Abstract
Rhinoscleroma is a chronic granulomatous infection of the upper airways caused by the bacterium Klebsiella pneumoniae subsp. rhinoscleromatis. The disease is endemic in tropical and subtropical areas, but its diagnosis remains difficult. As a consequence, and despite available antibiotherapy, some patients evolve advanced stages that can lead to disfiguration, severe respiratory impairment and death by anoxia. Because identification of the etiologic agent is crucial for the definitive diagnosis of the disease, the aim of this study was to develop two simple PCR assays. We took advantage of the fact that all Klebsiella pneumoniae subsp. rhinoscleromatis isolates are (i) of capsular serotype K3; and (ii) belong to a single clone with diagnostic single nucleotide polymorphisms (SNP). The complete sequence of the genomic region comprising the capsular polysaccharide synthesis (cps) gene cluster was determined. Putative functions of the 21 genes identified were consistent with the structure of the K3 antigen. The K3-specific sequence of gene Kr11509 (wzy) was exploited to set up a PCR test, which was positive for 40 K3 strains but negative when assayed on the 76 other Klebsiella capsular types. Further, to discriminate Klebsiella pneumoniae subsp. rhinoscleromatis from other K3 Klebsiella strains, a specific PCR assay was developed based on diagnostic SNPs in the phosphate porin gene phoE. This work provides rapid and simple molecular tools to confirm the diagnostic of rhinoscleroma, which should improve patient care as well as knowledge on the prevalence and epidemiology of rhinoscleroma.
Author Summary
In humans, the bacterium Klebsiella pneumoniae subsp. rhinoscleromatis (also called clone Rhinoscleromatis, as it evolved from a single Klebsiella pneumoniae ancestral strain) causes rhinoscleroma, a chronic infection of the nose and throat. Identification of the bacterium from biopsies or nasal secretions is essential for diagnosis, and currently relies on the biochemical characteristics of clone Rhinoscleromatis and on detection of its capsule of antigenic type K3. Our aim was to develop two identification tests based on amplification by polymerase chain reaction (PCR) of specific portions of the genome of clone Rhinoscleromatis. We established the sequence of the capsular polysaccharide synthesis cluster and identified one gene sequence that was unique to K3 strains. A PCR test that targets this gene was shown to be specific for K3 strains. We also exploited unique DNA signatures of clone Rhinoscleromatis to develop a second PCR test, which is specific for this clone, thus allowing distinction from other K. pneumoniae K3 strains. These novel and simple identification tests should help to diagnose rhinoscleroma and to understand the epidemiology of this disease.
Introduction
Rhinoscleroma is a chronic granulomatous infection of the nose and upper airways of humans. The relative rarity of the disease and low specificity of early symptoms make the clinical diagnosis of rhinoscleroma difficult [1]. As a consequence, some patients evolve advanced stages that can lead to severe respiratory impairment [2]. The most characteristic feature of the disease is the observation in biopsies of big foamy cells, called Mikulicz cells, in which bacilli are seen after hematoxilin-eosin staining [3]. Hence, the specific diagnosis of rhinoscleroma currently relies on the combination of histological and bacteriological examinations, with the presence of Mikulicz cells and the identification of the causative agent, the bacterium Klebsiella pneumoniae subsp. rhinoscleromatis [4] from tissue culture, nasal swabs or blood culture [4], [5].
Initially, species status (Klebsiella rhinoscleromatis) was given to the agent of rhinoscleroma. However, given high similarity based on DNA-DNA hybridization [6], it is now regarded as a subspecies of Klebsiella pneumoniae [7]. Multiple gene sequencing confirmed the close relatedness of the agent of rhinoscleroma with K. pneumoniae [8]–[10]. Currently, the definitive identification of the organism is difficult. Different from K. pneumoniae sensu stricto (i.e., K. pneumoniae subsp. pneumoniae), which can be encountered in many hosts, environments and clinical sources [11], K. pneumoniae subsp. rhinoscleromatis is strictly associated with humans and has, to our knowledge, only been isolated from cases of rhinoscleroma. As K. pneumoniae subsp. rhinoscleromatis is not considered as a commensal of the respiratory tract, its identification is key to the definitive diagnosis of the disease [12], [13]. This infectious agent is metabolically less versatile than other K. pneumoniae strains, but definitive identification based on biochemical properties (ONPG negative, no acid production from lactose, urease, LDC and citrate negative) is rendered difficult due to variation in metabolic characteristics of K. pneumoniae strains [9], [11]. Furthermore, members of a third subspecies, K. pneumoniae subsp. ozaenae, which are associated with an atrophic rhinitis known as ozaena, also have reduced metabolic capacities [8].
Klebsiella strains are typically surrounded by a polysaccharidic capsule of considerable thickness that is responsible for the mucoid aspect of colonies. Klebsiella capsular serotyping (K typing) distinguishes at least 77 K types [7], [14]. As all strains of K. pneumoniae subsp. rhinoscleromatis are of capsular type 3 (K3), K typing is used for confirmatory identification of K. pneumoniae subsp. rhinoscleromatis and allows distinction from K. pneumoniae subsp. ozaenae strains, which are mostly of type K4 and more rarely of types K5 and K6 [7]. However, K typing is technically cumbersome and a small proportion of K. pneumoniae subsp. pneumoniae strains (including the type strain CIP 82.91T = ATCC 13883T) are of type K3 as well, meaning that capsular typing alone cannot provide a definitive identification of K. pneumoniae subsp. rhinoscleromatis. Restriction fragment length polymorphism (RFLP) of the amplified capsular polysaccharide synthesis (cps) region [15] distinguished, among strains of the K3 capsular type, four distinct banding patterns (C-patterns C3a to C3d) that differ by one or a few DNA fragments [8]. All K. pneumoniae subsp. rhinoscleromatis isolates have C-pattern C3a, but some K. pneumoniae subsp. pneumoniae isolates also have this pattern, thus impairing the use of this method to confirm identification of the agent of rhinoscleroma.
