herde M, Wellerhaus K, Sonntag S, Willecke K, Herbison AE. Role of connexin36 gap junctions in the gonadotropin-releasing hormone neuronal network. Endocrinology. 2011 doi: 10.1210/en.2010-1311. (under revision) [DOI] [PubMed] [Google Scholar]
- 138.Terasawa E, Keen KL, Grendell RL, Golos TG. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol Endocrinol. 2005;19(11):2736–47. doi: 10.1210/me.2005-0034. [DOI] [PubMed] [Google Scholar]
- 139.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444(7120):707–12. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 140.Spitzer NC, Kingston PA, Manning TJ, Conklin MW. Outside and in: development of neuronal excitability. Curr Opin Neurobiol. 2002;12(3):315–23. doi: 10.1016/s0959-4388(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 141.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20(11):523–9. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 142.Foster DL, Mickelson IH, Ryan KD, Coon GA, Drongowski RA, Holt JA. Ontogeny of pulsatile luteinizing hormone and testosterone secretion in male lambs. Endocrinology. 1978;102(4):1137–46. doi: 10.1210/endo-102-4-1137. [DOI] [PubMed] [Google Scholar]
- 143.Plant TM. Pulsatile luteinizing hormone secretion in the neonatal male rhesus monkey (Macaca mulatta) J Endocrinol. 1982;93(1):71–4. doi: 10.1677/joe.0.0930071. [DOI] [PubMed] [Google Scholar]
- 144.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24(5):668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 145.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–10. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 146.Nicolaidis S. Prenatal imprinting of postnatal specific appetites and feeding behavior. Metabolism. 2008;57(Suppl 2):S22–6. doi: 10.1016/j.metabol.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Gibson MJ, Miller GM, Silverman AJ. Pulsatile luteinizing hormone secretion in normal female mice and in hypogonadal female mice with preoptic area implants. Endocrinology. 1991;128(2):965–71. doi: 10.1210/endo-128-2-965. [DOI] [PubMed] [Google Scholar]