Abstract
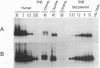
A variety of Alu subfamilies amplified in primate genomes at different evolutionary time periods. Alu Sb2 belongs to a group of young subfamilies with a characteristic two-nucleotide deletion at positions 65/66. It consists of repeats having a 7-nucleotide duplication of a sequence segment involving positions 246 through 252. The presence of Sb2 inserts was examined in five genomic loci in 120 human DNA samples as well as in DNAs of higher primates. The lack of the insertional polymorphism seen at four human loci and the absence of orthologous inserts in apes indicated that the examined repeats retroposed early in the human lineage, but following the divergence of great apes. On the other hand, similar analysis of the fifth locus (butyrylcholinesterase gene) suggested contemporary retropositional activity of this subfamily. By a semi-quantitative PCR, using a primer pair specific for Sb2 repeats, we estimated their copy number at about 1500 per human haploid genome; the corresponding numbers in chimpanzee and gorilla were two orders of magnitude lower, while in orangutan and gibbon the presence of Sb2 Alu was hardly detectable. Sequence analysis of PCR-amplified Sb2 repeats from human and African great apes is consistent with the model in which the founding of Sb2 subfamily variants occurred independently in chimpanzee, gorilla and human lineages.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batzer M. A., Deininger P. L. A human-specific subfamily of Alu sequences. Genomics. 1991 Mar;9(3):481–487. doi: 10.1016/0888-7543(91)90414-a. [DOI] [PubMed] [Google Scholar]
- Batzer M. A., Gudi V. A., Mena J. C., Foltz D. W., Herrera R. J., Deininger P. L. Amplification dynamics of human-specific (HS) Alu family members. Nucleic Acids Res. 1991 Jul 11;19(13):3619–3623. doi: 10.1093/nar/19.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzer M. A., Kilroy G. E., Richard P. E., Shaikh T. H., Desselle T. D., Hoppens C. L., Deininger P. L. Structure and variability of recently inserted Alu family members. Nucleic Acids Res. 1990 Dec 11;18(23):6793–6798. doi: 10.1093/nar/18.23.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J. Rates of DNA sequence evolution differ between taxonomic groups. Science. 1986 Mar 21;231(4744):1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E., Zielinski R., Boyd C., Dugaiczyk A. Structure, polymorphism, and novel repeated DNA elements revealed by a complete sequence of the human alpha-fetoprotein gene. Biochemistry. 1987 Mar 10;26(5):1332–1343. doi: 10.1021/bi00379a020. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. P., Rommens J. M., Andrew S. E., Hutchinson G. B., Lin B., Theilmann J., Graham R., Glaves M. L., Starr E., McDonald H. Identification of an Alu retrotransposition event in close proximity to a strong candidate gene for Huntington's disease. Nature. 1993 Mar 25;362(6418):370–373. doi: 10.1038/362370a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson G. B., Andrew S. E., McDonald H., Goldberg Y. P., Graham R., Rommens J. M., Hayden M. R. An Alu element retroposition in two families with Huntington disease defines a new active Alu subfamily. Nucleic Acids Res. 1993 Jul 25;21(15):3379–3383. doi: 10.1093/nar/21.15.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J. A new subfamily of recently retroposed human Alu repeats. Nucleic Acids Res. 1993 May 11;21(9):2252–2252. doi: 10.1093/nar/21.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J., Milosavljevic A. Reconstruction and analysis of human Alu genes. J Mol Evol. 1991 Feb;32(2):105–121. doi: 10.1007/BF02515383. [DOI] [PubMed] [Google Scholar]