Our recent population genetics study [8] showed that isolates of K. pneumoniae subsp. rhinoscleromatis are genetically homogeneous and form a unique clonal family (clonal complex CC67) that can be distinguished, based on the sequence of housekeeping genes, from all other K. pneumoniae members, including K. pneumoniae subsp. ozaenae. Hence, these isolates can be regarded as members of a clone that evolved recently from its ancestor, and we hereafter use the designation clone Rhinoscleromatis for this recent phylogenetic entity, as this clone comprises all studied isolates that belong to K. pneumoniae subsp. rhinoscleromatis, and only these isolates. The loss of metabolic abilities by clone Rhinoscleromatis was proposed to reflect its ecological specialization to the human respiratory tract [8]. Based on multilocus sequence typing (MLST), K. pneumoniae subsp. pneumoniae strains of the capsular type K3 are genetically heterogeneous but, importantly, all are clearly distinct from clone Rhinoscleromatis. Indeed, the presence of capsular type K3 in different genetic backgrounds can be explained by past events of horizontal transfer of the cps region [8]. Interestingly, two synonymous SNPs located at positions 48 and 216 on the phosphoprotein gene phoE, were observed in all isolates of clone Rhinoscleromatis, but in no other K. pneumoniae strain. Phosphoporin E is an outer membrane pore protein with a recognition site for negatively charged compounds, the expression of which is induced under phosphate limitation.
The aims of this study were (1) To characterize the structure and gene content of the Klebsiella K3 cps cluster and to use its unique features to set up a specific PCR assay for K3 strains; and (2) To develop a specific molecular assay to identify members of clone Rhinoscleromatis and distinguish them from other K. pneumoniae strains, including those of capsular type K3.
Methods
Identification and sequencing of strain SB3432
The clone Rhinoscleromatis strain SB3432 was isolated in 2004 in the Avicenne hospital, Bobigny, France from a biopsy of the left nasal cavity of an 11-year old patient diagnosed with rhinoscleroma. Identification was initially achieved using API NE and API20E strips (BioMerieux, Marcy-l'Etoile, France), and by biochemical characteristics [11]. MLST [16] confirmed this strain to be 100% identical at seven housekeeping genes to the type strain of K. pneumoniae subsp. rhinoscleromatis CIP 52.210T ( = ATCC 13884T). The cps PCR-RFLP pattern was determined as C3a by the molecular serotyping method [15]. In the frame of the complete genome sequencing of Rhinoscleromatis strain SB3432, the cps cluster sequence was determined. The K3 cps cluster sequence was deposited in GenBank/EMBL/DDBJ databases under accession number FQ311478.
Annotation of the K3 cps cluster
Open reading frames (ORFs) encoding proteins of ≥40 amino acids were evaluated for coding potential using the Genemark program [17]. Other criteria included similarity to other Klebsiella and gene overlaps were used to propose a start codon. Homology searches were also conducted using TBLASTX [18] on Uniprot [19] and COG dababases [20]. Finally, each putative CDS was curated manually through visual inspection within the CAAT-Box programme [21]. Topology prediction of membrane proteins was achieved with the TopPred program [22].
Comparison of cps clusters
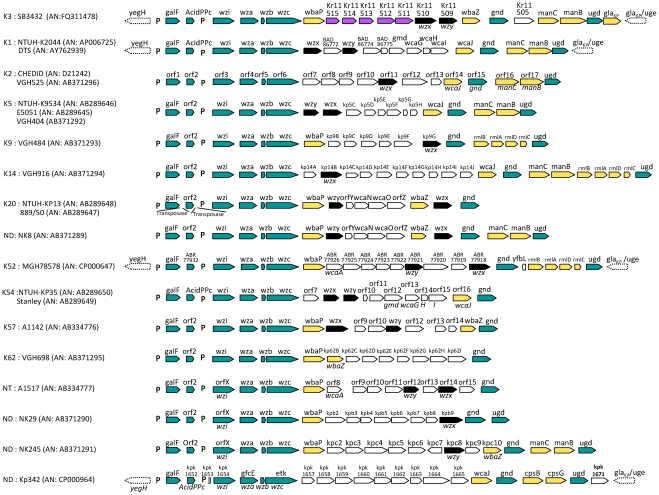
In order to compare the cps region encoding a capsular type K3 with previously sequenced cps regions of K. pneumoniae, all genes from galF (UTP-glucose-1-phosphate uridylyltransferase) to glaKP (homonym of uge [23], [24], encoding an UDP-galacturonic acid C4-epimerase) were submitted to pairwise comparison using clustalW2 algorithm (http://www.ebi.ac.uk/Tools/clustalw2/index.html). Previously published cps regions of Klebsiella strains were used: serotype K1 (strains NTUH-K2044 [25] and DTS [26]), K2 (strains CHEDID [27] and VGH525 [28]), K5 (strains NTUH-K9534, E5051 [29] and VGH404 [28]), K9 (strain VGH484 [28]), K14 (strain VGH916 [28]), K20 (strains NTUH-KP13 and 889/50 [29]), K52 (strain MGH78578), K54 (strain NTUH-KP35 [29]), K57 (strain A1142 [30]), K62 (strain VGH698 [28]) as well as a non-typeable strain (A1517 [30]) and four strains with undetermined serotype (NK8, NK29, NK245 [28] and Kp342 [31]). Accession numbers are given on Figure 1 .
Figure 1. Gene composition of the cps region of Klebsiella capsular serotype K3 and comparison with other cps regions.
