Abstract
Pure soluble, recombinant and synthetic antigens, despite their better tolerability, are unfortunately often much less immunogenic than live or killed whole organism vaccines. Thus, the move towards the development of safer subunit vaccines has created a major need for more potent adjuvants. In particular, there is an urgent need for adjuvants capable of boosting cellular (Th1) immunity but without unacceptable toxicity. The adjuvant activity of aluminium compounds (aluminium phosphate or hydroxide) was first described by Glenny and colleagues in 1926. Surprisingly, despite the description of over one hundred adjuvants in the scientific literature, alum remains the only adjuvant approved for human use in the USA. Unfortunately, alum has no effect on cellular immunity and is faced with increasing concerns regarding potential for cumulative aluminium toxicity. Why then has alum not been replaced in human vaccines? Despite the enormous number of candidates, potency has invariably been associated with increased toxicity, and this more than anything else has precluded their use, particularly in prophylactic vaccines where safety issues are paramount. Hence, there is a major unmet need for a safe efficacious adjuvant capable of boosting cellular plus humoral immunity. The extensive data on inulin-based adjuvants indicate that these are excellent candidates to replace alum as the adjuvant of choice for many vaccines. Particular advantages offered by inulin-based adjuvants is that they induce cellular in addition to humoral immunity and offer excellent safety, tolerability, ease of manufacture and formulation. Thus, adjuvants based on inulin have enormous potential for use in vaccines against both pathogens and cancer.
Keywords: Adjuvant, Vaccine, Inulin, Complement, Cellular, Immune, Th1, Th2
1. Introduction
Some of the features involved in adjuvant selection are: the antigen, the species to be vaccinated, the route of administration, the likelihood of side effects and the requirement for a cell-mediated or humoral antibody response [1,2]. Ideally, adjuvants should promote an appropriate immune response, (Th1 or Th2), be stable with long shelf life, biodegradable, cheap to produce and not themselves immunogenic [3]. Freund et al. in 1936, developed an emulsion of water and mineral oil containing killed Mycobacteria, thereby creating Freund’s complete adjuvant (FCA), which remains amongst the most potent of known adjuvants and a particularly powerful stimulant of both cellular and humoral immunity [4]. Unfortunately FCA causes severe reactions and is too toxic for human use. A persuasive argument in favour of inulin-based adjuvants is that they can provide immune responses matching FCA without the toxicity (Fig. 1).
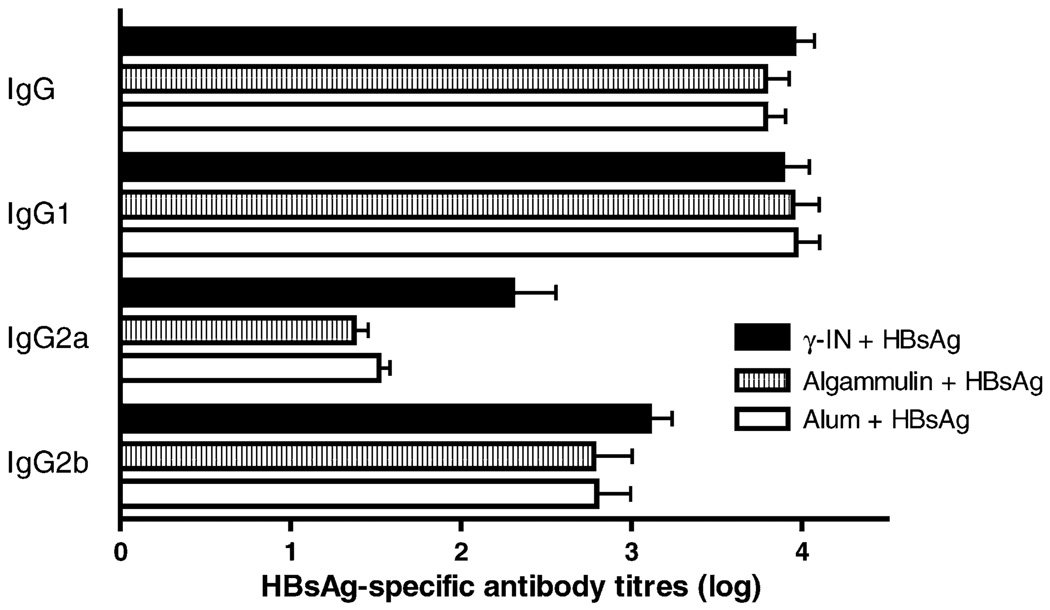
Fig. 1.
