Abstract
Background and Aims
Masting, i.e. synchronous but highly variable interannual seed production, is a strong sink for carbon and nutrients. It may, therefore, compete with vegetative growth. It is currently unknown whether increased atmospheric CO2 concentrations will affect the carbon balance (or that of other nutrients) between reproduction and vegetative growth of forest species. In this study, reproduction and vegetative growth of shoots of mature beech (Fagus sylvatica) trees grown at ambient and elevated atmospheric CO2 concentrations were quantified. It was hypothesized that within a shoot, fruiting has a negative effect on vegetative growth, and that this effect is ameliorated at increased CO2 concentrations.
Methods
Reproduction and its competition with leaf and shoot production were examined during two masting events (in 2007 and 2009) in F. sylvatica trees that had been exposed to either ambient or elevated CO2 concentrations (530 µmol mol−1) for eight consecutive years, between 2000 and 2008.
Key Results
The number of leaves per shoot and the length of terminal shoots was smaller or shorter in the two masting years compared with the one non-masting year (2008) investigated, but they were unaffected by elevated CO2 concentrations. The dry mass of terminal shoots was approx. 2-fold lower in the masting year (2007) than in the non-masting year in trees growing at ambient CO2 concentrations, but this decline was not observed in trees exposed to elevated CO2 concentrations. In both the CO2 treatments, fruiting significantly decreased nitrogen concentration by 25 % in leaves and xylem tissue of 1- to 3-year-old branches in 2009.
Conclusions
Our findings indicate that there is competition for resources between reproduction and shoot growth. Elevated CO2 concentrations reduced this competition, indicating effects on the balance of resource allocation between reproduction and vegetative growth in shoots with rising atmospheric CO2 concentrations.
Keywords: Beech, carbon autonomy, CO2 enrichment, Fagus sylvatica, mast seeding, nitrogen, resource allocation, trade-off, vegetative growth
INTRODUCTION
Plant life history functions, including growth, maintenance and reproduction, all require resources. If resources such as carbon (C) and nitrogen (N) are limited, their allocation to reproduction may occur at the expense of other functions, generally referred to as the ‘cost of reproduction’ (Obeso, 2002). In perennial woody plants, the cost of reproduction for the whole plant has been estimated from annual tree-ring increments (Obeso, 1997; Genet et al., 2010), while local trade-offs, for single shoots, can be analysed by comparing current shoot elongation in fruit-bearing and non-reproductive shoots (Obeso, 1997; Suzuki, 2001; Kawamura and Takeda, 2006). Shoots can be considered as the modular unit of a tree crown. Resource allocation within branchlets might respond to local trade-offs (e.g. leaf size and number, shoot growth and fruit production) but is probably integrated at individual trees (as measured by tree-ring growth, for example) (Miyazaki et al., 2002; Obeso, 2002; Ishihara and Kikuzawa, 2009).
Mast seeding or masting, i.e. synchronous, but highly variable, interannual seed production by plant populations, is a characteristic of many perennial species globally, including tropical and temperate trees and temperate herbs (Shibata et al., 1998; Kelly and Sork, 2002). This complex phenomenon, the results of many endogenous and exogenous factors, has been explained by ultimate evolutionary advantages and proximate causes (Kelly and Sork, 2002; Piovesan and Adams, 2005). Although the physiological mechanism behind the masting phenomenon is not fully understood, it is generally considered to be related to temporal variations in individual resource budgets and the associated costs of reproduction, which is strongly affected by climatic conditions (Hilton and Packham, 2003; Richardson et al., 2005). It is assumed in most resource-driven models of masting that C is the main limiting internal resource for seed production (Isagi et al., 1997; Satake and Iwasa, 2000). Only recently, mobile carbohydrate stores have been shown to control mast flowering in Astragalus scaphoides, a perennial herb (Crone et al., 2009). Because masting is a consequence of physiological controls of reproduction, under environmental influence, differences in plant resource acquisition and allocation could dramatically change patterns of seed production. If C is the resource limiting masting in Fagus sylvatica, increased atmospheric carbon dioxide (CO2) concentrations could lead to a higher frequency of masting events and thus greater seed production. Interestingly, Övergaard et al. (2007) found that the average interval between masting events of F. sylvatica in Sweden has been reduced to 2·5 years during the last 30 years compared with an average of 5·0 years from the end of the 17th century up to the 1960s, although other environmental factors may also be involved. However, to date, only a few studies have experimentally tested the reproductive response of forest tree species to elevated CO2 (LaDeau and Clark, 2001; Stiling et al., 2004; Körner et al., 2005; Way et al., 2010). The competition between reproduction and shoot growth as atmospheric CO2 concentrations increase has received little attention. Yet, seed production and seed quality are important determinants of natural regeneration and maintenance of species diversity.