The comparative strains represent 11 serotypes (K1, K2, K3, K5, K9, K14, K20, K52, K54, K57 and K62), one new serotype (NT) and four undetermined serotypes (ND). Arrows with dotted lines belong to flanking regions of the cps cluster. Green arrows represent highly conserved genes present in all cps regions. Yellow arrows correspond to genes of the K3 cps with highly conserved sequences, but which presence/absence depends on capsular type. Serotype K3 genes having weaker or no homology with the other Klebsiella cps regions are represented by purple arrows. Black arrows correspond to genes wzx and wzy, which are highly variable at the sequence level. In the K3 cps region, the suggested names for the ORFs in green and yellow are based on amino-acid homology superior to 78%, with at least one annotated gene from another Klebsiella cps regions. For the previously published cps regions, names indicated above each gene correspond to annotations found in public databases, whereas names in italic under the genes are suggestions based on our pairwise comparisons (>65% amino acid identity except for wzx and wzy). The suggested wzx and wzy annotations are based on 30% to 48% and 23% to 26% of amino-acid similarity on most of their sequence length with the respective proteins of E. coli. As noted earlier [28], the presence of genes wbaP and wcaJ appeared mutually exclusive. P: promoter [27]; AN: Accession number.
PCR assay for K3 Klebsiella strains
Primers Kr11509-F (5′- TAG GCA ATT GAC TTT AGG TG - 3′) and Kr11509-R (5′- AGT GAA TCA GCC TTC ACC T - 3′) were designed inside the sequence of ORF Kr11509 (wzy) to allow PCR amplification of a 549 bp fragment. The PCR mix contained 0.85 U of Taq polymerase (Invitrogen), 100 µM of each deoxynucleoside triphosphate, 0.2 µM of each primer, 40 ng of DNA, 1.5 mM MgCl2, 20 mM Tris-Hcl (pH 8.4) and 50 mM KCl in a final volume of 50 µl. Samples were submitted to an initial denaturation step (30 sec at 94°C), followed by 35 amplification cycles (30 sec at 94°C, 30 sec at 52°C, 30 sec at 72°C) and a final elongation step (5 min at 72°C). A MasterCycler ep Gradient S thermocycler (Eppendorf) was used. This K3 PCR assay was tested on 164 Klebsiella strains from a previous study [8], including 16 clone Rhinoscleromatis isolates and 14 K3 K. pneumoniae subsp. pneumoniae strains (Table S1). As a control that DNA was PCR-amplifiable, rpoB (RNA polymerase beta-subunit) PCR was performed as described previously [16]. Water was used as template for negative controls.
phoE PCR assay for clone Rhinocleromatis
Primer pair phoE-rhiF (5′-GCG GCA GCG ACT TCG CCG TA-3′) and phoE-rhiR (5′-GTT CTG CGC TTT GTT GGC AAA C-3′) were designed so that their 3′ base corresponded to the two Rhinoscleromatis-specific SNP positions. PCR mix composition and the thermocycler used were identical to those used for K3 PCR. Samples were submitted to an initial denaturation step (30 sec at 94°C), followed by 35 amplification cycles (30 sec at 94°C, 30 sec at 58°C, 30 sec at 72°C) and a final elongation step (5 min at 72°C). This PCR assay was tested on 42 Klebsiella strains, including 16 clone Rhinoscleromatis isolates, 20 K. pneumoniae subsp. pneumoniae strains, five K. pneumoniae subsp. ozaenae strains and one K. planticola (Table S1). rpoB PCR was used as positive control, while water was used for negative controls.
Animal infections
Female Balb/cJ mice were used at the age of 6–8 weeks and housed in dedicated biosafety level 2 animal facilities of Institut Pasteur. Mice received food and water ad libidum. All animal experiments were carried according to the French national guidelines for animal experiments and were approved by the Animal Care Committee of the Institut Pasteur with the agreement number 05-59. Mice were infected by intra-nasal inoculation of 2.106 or 2.107 K. pneumoniae subsp. rhinoscleromatis strain SB3432. The control group was inoculated with the same volume of saline water. Five days after infection, lungs were surgically removed for bacterial load quantification and total DNA extraction. Briefly, lungs were collected and homogenized mechanically in 3 ml of ice-cold Hepes buffer supplemented with 0.2 mM EDTA and 0.1% bovine serum albumin. Bacterial counts were determined as colony forming units (CFU) by plating serial dilutions of the lung extract. Total DNA was extracted using a Genomic DNA Isolation NucleoBond kit (Macherey-Nagel), following the manufacturer's instructions.
Results
1. K3 cps operon structure and identification of a K3-specific gene sequence
We determined the complete sequence of the cps region of the capsular type K3 K. pneumoniae subsp. rhinoscleromatis strain SB3432. The 21 ORFs that are likely to represent coding sequences in the region between galF and glaKP were compared with the cps region of the other Klebsiella capsular types sequenced to date ( Table 1 , Figure 1 ) [25]–[31]. Eight genes (galF, acid phosphatase gene, wzi, wza, wzb, wzc, gnd and ugd; green arrows in Figure 1 ) were highly conserved, with homologs in all Klebsiella cps regions and in the group 1 capsule locus of E. coli [32]. The JUMPstart (for “just upstream of many polysaccharide starts”) element, involved in transcriptional antitermination of the cps cluster [33], is present upstream of gene wzi. In all strains for which the sequence downstream of gnd was available, gene ugd was found and considered to be the last gene of the cps cluster [28], [29]. In the three fully sequenced strains NTUH-K2044, MGH78578 and Kp342, ugd is followed by gene glaKP ( Figure 1 ), also known as uge [23] and involved in galacturonic acid synthesis [24]. This gene is in the opposite direction and considered to be part of the cluster for synthesis of the LPS core [24], [34], [35]. In the K3 cps cluster, this glaKP gene is preceded by another, face-to-face copy of glaKP ( Figure 1 ). Because the K3 capsular polysaccharide contains a galacturonic acid, which is rare among known Klebsiella polysaccharide structures [7], the forward glaKP copy might represent the last gene of the K3 cps cluster.