γ-Inulin potentiates Th1 response to HBsAg. Groups of C57/B6 mice were immunized with Hepatitis B surface antigen (HBsAg) (1 µg/mouse) in γ-inulin, algammulin or alum. After the second immunization total HBsAg-specific IgG2a were significantly higher for the γ-inulin group whereas total IgG and IgG1 titers were comparable in all groups.
2. Safety of inulin-based adjuvants
The benefits flowing from adjuvant incorporation into any vaccine formulation have to be balanced with the risk of adverse reactions induced by these compounds. Unfortunately, strong adjuvant activity is often correlated with increased toxicity, as exemplified by FCA. A major challenge in adjuvant research is to increase adjuvant activity while reducing toxicity [5]. Adverse reactions to adjuvants include local pain, inflammation, injection site necrosis, lymphadenopathy, granulomas or sterile abscesses. Systemic reactions include nausea, fever, adjuvant arthritis, uveitis, anaphylaxis, organ specific toxicity and immunotoxicity, immunosuppresion or autoimmune diseases [1,6]. There are also increasing community concerns regarding the use of metals, such as aluminium in parenteral vaccines due to possible links to Alzheimers disease and other neurodegenerative disorders. To date inulin-based adjuvants have been tested with a wide range of different antigens in multiple animal species with no significant toxicity.
3. Alternatives to γ-inulin adjuvants
Adjuvants can be classified according to their source, action mechanisms or physico chemical properties [1,7]. Although many adjuvants have been proposed in each of the above classes over the years, these have failed to be successful in humans, chiefly because of toxicity, poor immunogenicity, manufacturing difficulties, instability or cost.
4. Toxicity of alum-based adjuvants
There is a high proportion of moderate to severe granulomas when alum-based vaccines are injected subcutaneously or intradermally [8,9]. Other limitations of alum adjuvants are increased IgE production, allergenicity and neurotoxicity [8,10–12]. Under conditions of reduced renal function, aluminium is accumulated in the body and becomes highly toxic causing fatal neurological syndrome and dialysis-associated dementia. Aluminium intoxication has also been associated with amyotrophic lateral sclerosis and Alzheimer’s disease.
5. Alternative human adjuvants
Calcium phosphate has been used for diphtheria-tetanus-pertussis vaccines but overall it is a weak adjuvant thereby limiting its broader use. The saponin Quil A, an aqueous extract from the bark of Quillaja saponaria and extracts, mainly QS-21, have been studied as alternatives to alum when strong cell-mediated responses are required [13,14]. In addition to pain on injection, severe local reactions and granulomas, toxicity includes severe haemolysis [5,15–17] making such adjuvants unsuitable for human uses other than for life threatening diseases, such as HIV infection or cancer [18]. Muramyl dipeptide (MDP) [19] and other derivatives from Gram-negative bacteria, such as lipopolysaccharides (LPS) and monophosphoryl lipid A [20] have also been used as human adjuvants although toxicity remains the single biggest barrier to the use of such adjuvants for human prophylactic vaccines. Oil and water emulsions including Montanide, Adjuvant 65, and Lipovant although good at inducing cellular immunity are similarly too toxic for human prophylactic vaccines [21,22]. Hence, adjuvant toxicity is the biggest single factor behind the reason why alum remains the only adjuvant approved for human use by the FDA.
6. Advantages of inulin-based adjuvants
Inulin is a natural storage polysaccharide of Compositae, and is approved for parenteral human use for renal function studies [23]. It contains only fructose with small amounts of glucose and is essentially a linear (unbranched) b-d-(2 → 1) polyfructofuranosyl a-d-flucose. γ-Inulin and related compounds, such as algamulin have been successfully tested in combination with antigens including ovalbumin, tetanus toxoid, syncytial respiratory virus, E7 protein of Human Papilloma Virus, glycoprotein D from Herpes Virus 2, Hepatitis B surface antigen, Influenza, Haemophilus influenzae and Plasmodium falciparum antigens across a wide range of species including mice, rats, rabbits, dogs, horses, monkeys, and man [24–26]. Inulin-derived adjuvants produce strong Th1 and Th2 immune responses as demonstrated by antibody isotyping (Fig. 1). Of note, no major toxicity of inulin has been demonstrated in any of the species tested, with the only significant finding being the occasional development of small granuloma when very high doses are injected subcutaneously. This excellent tolerability contrasts markedly with the experience of other Th1 adjuvants.