Here, we describe an investigation of the effect of 8 years of continuous free-air CO2 enrichment (530 µmol mol−1) at the Swiss Canopy Crane (SCC) site on the reproductive effort and seed traits of F. sylvatica and the competition between reproduction and vegetative growth during the growing season. Previous studies at the SCC site found that net photosynthesis in fully sunlit, upper canopy foliage was stimulated by approx. 40 % compared with ambient controls (Zotz et al., 2005; Bader et al., 2010). However, the fate of these surplus C assimilates remained uncertain, since no significant increase in growth was found when evaluated as stem basal area increment (Körner et al., 2005; Asshoff et al., 2006). Fruits might be an additional C sink for surplus photoassimilates under elevated CO2 conditions, although a previous coarse estimate of the biomass of seeds and vegetative infructescence tissues (e.g. cupules) at the SCC site, based on litter trap data, revealed no significant difference between trees growing at ambient and elevated CO2 concentrations (Körner et al., 2005). In the current study, seed production was quantified by investigating individual shoots of F. sylvatica trees at the SCC site. Therefore, the main objectives of this study were to: (a) investigate trade-offs between seed production and vegetative growth within individual shoots; and (b) evaluate effects of elevated CO2 on these trade-offs in F. sylvatica 1 year after the end of an 8 year period of CO2 enrichment.
MATERIALS AND METHODS
Study site and CO2 enrichment
The study was conducted at the SCC site close to Hofstetten, 15 km south of Basel, Switzerland (47°28′N, 7°30′E, 550 m a.s.l.). It is a mature temperate deciduous forest, with trees ages between 80 and 120 years, tree heights between 30 and 35 m, a tree density (breast height diameter ≥0·1 m) of 415 trees ha−1 and a stem basal area of about 46 m2 ha−1. The stand is characterized by dominant Fagus sylvatica and Quercus petraea, with Carpinus betulus, Tilia platyphyllos, Acer campestre, Prunus avium and four conifers (Abies alba, Larix decidua, Picea abies and Pinus sylvestris) as companion species. The climate is typical of the humid temperate zone, characterized by mild winters and moderately warm summers. Soils are of the rendzina type on calcareous bedrock (a silty loam with an accessible profile depth of approx. 30 cm and a pH of approx. 5·8 in the top 10 cm of the profile). Further details regarding the site are presented in Pepin and Körner (2002).
At the SCC site, 13 of the 64 broad-leaved trees within the reach of the crane were exposed to elevated CO2 between late September 2000 and October 2008, using the so-called web-FACE technology (Pepin and Körner, 2002). On average, the treated trees experienced CO2 concentrations of 530 µmol mol−1 during daytime hours throughout the growing season (March–October; Körner et al., 2005). Control trees, which grew in the crane area but at sufficient distance from the CO2 release to avoid elevated CO2, were permanently at ambient atmospheric CO2 concentrations. The CO2 enhancement ended at leaf fall of the deciduous species in October of 2008.
Three F. sylvatica trees previously exposed to elevated CO2 concentrations and four individuals exposed to ambient CO2 concentrations were selected for this study. In autumn 2008, the presence of floral buds was confirmed on all selected trees, indicating mast fruiting in 2009. All of the leaves of F. sylvatica trees are pre-formed in the winter buds (Eschrich et al., 1989). Thus, the numbers of leaves per shoot and even the numbers of cell layers in the palisade tissues within the leaves on the trees in 2009 were fixed during summer 2008, i.e. while still exposed to elevated CO2 concentrations. Trees under both elevated and ambient CO2 concentrations mast fruited in 2007, and this was followed by a non-fruiting year in 2008, and a masting year again in 2009. The biomass of seeds and vegetative infructescence tissues (e.g. cupules) collected from litter traps was 30·8 ± 9·6, 0·36 ± 0·14 and 31·2 ± 5·3 g m−2 in 2007, 2008 and 2009, respectively.
Shoot and fruit sampling
All shoots were investigated and sampled by means of a crane gondola. In July 2009, four 5-year-old fully sunlit branches from the upper crown of each tree were chosen, within which all shoots were classified according to their age. The numbers of leaves and fruits per shoot were counted directly for shoots in 2009 and indirectly from petiole scars for shoots from the 2007 and 2008 seasons. All measurements in July 2009 were conducted non-destructively in situ.
In September 2009, two 5-year-old branches were harvested from each tree and kept under cool conditions during transport. In the laboratory, each branch was separated into shoot segments developed in 2009, 2008 and 2007. The numbers of leaves and fruits per shoot for the year 2009 were counted, and total leaf areas per shoot were measured using a leaf area meter (Li-3100, LI-COR, Lincoln, NE, USA). In addition, the length of each terminal shoot was measured for the years 2007–2009. All samples were dried at 80 °C for 48 h and the dry mass was recorded for each. For chemical analyses, the same tissues of both sampled branches were pooled for each tree before grinding in a steel ball mill (MM2000, Retsch, Haan, Germany). The C and N concentrations were measured after combustion in a CHN Analyzer (Vario EL III, Elementar, Hanau, Germany).