Table 1. Composition of the capsular type K3 cps cluster.
| Putative function | Gene | Gene name/annotation | Best BLAST hit | Amino-acid identity | Species (serotype/strain name) 1 |
| Polymerization and surface assembly | wzi | Outer membrane protein | wzi | 99% | Kp (K20, K52, K57, NK8, NK29, NK245) |
| Polymerization and surface assembly | wza | Multimeric putative translocation channel | wza | 92% | Kp (all serotypes except K2 and NK8) |
| Polymerization and surface assembly | wzb | Protein tyrosine phosphatase | wzb | 78% | E. coli (K30), Kp (K20, NK8) |
| Polymerization and surface assembly | wzc | Inner membrane tyrosine autokinase | wzc | 81% | E. coli (K30), Kp (K20, NK8) |
| Polymerization and surface assembly | Kr11510 | Putative flippase wzx | wzx | 25% | Streptococcus pneumoniae (23% on E. coli wzx; 24% on K57 wzx) |
| Polymerization and surface assembly | Kr11509 | Putative polymerase wzy | wzy | 15% | Kp (K20, K54, K57) |
| D-galactose synthesis | galF | UTP-glucose-1-phosphate uridylyltransferase | galF | 100% | Kp (K1, K52) |
| Unknown | AcidPPC | acid phosphatase homolog | AcidPPc | 100% | Kp (K14) |
| Unknown | gnd | gluconate-6-phosphate dehydrogenase | gnd | 99% | Kp (K14, K54) |
| Synthesis of UDP-glucuronic acid from UDP-glucose | ugd | UDP-glucose 6-dehydrogenase | ugd | 100% | Kp (K9, NK245, Kp342), E. coli |
| Transfer of D-galactose on undecaprenyl-phosphate | wbaP | Gal::undecaprenolphosphate Gal-1-P transferase | wbaP | 84% | E. coli K30, Kp (K20, NK8) |
| Transfer of the first mannose residue on the galactose residue | wbaZ | Mannosyl transferase | wbaZ | 76% | Kp (K57) |
| Synthesis of GDP-mannose from mannose-1-phosphate | manC | GDP-mannose phosphorylase | manC/cpsB | 99% | Kp (K2, K14, NK245, Kp342) |
| Synthesis of mannose-1-phosphate from mannose-6-phosphate | manB | Phosphomannomutase | manB/cpsG | 99% | Kp (K1, K2, K5, K14, K62, NK245, NK8, Kp342) |
| Synthesis of UDP-galacturonic acid from UDP-glucuronic acid | glaKp/uge | UDP-galacturonic C4-epimerase | uge | 86% | Kp (Kp342) |
| Transfer of the second and third mannose residue | Kr11515 | Putative mannosyl transferase | Mannosyl transferase | 47% | Salmonella enterica subsp. enterica |
| Unknown | Kr11511 | Putative mannosyl transferase | Mannosyl transferase | 39% | Bacteroides fragilis |
| Transfer of the UDP-galacturonic acid on the second mannose residue | Kr11513 | Putative group 1 glycosyl transferase | Group 1 glysosyl transferase | 55% | Serratia proteamaculans |
| Unknown | Kr11514 Kr11512 | Hypothetical protein | Hypothetical protein | 47% | Serratia proteamaculans |
| Transposition of mobile genetic element | Kr11505 | Transposase | Transposase | 98% | E. coli |
Kp, Klebsiella pneumoniae.
Four other genes of the K3 cps region (wbaP, wbaZ, manB and manC; Figure 1 , yellow arrows) were highly similar to genes in other cps clusters ( Table 1 ), but their presence depended on K type ( Figure 1 ). Further, five genes of the K3 cps cluster (Kr11511 to Kr11515; Figure 1 , purple arrows) had weaker but nevertheless significant homology ( Table 1 ) with genes previously described in Klebsiella cps clusters, or with genes in other species. The K3 cps cluster also encodes a putative transposase (Kr11505), located between gnd and manC but not found in the other cps regions.
Finally, the sequence of both wzx (flippase) and wzy (polymerase) genes, necessary for polysaccharide synthesis in E. coli, is known to be highly variable among capsular types, rendering their identification problematic [28], [34]. The ∼25% identity with Wzx in different species might indicate that ORF Kr11510 corresponds to the flippase gene ( Table 1 ). Likewise, ORF Kr11509 showed 9.9% to 15.8% similarity with Wzy proteins in other Klebsiella cps clusters, which is the range of the similarity among other Klebsiella Wzy proteins (9.2% to 19%). Moreover, topology prediction revealed that Kr11509 encodes a protein with eleven transmembrane domains, which is typical for Wzy sugar polymerases. As no other gene in the cps operon or elsewhere in the genome presented homology with wzy, we hypothesize that ORF Kr11509 encodes the K3 capsule polymerase.
2. Development of a PCR assay specific for K3 Klebsiella strains
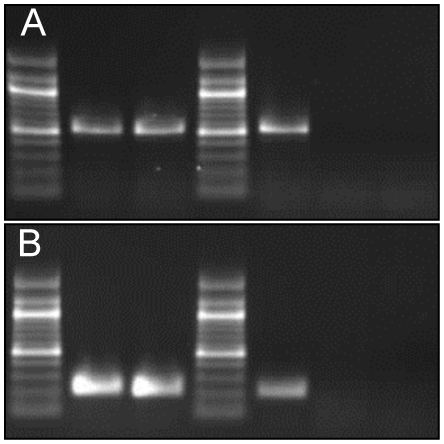
In order to develop a K3-specific PCR test, a pair of PCR primers (Kr11509-F and Kr11509-R) was designed to amplify a region of ORF Kr11509 (wzy). The PCR was assayed on 16 isolates of clone Rhinoscleromatis (all being K3), 14 K3 K. pneumoniae subsp. pneumoniae strains of C-patterns C3a to C3d, and 134 Klebsiella strains that included reference strains of the 76 other K types (Table S1). PCR amplification was positive in all K3 strains (30/30, 100%), and negative for all other strains (Table S1 and Figure 2A ). The rpoB PCR, used as positive control, was positive in all strains, while water controls were PCR negative. Therefore, this PCR assay was specific for K3 strains.