7. Regulatory requirements for adjuvant approval
Significant regulatory and other hurdles exist to approval of new adjuvants. In addition to pre-clinical studies on the adjuvant itself, the combined antigen-adjuvant formulation also needs to be subjected to toxicology prior to commencement of phase I clinical trials [27]. Pre-clinical toxicology evaluation is normally conducted in a small animal species, such as mice, rats, or rabbits and should use the same administration route proposed for human use. The dose and frequency of vaccination for pre-clinical toxicology should be similar or higher to the proposed dose for humans in order to maximize the ability to identify potential safety problems [27]. Nevertheless, many adjuvants appear to be able to pass these animal tests and yet still turn out to be unsatisfactory once administered to humans. It is, therefore, reassuring that in a pilot Phase 1 human study an inulin-based adjuvant was demonstrated to be safe and effective with minimal toxicity [28]. Results on the capacity of inulin-based adjuvants to enhance the immune response consistently show that inulin adjuvants are equal or superior to alum at eliciting antibody responses, and in some instances are even equal in potency to the gold standard, FCA. In addition, they have the benefit over alum that they also stimulate cellular immunity as reflected by Th1 antibody isotype induction. This justifies further development of inulin-based adjuvants for use in prophylactic and therapeutic vaccines.
8. Conclusions
The move away from live or whole killed vaccines to poorly immunogenic purified subunit vaccines requires the development of more potent adjuvants that are nevertheless free of significant toxicity. Many pathogens including viruses, such as HIV require cellular immunity for protection. The currently available human adjuvant, namely alum, is ineffective for this purpose. Whilst several hundred different adjuvants have been proposed over the last few decades, the vast majority have not been successful in being approved for human use, with limitations including lack of efficacy, unacceptable local or systemic toxicity, difficulty of manufacture, poor stability, and prohibitive cost. Inulin-based adjuvants are relatively unique in exhibiting few of these limitations and have the advantage that they are potent inducers of both cellular and humoral immunity, making them suitable for a wide spectrum of prophylactic and therapeutic vaccines.
Acknowledgements
The contributing author NP holds shares in Vaxine Pty Ltd., a privately owned vaccine company. Vaxine has proprietary interests in the inulin-based adjuvant technology described in this paper. Work described in this paper was funded by grants to Vaxine from the Biotechnology Innovation Fund and the ACT Innovation Grants Scheme. Diego Silva is thanked for his assistance in designing the figures.
References
- 1.Allison AC, Byars NE. Immunological adjuvants: desirable properties and side-effects. Mol Immunol. 1991;28(3):279–284. doi: 10.1016/0161-5890(91)90074-t. [DOI] [PubMed] [Google Scholar]
- 2.Edelman R. The development and use of vaccine adjuvants. Mol Biotechnol. 2002;21(2):56–67. doi: 10.1385/MB:21:2:129. [DOI] [PubMed] [Google Scholar]
- 3.Edelman R. Vaccine adjuvants. Rev Infect Dis. 1980;2(3):370–383. doi: 10.1093/clinids/2.3.370. [DOI] [PubMed] [Google Scholar]
- 4.Freund J, Casals J, Hosmer E. Sensitization and antibody formation after injection of tubercle bacili and parafin oil. Proc Soc Exp Biol Med. 1937;37:509–513. [Google Scholar]
- 5.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants: a balance between toxicity and adjuvanticity. Vaccine. 1993;11(3):293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 6.Waters RV, Terrell TG, Jones GH. Uveitis induction in the rabbit by muramyl dipeptides. Infect Immun. 1986;51(3):816–825. doi: 10.1128/iai.51.3.816-825.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogel FR. Adjuvants in perspective. Dev Biol Stand. 1998;92:241–248. [PubMed] [Google Scholar]