Statistical analyses
The effects of all of the independent variables (i.e. elevated CO2, fruiting and year) on all dependent variables measured were evaluated by fitting generalized linear mixed models (GLMMs) on the basis of restricted maximum likelihood, using SAS/STAT 9·1 software (PROC GLIMMIX, SAS Institute, Cary, NC, USA). Poisson (count data), binomial (binary data), log-normal (length and dry mass data) or normal (C and N concentrations) distributions were assumed for the errors of the dependent variables, and the effect of individual trees and that of branches within individuals were included in the models as random effects. The degrees of freedom of the denominator in type III tests of the fixed effects (Wald-type tests) were approximated using the method of Kenward and Roger (1997). Results were considered significant when the P-value was <0·05 unless otherwise mentioned.
RESULTS
Fruiting efforts and seed traits
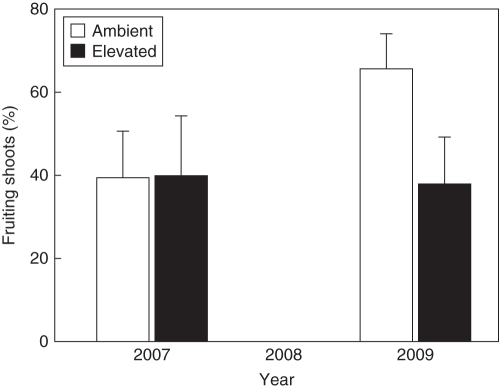
The proportion of fruit-bearing shoots was significantly greater in 2009 than in 2007, but only for trees growing at ambient CO2 before 2009, i.e. there was a significant interaction between CO2 treatment and year (Fig. 1, Table 1). In contrast, the number of fruits per fruit-bearing shoot was similar between the two recorded masting years for trees growing at both ambient and elevated CO2 (data not shown). CO2 enrichment in the preceding years had no significant carry-over effect on either dry mass of seed and cupule or their respective N and C concentrations (Table 2).
Fig. 1.
Comparison of the estimated percentage of fruit-bearing shoots of Fagus sylvatica trees (means ± s.e.) exposed to ambient (n = 4) and elevated (n = 3) CO2 concentrations, as indicated. Masting occurred in 2007 and 2009, whereas 2008 was a non-masting year. The results of the statistical analysis are shown in Table 1.
Table 1.
Summary statistics for the type III test of fixed effects (Wald-type test) and the estimated random effects in the generalized linear mixed model for data presented in Table 3 and Figs 1–3
| Random effect |
|||||||
|---|---|---|---|---|---|---|---|
| Variables in Table 3 and Figs 1-3 | Fixed effect | d.f. | den d.f. | F | P | Individuals | Branches in individuals |
| Table 3 | |||||||
| Leaf number | Fruiting | 1 | 239·0 | 19·16 | <0·0001 | 0 | 0 |
| CO2 | 1 | 239·0 | 1·04 | 0·308 | |||
| Fruiting × CO2 | 1 | 239·0 | 1·74 | 0·189 | |||
| Leaf area | Fruiting | 1 | 229·5 | 16·97 | <0·0001 | 0·0355 | 0·0127 |
| CO2 | 1 | 5·0 | 0·85 | 0·399 | |||
| Fruiting × CO2 | 1 | 229·5 | 0·14 | 0·704 | |||
| LMA | Fruiting | 1 | 230·0 | 32·88 | < 0·0001 | 0·0224 | 0·0040 |
| CO2 | 1 | 5·1 | 1·23 | 0·318 | |||
| Fruiting × CO2 | 1 | 230·0 | 2·14 | 0·145 | |||
| Figure 1 | |||||||
| Fruiting shoot | Year | 1 | 982·0 | 7·28 | 0·007 | 0·5360 | 0·0369 |
| CO2 | 1 | 5·1 | 1·64 | 0·255 | |||
| Year × CO2 | 1 | 982·0 | 5·25 | 0·022 | |||
| Figure 2 | |||||||
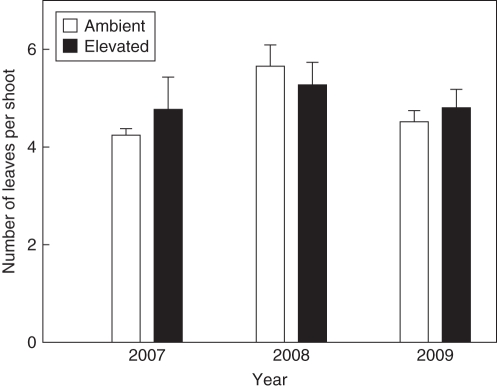
| Leaf number | Year | 2 | 1297·0 | 14·29 | < 0·0001 | 0·0121 | 0·0023 |