Figure 2. K3 and phoE PCR assays.
(A) Kr11509 (wzy) PCR amplification, which is specific for serotype K3 Klebsiella isolates. Serotype K3 isolates of K. pneumoniae subsp. pneumoniae (lane 2: strain SB3204 = CIP 52.146; lane 3: strain SB3206 = CIP 82.91T) and subsp. rhinoscleromatis (lane 5: strain SB3432) showed the expected PCR product of 549 bp. This result is representative of the 16 clone Rhinoscleromatis isolates and the 14 K3 K. pneumoniae subsp. pneumoniae strains tested in this study (Table S1). No PCR amplification was obtained with the 134 non-K3 Klebsiella isolates tested (Table S1) representing the 76 other serotypes, as shown for serotype K2 (lane 6: strain SB3341 = CIP 52.145) and serotype K4 (lane 7: strain SB3220 = K. pneumoniae subsp. ozaenae CIP 52.211T). Lanes 1 and 4: 100 bp ladder (New England Biolabs). (B) phoE PCR amplification, which is specific for members of clone Rhinoscleromatis. PCR performed with strains belonging to clone Rhinoscleromatis (lane 2: strain SB167 = C5046; lane 3: strain SB1782 = K. pneumoniae subsp. rhinoscleromatis CIP 52.210T and lane 5: strain SB3432) show the expected PCR amplification product of 209 bp. This product was observed for the 16 clone Rhinoscleromatis isolates tested in this study. PCR with non-Rhinoscleromatis Klebsiella strains (Table S1), including strains of serotype K3 and belonging to K. pneumoniae subsp. ozaenae gave no amplification (lane 6: SB3206 = CIP 82.91T; lane 7: K. pneumoniae subsp. ozaenae SB3220 = CIP 52.211T). Lanes 1 and 4: 100-bp ladder (New England Biolabs).
3. Development of a PCR assay specific for clone Rhinoscleromatis
To develop a PCR assay that distinguishes clone Rhinoscleromatis from all other K. pneumoniae clones, MLST data [8] were analyzed in order to find diagnostic single nucleotide polymorphisms (SNP) for clone Rhinoscleromatis. Two SNPs were identified on the phosphoporin gene phoE: the cytosines in position 48 and 216 (relative to position 1 of the MLST template, see www.pasteur.fr/mlst) observed in all K. pneumoniae subspecies pneumoniae and K. pneumoniae subspecies ozaenae strains, were replaced by an adenine and a guanine, respectively, in clone Rhinoscleromatis. The two SNPs are synonymous and correspond to positions 609 and 777 of the phosphoprotein phoE gene on the complete genome sequence of strain NTUH-K2044 (Accession number AP006725.1). We sought to exploit these two diagnostic SNPs located 168 bp apart on gene phoE, by developing an allele-specific PCR assay. The resulting phoE PCR was assayed on the 16 clone Rhinoscleromatis isolates, on 14 K. pneumoniae subsp. pneumoniae K3 strains, as well as on five K. pneumoniae subsp ozaenae strains, on six K. pneumoniae subsp. pneumoniae K1 and K2 isolates and on one K. planticola (Table S1). The expected PCR product was observed only after amplification of DNA from strains that were determined to belong to clone Rhinoscleromatis based on MLST, while all other strains were negative (Table S1 and Figure 2B ). The rpoB control PCR was positive in all strains and water controls were PCR negative. Hence, the phoE PCR assay was specific for clone Rhinoscleromatis, and positive for all strains of this clone.
4. Specificity, sensitivity and direct detection from biological samples
To investigate the potential use of the two PCR assays for direct diagnosis of rhinoscleroma, we first checked their specificity towards different pathogens. Both K3 and phoE PCR assays were negative at various dilutions of total DNA extracts from the bacterial throat commensals and pathogens Neisseria meningitidis, Streptococcus pneumoniae, Streptococcus mitis, Streptococcus salivarius, Streptococcus mutans and Staphylococcus epidermidis, whereas control 16S rRNA gene PCR amplifications of these extracts were positive (not shown). The sensitivity of the two PCR assays was tested by serial dilutions. The lowest quantity of total DNA extract yielding a clearly visible PCR product on ethidium bromide-stained agarose gels was 0.1 ng (phoE PCR) or 1 ng (K3 PCR), corresponding to 2.105 and 2.106 bacterial chromosomes, respectively. We then controlled and observed that human DNA alone did not yield any PCR product, even with large template amounts. We also demonstrated the robustness of the PCR assays to the presence of human DNA, as amplification from the clone Rhinoscleromatis DNA was still positive in the presence of up to 10-fold (K3 PCR) and 1000-fold (phoE PCR) excess of human DNA. Finally, we tested direct detection of the rhinoscleroma bacillus from lungs of experimentally infected mice (Fevre et al., in preparation). Five days post-infection with 2.106 or 2.107 bacilli, the number of the infecting agents was quantified from lung extracts. There were 4.107 and 8.109 CFUs per lung, respectively. In the same samples, the two PCR assays were strongly positive from total lung DNA of infected mice at DNA amounts of 0.1 ng (phoE PCR) and 1 ng (K3 PCR) and above. As the total DNA amount of lungs carrying 8.109 cfu is 1.5 mg, this indicates that we can detect in vivo around 5.103 bacteria per lung.