- Labuda D., Sinnett D., Richer C., Deragon J. M., Striker G. Evolution of mouse B1 repeats: 7SL RNA folding pattern conserved. J Mol Evol. 1991 May;32(5):405–414. doi: 10.1007/BF02101280. [DOI] [PubMed] [Google Scholar]
- Labuda D., Striker G. Sequence conservation in Alu evolution. Nucleic Acids Res. 1989 Apr 11;17(7):2477–2491. doi: 10.1093/nar/17.7.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuda D., Zietkiewicz E. Evolution of secondary structure in the family of 7SL-like RNAs. J Mol Evol. 1994 Nov;39(5):506–518. doi: 10.1007/BF00173420. [DOI] [PubMed] [Google Scholar]
- Leeflang E. P., Chesnokov I. N., Schmid C. W. Mobility of short interspersed repeats within the chimpanzee lineage. J Mol Evol. 1993 Dec;37(6):566–572. doi: 10.1007/BF00182742. [DOI] [PubMed] [Google Scholar]
- Leeflang E. P., Liu W. M., Chesnokov I. N., Schmid C. W. Phylogenetic isolation of a human Alu founder gene: drift to new subfamily identity [corrected]. J Mol Evol. 1993 Dec;37(6):559–565. doi: 10.1007/BF00182741. [DOI] [PubMed] [Google Scholar]
- Matera A. G., Hellmann U., Schmid C. W. A transpositionally and transcriptionally competent Alu subfamily. Mol Cell Biol. 1990 Oct;10(10):5424–5432. doi: 10.1128/mcb.10.10.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani K., Hada T., Yamamoto Y., Kaneko T., Shigeto Y., Ohue T., Furuyama J., Higashino K. Inactivation of the cholinesterase gene by Alu insertion: possible mechanism for human gene transposition. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11315–11319. doi: 10.1073/pnas.88.24.11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C., Maraia R. Transcriptional regulation and transpositional selection of active SINE sequences. Curr Opin Genet Dev. 1992 Dec;2(6):874–882. doi: 10.1016/s0959-437x(05)80110-8. [DOI] [PubMed] [Google Scholar]
- Shen M. R., Batzer M. A., Deininger P. L. Evolution of the master Alu gene(s). J Mol Evol. 1991 Oct;33(4):311–320. doi: 10.1007/BF02102862. [DOI] [PubMed] [Google Scholar]
- Sibley C. G., Ahlquist J. E. DNA hybridization evidence of hominoid phylogeny: results from an expanded data set. J Mol Evol. 1987;26(1-2):99–121. doi: 10.1007/BF02111285. [DOI] [PubMed] [Google Scholar]
- Trabuchet G., Chebloune Y., Savatier P., Lachuer J., Faure C., Verdier G., Nigon V. M. Recent insertion of an Alu sequence in the beta-globin gene cluster of the gorilla. J Mol Evol. 1987;25(4):288–291. doi: 10.1007/BF02603112. [DOI] [PubMed] [Google Scholar]
- Vidaud D., Vidaud M., Bahnak B. R., Siguret V., Gispert Sanchez S., Laurian Y., Meyer D., Goossens M., Lavergne J. M. Haemophilia B due to a de novo insertion of a human-specific Alu subfamily member within the coding region of the factor IX gene. Eur J Hum Genet. 1993;1(1):30–36. doi: 10.1159/000472385. [DOI] [PubMed] [Google Scholar]
- Wallace M. R., Andersen L. B., Saulino A. M., Gregory P. E., Glover T. W., Collins F. S. A de novo Alu insertion results in neurofibromatosis type 1. Nature. 1991 Oct 31;353(6347):864–866. doi: 10.1038/353864a0. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz E., Labuda M., Sinnett D., Glorieux F. H., Labuda D. Linkage mapping by simultaneous screening of multiple polymorphic loci using Alu oligonucleotide-directed PCR. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8448–8451. doi: 10.1073/pnas.89.18.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietkiewicz E., Makałowski W., Mitchell G. A., Labuda D. Phylogenetic analysis of a reported complementary DNA sequence. Science. 1994 Aug 19;265(5175):1110–1111. doi: 10.1126/science.8066452. [DOI] [PubMed] [Google Scholar]
- Zietkiewicz E., Sinnett D., Richer C., Mitchell G., Vanasse M., Labuda D. Single-strand conformational polymorphisms (SSCP): detection of useful polymorphisms at the dystrophin locus. Hum Genet. 1992 Jun;89(4):453–456. doi: 10.1007/BF00194322. [DOI] [PubMed] [Google Scholar]