| CO2 | 1 | 5·3 | 0·12 | 0·747 | |||
| Year × CO2 | 2 | 1297·0 | 1·22 | 0·296 | |||
| Figure 3 | |||||||
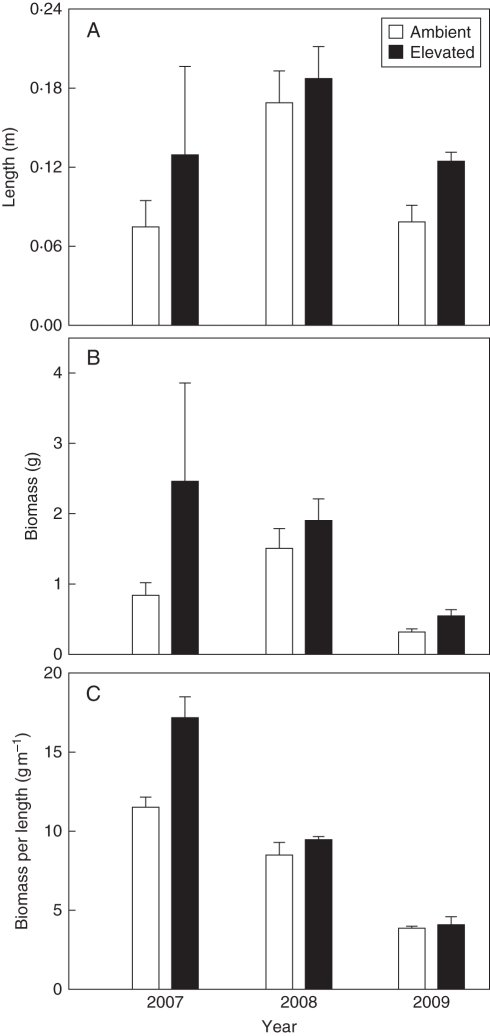
| Length | Fruiting | 2 | 24·0 | 8·02 | 0·002 | 0·0297 | 0·0117 |
| CO2 | 1 | 5·0 | 2·22 | 0·196 | |||
| Fruiting × CO2 | 2 | 24·0 | 0·31 | 0·739 | |||
| Biomass | Fruiting | 2 | 24·0 | 22·20 | < 0·0001 | 0 | 0·1330 |
| CO2 | 1 | 12·0 | 3·21 | 0·098 | |||
| Fruiting × CO2 | 2 | 24·0 | 0·61 | 0·549 | |||
| Biomass per length | Fruiting | 2 | 24·0 | 205·73 | < 0·0001 | 0 | 0·0363 |
| CO2 | 1 | 12·0 | 1·98 | 0·185 | |||
| Fruiting × CO2 | 2 | 24·0 | 4·03 | 0·031 | |||
The degrees of freedom of the denominator (den d.f.) were approximated using Kenward and Roger's method.
Significant values are highlighted in bold.
Table 2.
Dry mass (DM), and nitrogen (N) and carbon (C) concentrations of individual seeds and cupules of trees exposed to ambient or elevated CO2 concentrations sampled on 8 September 2009
| Seeds |
Cupules |
|||||
|---|---|---|---|---|---|---|
| CO2 concentration | DM (g) | N (%) | C (%) | DM (g) | N (%) | C (%) |
| Ambient | 0·17 ± 0·03 | 3·45 ± 0·14 | 58·0 ± 0·8 | 0·76 ± 0·06 | 0·21 ± 0·02 | 46·8 ± 1·0 |
| Elevated | 0·20 ± 0·02 | 3·21 ± 0·17 | 56·4 ± 0·9 | 1·01 ± 0·17 | 0·25 ± 0·00 | 45·9 ± 0·7 |
| d.f. | 1 | 1 | 1 | 1 | 1 | 1 |
| den d.f. | 5·0 | 5·0 | 5·0 | 5·0 | 5·0 | 5·0 |
| F | 0·95 | 1·19 | 1·86 | 3·88 | 2·62 | 0·49 |
| P-value | 0·373 | 0·325 | 0·231 | 0·106 | 0·167 | 0·514 |
Values shown are means ± s.e. from four trees exposed to ambient CO2 and three trees previously exposed to elevated CO2. A generalized linear model was used for statistical analysis.
Leaf traits
In 2009, fruit-bearing shoots had significantly more leaves with greater individual leaf area and leaf dry mass per area (LMA) than non-fruiting shoots (Table 3). Since this difference in leaf traits between fruiting and non-fruiting branches also persisted within a 5-year-old branch of an individual tree (data not shown), they are unlikely to be due to factors associated with different crown positions (e.g. sun exposure). The interannual comparison revealed that there were fewer leaves per shoot in the masting years than in the non-masting year (Fig. 2, Table 1). Leaf number per shoot was not affected by CO2 enrichment (Fig. 2, Table 3).
Table 3.
Effects of fruiting and CO2 treatment on number of leaves per shoot, individual leaf area and leaf dry mass per area (LMA) from non-fruiting and fruiting shoots sampled on 8 September 2009
| Shoot type | No. of leaves per shoot | Leaf area (m2 × 10−4) | LMA (g m−2) |
|---|---|---|---|
| Ambient CO2 | |||
| Non-fruiting | 3·2 ± 0·2 | 7·88 ± 0·56 | 85·59 ± 5·98 |
| Fruiting | 4·7 ± 0·2 | 8·52 ± 0·51 | 96·83 ± 6·81 |
| Elevated CO2 | |||
| Non-fruiting | 3·3 ± 0·3 | 9·18 ± 1·57 | 100·66 ± 9·97 |
| Fruiting | 4·5 ± 0·2 | 10·30 ± 1·69 | 107·57 ± 7·48 |
Values shown are the means ± s.e. from four trees exposed to ambient CO2 and three trees previously exposed to elevated CO2. The results of the statistical analyses are shown in Table 1.