Discussion
Rhinoscleroma remains difficult to diagnose [5], [36], [37]. The clinical symptoms depend on the region of the respiratory tract that is infected, and are often non-specific, mimicking those of other nasal disorders [37], [38]. Failure to diagnose rhinoscleroma is unfortunate, as treatments consisting in prolonged antibiotherapy with quinolones [39] or trimetoprim-sulfometoxazole [5] are available. Identification of the causative organism provides definitive diagnostic, but culture from biopsy can fail in a substantial number of cases [4], [5]. To our knowledge, there is no available molecular detection test. As all isolates of clone Rhinoscleromatis are of serotype K3, we followed the ‘molecular serotyping’ strategy, whereby the serotype is deduced from the sequence characteristics of the cps cluster, e.g. PCR-based identification of K types [30], [40], [41]. For this purpose, we established the complete sequence of the cps cluster of a K3 Rhinoscleromatis strain. We then developed two specific PCR assays for the identification of the agent of rhinoscleroma.
The deduced gene content of the K3 cps cluster is highly consistent with the structure of the K3 capsular polysaccharide from Klebsiella [42], as depicted on Figure S1. Currently, the link between capsular type 3 and Klebsiella strain virulence remains to be established. As the capsule is a prominent factor in bacterial pathogenesis, the availability of the K3 cps sequence will facilitate research into the possible implication of the K3 capsule in the peculiar pathophysiological aspects of rhinoscleroma.
Given the high sequence variability of wzy among distinct K-types, this gene is a good target for K-type specific PCR assays [28], [41]. The positive PCR results obtained herein for all K3 strains, belonging either to K. pneumoniae subsp. pneumoniae or to clone Rhinoscleromatis, indicate that wzy is conserved among K3 isolates, consistent with the fact that the cps PCR-RFLP patterns of K3 strains are highly similar [15]. This PCR assay can therefore be used to identify strains with capsular type 3, which would be technically easier than performing Klebsiella K typing with antisera. However, even though they are rare, some K. pneumoniae subsp. pneumoniae isolates also express the K3 capsular type [8], hampering the use of this PCR assay alone to identify the agent of rhinoscleroma. In contrast, MLST circumscribes clone Rhinoscleromatis without ambiguity [8] and as the phosphoporin gene phoE harbored diagnostic SNPs of this clone, we were able to develop a PCR assay that showed complete specificity with no false negatives. This achievement confirms that MLST data can be used to set up simple and rapid assays for identification of particular clones [43]. The two nucleotide changes at phoE positions 48 and 216 evolved in the branch leading to clone Rhinoscleromatis from its ancestor K. pneumoniae, since these changes were not observed in >700 other Klebsiella (including K. granulomatis), Enterobacter or Raoultella strains (our unpublished data and http://www.pasteur.fr/mlst). As the two mutations do not induce amino-acid changes, they can be considered neutral, and consequently, evolutionarily stable. For the same reason, it would be very unlikely that both mutations would evolve separately in other species.
The PCR assays were positive even in the presence of an excess of human DNA, and we were able to detect the infecting agent from the lungs of experimentally infected mice. In contrast, the assays were negative when tested on other pathogens and commensal organisms of the throat. Based on these preliminary observations, it should be interesting to evaluate the clinical value of these assays for direct detection of the agent of rhinoscleroma from human biopsies or nasal secretions.
In summary, although the phoE assay is, on its own, specific for clone Rhinoscleromatis, the combination of phoE and K3 PCR assays is recommended to increase the level of confidence in identifying clone Rhinoscleromatis. When combined with the defining metabolic characteristics of K. pneumoniae subsp. rhinoscleromatis [11], these two new PCR assays will be useful in confirming the diagnostic of rhinoscleroma and will help defining the epidemiology of this neglected disease.
Supporting Information
Model of the biosynthesis, polymerization and surface assembly of the K3 capsular polysaccharide. A. Structure of the K3 polysaccharide [42]. B. Possible implication of genes of the cps region in the synthesis and expression of the K3 capsular polysaccharide. In Klebsiella and E. coli [28], [34], the synthesis of UDP-D-galactose from UDP-D-glucose requires protein GalE (UDP-galactose 4-epimerase), with GalF regulating its level [28]. Next, WbaP is involved in the transfer of the D-galactose to undecaprenyl-phosphate, the lipid carrier of repeat units [44]. Synthesis of D-mannose from mannose-6-phosphate is catalyzed by the products of genes manB and manC [44], which are located at the 3′ terminus of the K3 cps region. The (1→3) linkage of the D-mannose to D-galactose is performed by the mannosyl transferase encoded by wbaZ [34]. The linkage of the two additional D-mannose residues is presumably carried out by the putative mannosyl transferases Kr11515 and Kr11511. The remaining putative transferase of the K3 cps cluster with no assigned function, encoded by Kr11513, might be involved in the transfer of UDP-D-galacturonic acid residues on D-mannose. Synthesis of D-galacturonic acid residues is achieved by both Ugd and GlaKP enzymes. The product of gene ugd transforms UDP-D-glucose into UDP-D-glucuronic acid, which can be converted into UDP-D-galacturonic acid through the activity of GlaKP [45]. These capsule unit repeats are then translocated through the inner membrane by the flippase Wzx encoded by ORF Kr11510, and subsequently polymerized by Wzy encoded by ORF Kr11509. Finally, the expression of the polysaccharide on the cell surface requires the activity of proteins encoded by wza, wzb, wzc and wzi [44]. The names of the enzymes are squared; grey shades correspond to putative enzyme activity. D-Glc: D-glucose ; D-Gal: D-galactose, D-Man: D-mannose, D-GalA: D-galacturonic acid; D-GlcA : D-glucuronic acid ; P-und: undecaprenyl-phosphate.
(0.13 MB TIF)
Strains used in this study, and their characteristics.