- 8.Butler NR, Voyce MA, Burland WL, Hilton ML. Advantages of aluminium hydroxide adsorbed combined diphtheria, tetanus, and pertussis vaccines for the immunization of infants. Br Med J. 1969;1(645):663–666. doi: 10.1136/bmj.1.5645.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straw BE, MacLachlan NJ, Corbett WT, Carter PB, Schey HM. Comparison of tissue reactions produced by Haemophilus pleuropneumoniae vaccines made with six different adjuvants in swine. Can J Comp Med. 1985;49(2):149–151. [PMC free article] [PubMed] [Google Scholar]
- 10.Audibert FM, Lise LD. Adjuvants: current status, clinical perspectives and future prospects. Immunol Today. 1993;14(6):281–284. doi: 10.1016/0167-5699(93)90046-N. [DOI] [PubMed] [Google Scholar]
- 11.Goto N, Kato H, Maeyama J, Eto K, Yoshihara S. Studies on the toxicities of aluminium hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine. 1993;11(9):914–918. doi: 10.1016/0264-410x(93)90377-a. [DOI] [PubMed] [Google Scholar]
- 12.Bomford P. Aluminium salt: perspectives in their use as adjuvants. In: Poste GGACAG, editor. Immunological adjuvants and vaccines. New York: Plenum Press; 1989. pp. 35–41. [Google Scholar]
- 13.Edelman R. An update on vaccine adjuvants in clinical trial. AIDS Res Hum Retroviruses. 1992;8(8):1409–1411. doi: 10.1089/aid.1992.8.1409. [DOI] [PubMed] [Google Scholar]
- 14.Ronnberg B, Fekadu M, Morein B. Adjuvant activity of non-toxic Quillaja saponaria Molina components for use in ISCOM matrix. Vaccine. 1995;13(14):1375–1382. doi: 10.1016/0264-410x(95)00105-a. [DOI] [PubMed] [Google Scholar]
- 15.Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol. 1991;146(2):431–437. [PubMed] [Google Scholar]
- 16.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm Biotechnol. 1995;6:525–541. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 17.Rook GA. New meanings for an old word: adjuvanticity, cytokines and T cells. Immunol Today. 1993;14(2):95–96. doi: 10.1016/0167-5699(93)90072-S. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 19.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 20.Freund J. The mode of action of immunologic adjuvants. Bibl Tuberc. 1956;(10):130–148. [PubMed] [Google Scholar]
- 21.Byars N, Allison A. Immunologic adjuvants: general properties, advantages, and limitations. In: Z H, editor. Laboratory methods in immunology. CRC press; 1990. pp. 39–51. [Google Scholar]
- 22.Allison AC, Byars NE. Immunological adjuvants and their mode of action. Biotechnology. 1992;20:431–449. doi: 10.1016/b978-0-7506-9265-6.50025-7. [DOI] [PubMed] [Google Scholar]
- 23.Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999;55(5):1878–1884. doi: 10.1046/j.1523-1755.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- 24.Cooper PD. Vaccine adjuvants based on gamma inulin. Pharm Biotechnol. 1995;6:559–580. doi: 10.1007/978-1-4615-1823-5_24. [DOI] [PubMed] [Google Scholar]
- 25.Cooper PD, McComb C, Steele EJ. The adjuvanticity of Algammulin, a new vaccine adjuvant. Vaccine. 1991;9(6):408–415. doi: 10.1016/0264-410x(91)90127-r. [DOI] [PubMed] [Google Scholar]
- 26.Cooper PD, Steele EJ. Algammulin, a new vaccine adjuvant comprising gamma inulin particles containing alum: preparation and in vitro properties. Vaccine. 1991;9(5):351–357. doi: 10.1016/0264-410x(91)90063-c. [DOI] [PubMed] [Google Scholar]
- 27.Goldenthal K, Cavagnaro J, Alving C, Vogel F. Safety evaluation of vaccine adjuvants. National Cooperative Vaccine Development Working Group. AIDS Res Hum Retroviruses. 1993;9:S45–S49. [Google Scholar]
- 28.Frazer IH, Tindle RW, Fernando GJP, Malcolm K, Herd K, McFadyn S, et al. Safety and immunogenicity of HPV16E7/algammulin. In: Tindle RW, editor. Vaccines for human papillomavirus infection and anogenital disease. R.G Landes Company; 1999. [Google Scholar]