Fig. 2.
Interannual variations in number of leaves per current-year shoot of Fagus sylvatica trees (means ± s.e.) exposed to ambient (n = 4) and elevated (n = 3) CO2 concentrations, as indicated. Masting occurred in 2007 and 2009, whereas 2008 was a non-masting year. The results of the statistical analysis are shown in Table 1.
Leaf N concentrations on a dry mass basis were significantly lower for fruiting shoots than for non-fruiting shoots in September (Tables 4 and 5). This difference in leaf N persisted when the comparison was made on a leaf area basis (mg N cm−2; data not shown). Moreover, leaf N decreased significantly between the two occasions, and this decline was greater for fruit-bearing shoots than for non-fruiting shoots. In contrast, leaf C concentrations were similar for fruiting and non-fruiting shoots. Neither leaf N nor leaf C concentrations were affected by previous CO2 enrichment.
Table 4.
Effects of fruiting and previous CO2 treatment on nitrogen (N) and carbon (C) concentrations of leaves and xylem tissue of 1- to 3-year old branches from fruiting and non-fruiting shoots sampled on 22 July and 8 September 2009
| Leaf |
Xylem of branches |
|||||||
|---|---|---|---|---|---|---|---|---|
| N (%) |
C (%) |
N (%) |
C (%) |
|||||
| Shoot type | July | September | July | September | July | September | July | September |
| Ambient CO2 | ||||||||
| Non-fruiting | 2·41±0·19 | 2·21 ± 0·13 | 51·0 ± 0·5 | 50·8 ± 1·1 | 0·67 ± 0·17 | 0·62 ± 0·07 | 48·6 ± 0·6 | 44·7 ± 0·3 |
| Fruiting | 2·40 ± 0·11 | 1·58 ± 0·07 | 53·0 ± 0·8 | 48·1 ± 0·6 | 0·43 ± 0·04 | 0·28 ± 0·05 | 48·8 ± 0·3 | 45·8 ± 0·8 |
| Elevated CO2 | ||||||||
| Non-fruiting | 2·56 ± 0·08 | 2·19 ± 0·13 | 52·0 ± 0·4 | 49·6 ± 0·4 | 0·83 ± 0·05 | 0·72 ± 0·06 | 48·6 ± 0·4 | 45·5 ± 0·8 |
| Fruiting | 2·13 ± 0·20 | 1·65 ± 0·25 | 48·9 ± 2·4 | 51·4 ± 0·6 | 0·42 ± 0·03 | 0·56 ± 0·10 | 47·9 ± 1·1 | 46·3 ± 0·2 |
Values shown are the means ± s.e. from four trees exposed to ambient CO2 and three trees previously exposed to elevated CO2. The results of the statistical analyses are shown in Table 5.
Table 5.
Summary statistics for the type III test of fixed effects (Wald-type test) and the estimated random effects from individuals in the generalized linear mixed model for the data in Table 4
| N (%) |
C (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue | Fixed effect | d.f. | den d.f. | F | P | Random effect | d.f. | den d.f. | F | P | Random effect |
| Leaf | Fruiting | 1 | 16 | 35·19 | < 0·0001 | 0·0517 | 1 | 21 | 0·32 | 0·576 | 0 |
| CO2 | 1 | 5 | 0·01 | 0·932 | 1 | 21 | 0·08 | 0·782 | |||
| Fruiting × CO2 | 1 | 16 | 1·53 | 0·235 | 1 | 21 | 0·03 | 0·867 | |||
| Date | 1 | 16 | 47·25 | < 0·0001 | 1 | 21 | 2·09 | 0·163 | |||
| Date × CO2 | 1 | 16 | 0·47 | 0·503 | 1 | 21 | 2·19 | 0·154 | |||
| Fruiting × date | 1 | 16 | 8·99 | 0·009 | 1 | 21 | 0·16 | 0·689 | |||
| Xylem | Fruiting | 1 | 16 | 36·73 | <0·0001 | 0·0151 | 1 | 16 | 0·67 | 0·424 | 0·0978 |
| CO2 | 1 | 5 | 1·60 | 0·261 | 1 | 5 | 0·02 | 0·894 | |||
| Fruiting × CO2 | 1 | 16 | 0·01 | 0·942 | 1 | 16 | 0·55 | 0·468 | |||
| Date | 1 | 16 | 0·78 | 0·390 | 1 | 16 | 48·87 | <0·0001 | |||
| Date × CO2 | 1 | 16 | 1·52 | 0·236 | 1 | 16 | 1·58 | 0·227 | |||
| Fruiting × date | 1 | 16 | 0·28 | 0·605 | 1 | 16 | 2·15 | 0·162 | |||
The degrees of freedom of the denominator (den d.f.) were approximated using Kenward and Roger's method.