(0.05 MB XLS)
Acknowledgments
We thank Peter Reeves for helpful comments on the cps cluster composition and Brice Sperandio, Lluis Quintana-Murci, L. Touqui, M. Taha and P. Trieu-Cuot for providing human DNA, primers, and control bacterial DNAs.
Footnotes
The authors have declared that no competing interests exist.
This work was supported financially by Institut Pasteur and the Genoscope (Evry, France), as well as by a generous gift from the Conny-Maeva Charitable Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wechsler HL. Rhinoscleroma. Jama. 1964;190:847. doi: 10.1001/jama.1964.03070220053015. [DOI] [PubMed] [Google Scholar]
- 2.Hart CA, Rao SK. Rhinoscleroma. J Med Microbiol. 2000;49:395–396. doi: 10.1099/0022-1317-49-5-395. [DOI] [PubMed] [Google Scholar]
- 3.Canalis RF, Zamboni L. An interpretation of the structural changes responsible for the chronicity of rhinoscleroma. Laryngoscope. 2001;111:1020–1026. doi: 10.1097/00005537-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Gaafar HA, Bassiouny M, El Mofty M, Badour NM, Nour YA. Experimental intravenous inoculation of Klebsiella rhinoscleromatis bacilli in albino rats: a histopathological and bacteriological study. Acta Otolaryngol. 2000;120:279–285. doi: 10.1080/000164800750001099. [DOI] [PubMed] [Google Scholar]
- 5.Evrard I, Gruyer X, Desse P, Francois A, Marie JP, et al. Spheno-ethmoidal rhinoscleroma. Report of a case and review of the literature. Ann Otolaryngol Chir Cervicofac. 1998;115:85–88. [PubMed] [Google Scholar]
- 6.Brenner DJ, A.G. S, Fanning GR. Differentiation of Enterobacter aerogenes from Klebsiellae by deoxyribonucleic acid reassociation. Int J Syst Bacteriol. 1972;22:193–200. [Google Scholar]
- 7.Ørskov I, Ørskov F. Serotyping of Klebsiella. Methods Microbiol. 1984;14:117–133. [Google Scholar]
- 8.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, et al. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brisse S, Grimont F, Grimont PAD. The genus Klebsiella. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. The prokaryotes A handbook on the Biology of Bacteria. 3rd edition ed. New York: Springer; 2006. [Google Scholar]
- 10.Brisse S, Verhoef J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol. 2001;51:915–924. doi: 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- 11.Grimont PAD, Grimont F. Genus Klebsiella. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey's manual of Systematic Bacteriology. New York: Springer-Verlag; 2005. pp. 685–693. [Google Scholar]
- 12.Abalkhail A, Satti MB, Uthman MA, Al Hilli F, Darwish A, et al. Rhinoscleroma: a clinicopathological study from the Gulf region. Singapore Med J. 2007;48:148–151. [PubMed] [Google Scholar]
- 13.Dawlatly EE. Radiological diagnosis of rhinoscleroma–the ‘palatal sign’. J Laryngol Otol. 1991;105:968–970. doi: 10.1017/s0022215100117967. [DOI] [PubMed] [Google Scholar]
- 14.Ørskov I, Fife-Asbury MA. New Klebsiella antigen K 82 and the deletion of five of those previously assigned. Int J Syst Bacteriol. 1977;27:386–387. [Google Scholar]
- 15.Brisse S, Issenhuth-Jeanjean S, Grimont PA. Molecular serotyping of Klebsiella species isolates by restriction of the amplified capsular antigen gene cluster. J Clin Microbiol. 2004;42:3388–3398. doi: 10.1128/JCM.42.8.3388-3398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borodovsky M, Mills R, Besemer J, Lomsadze A. Prokaryotic gene prediction using GeneMark and GeneMark.hmm. Curr Protoc Bioinformatics Chapter. 2003;4:Unit4 5. doi: 10.1002/0471250953.bi0405s01. [DOI] [PubMed] [Google Scholar]
- 18.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Universal Protein Resource (UniProt) in 2010. Nucleic Acids Res. 38(Database issue):D142–148. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatusov RL. Phylogenetic classification of proteins encoded in complete genomes. 2001 doi: 10.1093/nar/29.1.22. Available: http://www.ncbi.nlm.nih.gov/COG/. Accessed 2009 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangeul L, Glaser P, Rusniok C, Buchrieser C, Duchaud E, et al. CAAT-Box, Contigs-Assembly and Annotation Tool-Box for genome sequencing projects. Bioinformatics. 2004;20:790–797. doi: 10.1093/bioinformatics/btg490. [DOI] [PubMed] [Google Scholar]
- 22.Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 23.Regue M, Hita B, Pique N, Izquierdo L, Merino S, et al. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect Immun. 2004;72:54–61. doi: 10.1128/IAI.72.1.54-61.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frirdich E, Whitfield C. Characterization of Gla(KP), a UDP-galacturonic acid C4-epimerase from Klebsiella pneumoniae with extended substrate specificity. J Bacteriol. 2005;187:4104–4115. doi: 10.1128/JB.187.12.4104-4115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol. 2009;191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh KM, Chang FY, Fung CP, Lin JC, Siu LK. magA is not a specific virulence gene for Klebsiella pneumoniae strains causing liver abscess but is part of the capsular polysaccharide gene cluster of K. pneumoniae serotype K1. J Med Microbiol. 2006;55:803–804. doi: 10.1099/jmm.0.46368-0. [DOI] [PubMed] [Google Scholar]
- 27.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, et al. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu HY, Fung CP, Liu YM, Wu KM, Chen YT, et al. Genetic diversity of capsular polysaccharide biosynthesis in Klebsiella pneumoniae clinical isolates. Microbiology. 2009;155:4170–4183. doi: 10.1099/mic.0.029017-0. [DOI] [PubMed] [Google Scholar]