Significant values are highlighted in bold.
Shoot traits
Both length and biomass of terminal shoots increased less when fruiting occurred, with significantly longer shoots in the non-masting year 2008 than in the two masting years 2007 and 2009 (Fig. 3, Table 1). The length of terminal shoots was not affected by the CO2 treatment irrespective of masting. In contrast, the biomass of terminal shoots was marginally affected by elevated CO2, with about half the biomass in the masting year 2007 compared with the non-masting year 2008 for trees at ambient CO2 but not at elevated CO2. However, since the biomass of an individual shoot depends on its length, and shoot length was affected by fruiting, CO2 effects on biomass increment were also analysed with respect to biomass per length. There was a significant interaction between CO2 enrichment and fruiting when the biomass per length of terminal shoots was considered.
Fig. 3.
Comparison of (A) length, (B) dry mass and (C) dry mass per length of terminal shoots of Fagus sylvatica trees (means ± s.e.) exposed to ambient (n = 4) and elevated (n = 3) CO2 concentrations, as indicated. Masting occurred in 2007 and 2009, whereas 2008 was a non-masting year. The results of the statistical analyses are shown in Table 1.
As with the leaves, N concentrations in the xylem of 1- to 3-year-old branches were significantly smaller for fruiting than non-fruiting shoots in trees exposed to both ambient and elevated CO2, while they were unaffected by CO2 treatment (Tables 4 and 5). In contrast, total C concentrations were unaffected by shoot type or CO2 treatment.
DISCUSSION
In our investigations of individual shoots, fruit-bearing shoots had more leaves with a larger surface area and greater LMA than non-fruiting shoots. These results suggest that fruits develop preferentially on shoots that can provide sufficient assimilates from current photosynthesis for seed production. This is in agreement with the hypothesis of carbon autonomy for fruiting of branchlets, as had already been suggested in an experimental study at the SCC site (Hoch, 2005). However, in contrast to the current study, numbers of leaves per shoot did not differ between fruiting and non-fruiting shoots in two other masting temperate tree species: Styrax obassia and Fagus crenata (Miyazaki et al., 2002; Han et al., 2008). These differences in results may be because a resource is autonomic at a different size or age of branchlets among different species, which may also depend on nutrient availability which would depend on the sites. In our study the differences in reproductive efforts between masting years are mainly controlled by the proportion of fruit-bearing shoots within the whole crown, but not by the number of fruits per fruiting shoot. This corroborates previous findings, which showed that cupules are more abundant on upper and/or outer branches in a moderate masting season, while they occur within the entire crown in a very heavy masting year (Hilton and Packham, 1997).
Competition between reproduction and vegetative growth in shoots
The numbers of leaves per shoot in masting years was smaller than in non-masting years, a relationship that also occurs in F. crenata (Han et al., 2008). The length of terminal shoots was also shorter in the masting years than in the non-masting year in trees grown in both ambient and elevated CO2. This might indicate that vegetative growth is suppressed by the high sink strength of reproductive structures. Biomass increase for an individual shoot results from both elongation and radial growth. Therefore, the ratio of biomass to shoot length is a proxy for the radial growth of a shoot, excluding its shortened length in masting years. If radial growth of shoots is the same in different years, one would expect that the same mass is added to terminal shoots each year for a given shoot length. However, dry mass per length of terminal shoots in trees at ambient CO2 was reduced in masting years (see Fig. 3C). Fagus is considered to be a flush-type genus, so most of the shoot elongation is completed early in the growing season, while shoot radial growth continues until late into the season (Kikuzawa, 2003). A previous study demonstrated that, in a masting year, non-structural carbohydrate in branches was significantly reduced during mid-season in F. sylvatica (Hoch et al., 2003), probably indicating an increased demand for carbon for fruiting, placing constraints on individual branches.
The N concentrations in dry matter of leaves were significantly smaller in fruiting shoots than in non-fruiting shoots in September. Moreover, between the two occasions, leaf N decreased more in fruiting than in non-fruiting shoots. These results suggest that the reduction in N concentration was not caused by leaf senescence, but by the high N sink strength resulting from seed production. Neighbouring foliage might serve as an N source for reproductive tissues, as has been demonstrated in Pseudotsuga menziesii and S. obassia (McDowell et al., 2000; Miyazaki et al., 2002). In addition, xylem N concentration of fruiting shoots was distinctly smaller than in non-fruiting shoots, implying that N for fruiting is supplied from the fruiting branchlets, but not from neighbouring non-fruiting parts of the crown.
CO2 effects on reproduction–growth trade-offs
In contrast to trees growing at ambient CO2, there was no decrease in dry mass per length of terminal shoots in the 2007 masting year compared with the 2008 non-masting year in trees exposed to elevated CO2. It is likely that enhanced photosynthesis at elevated CO2 concentrations (Zotz et al., 2005; Bader et al., 2010) alleviated the competition between seed production and shoot growth, of which the former is the strongest sink for newly produced photoassimilates in many species such as herbs and trees (Wardlaw, 1990; Hoch and Keel, 2006; Kudo and Ida, 2010). In contrast, CO2 enrichment had no effect on the numbers of leaves or fruits per shoot, which is consistent with previous results from the same site based on litter trap collections (Körner et al., 2005). In addition, no significant increase in tree-ring increment of F. sylvatica has been recorded under elevated CO2 at the SCC site (Körner et al., 2005; Asshoff et al., 2006). Our findings for small branchlets support the hypothesis of module specialization and physiological integration for reproductive events (Obeso, 2002): resource allocation within branchlets might respond to local autonomy and trade-offs (e.g. leaf number, shoot growth, fruiting effort and seed production), but is probably integrated at individual trees (e.g. degree of fruit loading or tree-ring growth).
It has been reported that the average frequency of masting in F. sylvatica has increased during the last 30 years (Hilton and Packham, 1997; Övergaard et al., 2007). Moreover, masting of F. sylvatica in two consecutive years, which was considered impossible in older literature, has recently been recorded by litter trap collections (Hilton and Packham, 1997; Körner et al., 2005; Övergaard et al., 2007). In the current study, both C and N resources were found to be autonomous within small branchlets, and elevated CO2 alleviated the competition for C between reproduction and vegetative growth, as indicated by some leaf and shoot traits. If resource balance controls masting in F. sylvatica, the ongoing rising atmospheric CO2 concentrations and N deposition may already have led to a higher frequency of masting events as observed over recent decades.
In conclusion, when masting in F. sylvatica occurred there were fewer leaves per shoot, and the increase in elongation and biomass of shoots was decreased, indicating competition for resources between the development of reproductive structures and growth within the shoot; the effect of elevated CO2 was to lessen the decrease in biomass of shoots caused by masting, so the competition may be ameliorated in the future as a result of rising atmospheric CO2 concentrations.
ACKNOWLEDGEMENTS
The authors thank Olivier Bignucolo for the CN analysis, Erwin Amstutz for operating the crane, and Christian Körner for his scientific advice. The authors thank the editor David Lawlor and the two anonymous reviewers for their valuable comments. All statistics were performed with SAS/STAT and the assistance of the Computer Center for Agriculture, Forestry and Fisheries Research, MAFFIN, Japan. CO2 enrichment and the crane infrastructure at the SCC site were funded via a grant to Ch. Körner from the Swiss National Science Foundation (SNF) and NCCR-Climate. This study was partly supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (nos 18580155 and 21380103 to Q.H.) and a research fellowship to Q.H. from the Co-operative Research Programme of the Organisation for Economic Co-operation and Development (OECD).
LITERATURE CITED
- Asshoff R, Zotz G, Körner C. Growth and phenology of mature temperate forest trees in elevated CO2. Global Change Biology. 2006;12:848–861. [Google Scholar]
- Bader M, Siegwolf R, Körner C. Sustained enhancement of photosynthesis in mature deciduous forest trees after 8 years of free air CO2 enrichment. Planta. 2010;232:1115–1125. doi: 10.1007/s00425-010-1240-8. [DOI] [PubMed] [Google Scholar]
- Crone EE, Miller E, Sala A. How do plants know when other plants are flowering? Resource depletion, pollen limitation and mast-seeding in a perennial wildflower. Ecology Letters. 2009;12:1119–1126. doi: 10.1111/j.1461-0248.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- Eschrich W, Burchardt R, Essiamah S. The induction of sun and shade leaves of the European beech (Fagus sylvatica L.): anatomical studies. Trees. 1989;3:1–10. [Google Scholar]
- Genet H, Bréda N, Dufrêne E. Age-related variation in carbon allocation at tree and stand scales in beech (Fagus sylvatica L.) and sessile oak (Quercus petraea (Matt.) Liebl.) using a chronosequence approach. Tree Physiology. 2010;30:177–192. doi: 10.1093/treephys/tpp105. [DOI] [PubMed] [Google Scholar]
- Han Q, Kabeya D, Iio A, Kakubari Y. Masting in Fagus crenata and its influence on the nitrogen content and dry mass of winter buds. Tree Physiology. 2008;28:1269–1276. doi: 10.1093/treephys/28.8.1269. [DOI] [PubMed] [Google Scholar]
- Hilton GM, Packham JR. A sixteen-year record of regional and temporal variation in the fruiting of beech (Fagus sylvatica L.) in England (1980–1995) Forestry. 1997;70:7–16. [Google Scholar]