- 29.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, et al. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis. 2007;45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 30.Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol. 2008;46:2231–2240. doi: 10.1128/JCM.01716-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fouts DE, Tyler HL, DeBoy RT, Daugherty S, Ren Q, et al. Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genet. 2008;4:e1000141. doi: 10.1371/journal.pgen.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahn A, Drummelsmith J, Whitfield C. Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae. J Bacteriol. 1999;181:2307–2313. doi: 10.1128/jb.181.7.2307-2313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs M, Reeves PR. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol. 1994;12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 34.Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 35.Frirdich E, Bouwman C, Vinogradov E, Whitfield C. The role of galacturonic acid in outer membrane stability in Klebsiella pneumoniae. J Biol Chem. 2005;280:27604–27612. doi: 10.1074/jbc.M504987200. [DOI] [PubMed] [Google Scholar]
- 36.Busch RF. Rhinoscleroma occurring with airway obstruction. Otolaryngol Head Neck Surg. 1993;109:933–936. doi: 10.1177/019459989310900524. [DOI] [PubMed] [Google Scholar]
- 37.Le Hir P, Marsot-Dupuch K, Bigel P, Elbigourmie TM, Jacquier I, et al. Rhinoscleroma with orbital extension: CT and MRI. Neuroradiology. 1996;38:175–178. doi: 10.1007/BF00604813. [DOI] [PubMed] [Google Scholar]
- 38.Kim NR, Han J, Kwon TY. Nasal rhinoscleroma in a nonendemic area: a case report. J Korean Med Sci. 2003;18:455–458. doi: 10.3346/jkms.2003.18.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badia L, Lund VJ. A case of rhinoscleroma treated with ciprofloxacin. J Laryngol Otol. 2001;115:220–222. doi: 10.1258/0022215011907028. [DOI] [PubMed] [Google Scholar]
- 40.Gierczynski R, Jagielski M, Rastawicki W, Kaluzewski S. Multiplex-PCR assay for identification of Klebsiella pneumoniae isolates carrying the cps loci for K1 and K2 capsule biosynthesis. Pol J Microbiol. 2007;56:153–156. [PubMed] [Google Scholar]
- 41.Turton JF, Baklan H, Siu LK, Kaufmann ME, Pitt TL. Evaluation of a multiplex PCR for detection of serotypes K1, K2 and K5 in Klebsiella sp. and comparison of isolates within these serotypes. FEMS Microbiol Lett. 2008;284:247–252. doi: 10.1111/j.1574-6968.2008.01208.x. [DOI] [PubMed] [Google Scholar]
- 42.Dutton GG, Parolis H, Joseleau JP, Marais MF. The use of bacteriophage depolymerization in the structural investigation of the capsular polysaccharide from Klebsiella serotype K3. Carbohydr Res. 1986;149:411–423. doi: 10.1016/s0008-6215(00)90061-2. [DOI] [PubMed] [Google Scholar]
- 43.Robertson GA, Thiruvenkataswamy V, Shilling H, Price EP, Huygens F, et al. Identification and interrogation of highly informative single nucleotide polymorphism sets defined by bacterial multilocus sequence typing databases. J Med Microbiol. 2004;53:35–45. doi: 10.1099/jmm.0.05365-0. [DOI] [PubMed] [Google Scholar]
- 44.Whitfield C, Paiment A. Biosynthesis and assembly of Group 1 capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. Carbohydr Res. 2003;338:2491–2502. doi: 10.1016/j.carres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Munoz R, Lopez R, de Frutos M, Garcia E. First molecular characterization of a uridine diphosphate galacturonate 4-epimerase: an enzyme required for capsular biosynthesis in Streptococcus pneumoniae type 1. Mol Microbiol. 1999;31:703–713. doi: 10.1046/j.1365-2958.1999.01211.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model of the biosynthesis, polymerization and surface assembly of the K3 capsular polysaccharide. A. Structure of the K3 polysaccharide [42]. B. Possible implication of genes of the cps region in the synthesis and expression of the K3 capsular polysaccharide. In Klebsiella and E. coli [28], [34], the synthesis of UDP-D-galactose from UDP-D-glucose requires protein GalE (UDP-galactose 4-epimerase), with GalF regulating its level [28]. Next, WbaP is involved in the transfer of the D-galactose to undecaprenyl-phosphate, the lipid carrier of repeat units [44]. Synthesis of D-mannose from mannose-6-phosphate is catalyzed by the products of genes manB and manC [44], which are located at the 3′ terminus of the K3 cps region. The (1→3) linkage of the D-mannose to D-galactose is performed by the mannosyl transferase encoded by wbaZ [34]. The linkage of the two additional D-mannose residues is presumably carried out by the putative mannosyl transferases Kr11515 and Kr11511. The remaining putative transferase of the K3 cps cluster with no assigned function, encoded by Kr11513, might be involved in the transfer of UDP-D-galacturonic acid residues on D-mannose. Synthesis of D-galacturonic acid residues is achieved by both Ugd and GlaKP enzymes. The product of gene ugd transforms UDP-D-glucose into UDP-D-glucuronic acid, which can be converted into UDP-D-galacturonic acid through the activity of GlaKP [45]. These capsule unit repeats are then translocated through the inner membrane by the flippase Wzx encoded by ORF Kr11510, and subsequently polymerized by Wzy encoded by ORF Kr11509. Finally, the expression of the polysaccharide on the cell surface requires the activity of proteins encoded by wza, wzb, wzc and wzi [44]. The names of the enzymes are squared; grey shades correspond to putative enzyme activity. D-Glc: D-glucose ; D-Gal: D-galactose, D-Man: D-mannose, D-GalA: D-galacturonic acid; D-GlcA : D-glucuronic acid ; P-und: undecaprenyl-phosphate.
(0.13 MB TIF)
Strains used in this study, and their characteristics.
(0.05 MB XLS)