- Hilton GM, Packham JR. Variation in the masting of common beech (Fagus sylvatica L.) in northern Europe over two centuries (1800–2001) Forestry. 2003;76:319–328. [Google Scholar]
- Hoch G. Fruit-bearing branchlets are carbon autonomous in mature broad-leaved temperate forest trees. Plant, Cell and Environment. 2005;28:651–659. [Google Scholar]
- Hoch G, Keel SG. 13C labelling reveals different contributions of photoassimilates from infructescences for fruiting in two temperate forest tree species. Plant Biology. 2006;8:606–614. doi: 10.1055/s-2006-924279. [DOI] [PubMed] [Google Scholar]
- Hoch G, Richter A, Körner C. Non-structural carbon compounds in temperate forest trees. Plant, Cell and Environment. 2003;26:1067–1081. [Google Scholar]
- Isagi Y, Sugimura K, Sumida A, Ito H. How does masting happen and synchronize? Journal of Theoretical Biology. 1997;187:231–239. [Google Scholar]
- Ishihara MI, Kikuzawa K. Annual and spatial variation in shoot demography associated with masting in Betula grossa: comparison between mature trees and saplings. Annals of Botany. 2009;104:1195–1205. doi: 10.1093/aob/mcp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Takeda H. Cost and probability of flowering at the shoot level in relation to variability in shoot size within the crown of Vaccinium hirtum (Ericaceae) New Phytologist. 2006;171:69–80. doi: 10.1111/j.1469-8137.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- Kelly D, Sork VL. Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics. 2002;33:427–447. [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- Kikuzawa K. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Functional Ecology. 2003;17:29–38. [Google Scholar]
- Körner C, Asshoff R, Bignucolo O, et al. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 2005;309:1360–1362. doi: 10.1126/science.1113977. [DOI] [PubMed] [Google Scholar]
- Kudo G, Ida TY. Carbon source for reproduction in a spring ephemeral herb, Corydalis ambigua (Papaveraceae) Functional Ecology. 2010;24:62–69. [Google Scholar]
- LaDeau SL, Clark JS. Rising CO2 levels and the fecundity of forest trees. Science. 2001;292:95–98. doi: 10.1126/science.1057547. [DOI] [PubMed] [Google Scholar]
- McDowell SCL, McDowell NG, Marshall JD, Hultine K. Carbon and nitrogen allocation to male and female reproduction in Rocky Mountain Douglas-fir (Pseudotsuga menziesii var. glauca, Pinaceae) American Journal of Botany. 2000;87:539–546. [PubMed] [Google Scholar]
- Miyazaki Y, Hiura T, Kato E, Funada R. Allocation of resources to reproduction in Styrax obassia in a masting year. Annals of Botany. 2002;89:767–772. doi: 10.1093/aob/mcf107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JR. Costs of reproduction in Ilex aquifolium: effects at tree, branch and leaf levels. Journal of Ecology. 1997;85:159–166. [Google Scholar]
- Obeso JR. The costs of reproduction in plants. New Phytologist. 2002;155:321–348. doi: 10.1046/j.1469-8137.2002.00477.x. [DOI] [PubMed] [Google Scholar]
- Övergaard R, Gemmel P, Karlsson M. Effects of weather conditions on mast year frequency in beech (Fagus sylvatica L.) in Sweden. Forestry. 2007;80:555–565. [Google Scholar]
- Pepin S, Körner C. Web-FACE: a new canopy free-air CO2 enrichment system for tall trees in mature forests. Oecologia. 2002;133:1–9. doi: 10.1007/s00442-002-1008-3. [DOI] [PubMed] [Google Scholar]
- Piovesan G, Adams JM. The evolutionary ecology of masting: does the environmental prediction hypothesis also have a role in mesic temperate forests? Ecological Research. 2005;20:739–743. [Google Scholar]
- Richardson SJ, Allen RB, Whitehead D, Carswell FE, Ruscoe WA, Platt KH. Climate and net carbon availability determine temporal patterns of seed production by Nothofagus. Ecology. 2005;86:972–981. [Google Scholar]
- Satake A, Iwasa Y. Pollen coupling of forest trees: forming synchronized and periodic reproduction out of chaos. Journal of Theoretical Biology. 2000;203:63–84. doi: 10.1006/jtbi.1999.1066. [DOI] [PubMed] [Google Scholar]
- Shibata M, Tanaka H, Nakashizuka T. Cause and consequences of mast seed production of four co-occurring Carpinus species in Japan. Ecology. 1998;79:54–64. [Google Scholar]
- Stiling P, Moon D, Hymus G, Drake B. Differential effects of elevated CO2 on acorn density, weight, germination, and predation among three oak species in a scrub-oak forest. Global Change Biology. 2004;10:228–232. [Google Scholar]
- Suzuki A. Resource allocation to vegetative growth and reproduction at shoot level in Eurya japonica (Theaceae): a hierarchical investment? New Phytologist. 2001;152:307–312. [Google Scholar]
- Wardlaw IF. Tansley Review No. 27. The control of carbon partitioning in plants. New Phytologist. 1990;116:341–381. doi: 10.1111/j.1469-8137.1990.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Way DA, Ladeau SL, McCarthy HR, et al. Greater seed production in elevated CO2 is not accompanied by reduced seed quality in Pinus taeda L. Global Change Biology. 2010;16:1046–1056. [Google Scholar]
- Zotz G, Pepin S, Körner C. No down-regulation of leaf photosynthesis in mature forest trees after three years of exposure to elevated CO2. Plant Biology. 2005;7:369–374. doi: 10.1055/s-2005-837635. [DOI] [PubMed] [Google Scholar]