INTRODUCTION
Because of the emergence of community-associated strains, invasive methicillin-resistant Staphylococcus aureus (MRSA) infections have increased substantially among pediatric patients(1, 2). At the present time, vancomycin remains the drug of choice for the treatment of invasive MRSA infections. However, increased minimum inhibitory concentration (MIC) even within the susceptible range is a risk factor for vancomycin treatment failure in adults with invasive MRSA infections(3-5). National increases in the median vancomycin MIC (so called “MIC creep”) and heteroresistance are additional concerns considering the reported cases associated with vancomycin failure(6-8). Accordingly, this shifting MIC pattern has led to recommendations of using increased vancomycin doses or alternative agents (e.g. linezolid, daptomycin, etc.) for invasive MRSA infections with isolates having MIC at the upper level of the susceptible range(5, 9, 10).
Area-under-the-concentration-time-curve (AUC) for 24 h divided by the MIC (AUC24/MIC) best predicts treatment outcomes when treating invasive MRSA infection in adults. Specifically, an AUC24/MIC >400 for vancomycin is associated with optimal outcomes in the treatment of adults(11). However, achievement of this target in MRSA isolates with MIC of 1-2 μg/ml often requires increased vancomycin dosing, particularly in those patients with normal renal function. Most children have increased renal function compared with adults and thus would be expected to be even less likely to achieve AUC24/MIC >400 in serious MRSA infection. It is unknown whether the recommended vancomycin dosing for children results in an AUC24/MIC >400.
At our institution treatment failures has anecdotally been observed in patients with invasive MRSA infections and initial vancomycin trough concentration < 5 μg/ml. We hypothesized that these failures could be due, in part, to inadequate vancomycin exposure, even with those susceptible isolates having MIC of 1 μg/ml. We predicted that outcomes could be improved by using an increased initial dose. The objective of this study was to estimate the AUC24/MIC for vancomycin using two recommended doses across the spectrum of MIC values encountered at our hospital.
MATERIAL AND METHODS
We developed a model to predict Vancomycin AUC24/MIC in children ages 2-12 years old. AUC24 was calculated as:
This calculated AUC24 was then divided by representative MIC of MRSA isolates (see ‘MIC for MRSA Isolates’ below) to obtain an AUC24/MIC. Neonates and infants were excluded because of their rapid maturational changes in renal function and uniqueness as a population compared to children with respect to vancomycin clearance.
Vancomycin Daily Dose
Major pediatric dosing references recommend a vancomycin daily dose of 40 mg/kg/day for empiric therapy (12-14). A dose of 60 mg/kg/day is recommended for central nervous system infections. Therefore, we calculated AUC24/MIC for vancomycin daily doses of both 40 and 60 mg/kg/day.
Vancomycin Clearance (CL)
Estimates for vancomycin CL were obtained by two approaches: 1) vancomycin CL calculated based on vancomycin serum levels and 2) vancomycin CL calculated using previously derived predictor models based on covariates (height, weight, serum creatinine, etc.).
1) Vancomycin CL estimates from vancomycin serum levels
Four studies were identified in which vancomycin CL was calculated based on direct measures of vancomycin levels in children(15-18). In one study(17) a recalculated vancomycin CL was required to account for vancomycin infusion time (19), which had not been taken into account by the authors. In this same study a weighted average of vancomycin CL from the three reported age groups (mean age of groups 3.9, 5.6, and 7.6 years) was calculated to obtain an overall mean vancomycin CL in children. The mean vancomycin CL for each study was: 0.114(15), 0.103(16), 0.157(17) and 0.110 L/hr/kg(18) respectively.
2) Vancomycin CL estimates from Predictor Models
Vancomycin CL was calculated using 7 previously derived predictor models and applied to a hypothetical population of children. Six models were derived from adults(20-25) and one from children(15). All models incorporated creatinine clearance (CrCL) as the major predictor of vancomycin CL (see Appendix). For each age, an average CrCL was calculated using the Schwartz Formula and reported mean serum creatinine, height, and weight in children(26-28). CrCL estimates were then denormalized by body surface area using the Mosteller formula(29) (BSA = √(Wt*Ht/3600)). The estimated CrCL values were then used in each predictor model to obtain vancomycin CL.
MIC for MRSA Isolates
For representative MIC of MRSA isolates, pediatric cultures at the University of California, San Francisco Children’s Hospital from July 2007 to June 2008 were reviewed. Vancomycin MIC was determined using a standard microtiter dilution technique with custom-made panels from the University of California Los Angeles (Clinical and Laboratory Standards Institute approved). The percentage of MRSA isolates with MIC ≤ 0.5, = 1.0, and = 2.0 μg/ml were 40, 59, and 1% respectively. The MIC50/90 was 1.0 μg/ml.
RESULTS
1) AUC24/MIC using vancomycin CL estimates from vancomycin serum levels
Using reported vancomycin CL in children, predicted AUC24/MIC for vancomycin at doses of 40mg/kg/day and 60 mg/kg/day are shown in the Table. For MRSA isolates with an MIC 0.5 μg/ml, both doses achieved AUC24/MIC >400. For isolates with an MIC of 1.0 μg/ml, the 40 mg/kg/day dose always predicted an AUC24/MIC <400. On the other hand, the 60 mg/kg/day dose achieved an AUC24/MIC >400 in three of the four predictions when MIC was 1.0 μg/ml. For MIC of 2.0 μg/ml, all AUC24/MIC predictions were well below 400 for both doses.
Table.
Predicted AUC24/MIC in children using reported vancomycin clearances from the literature. Values >400 noted in bold. MIC, μg/ml.
| Source | AUC24/MIC
|
|||||
|---|---|---|---|---|---|---|
| Dose 40 mg/kg/day | Dose 60 mg/kg/day | |||||
| MIC 0.5 | MIC 1.0 | MIC 2.0 | MIC 0.5 | MIC 1.0 | MIC 2.0 | |
| Schaad et al. | 515 | 258 | 129 | 773 | 386 | 193 |
|
| ||||||
| Wrisko et al. | 727 | 364 | 182 | 1091 | 545 | 273 |
|
| ||||||
| Lamarre et al. | 777 | 388 | 194 | 1165 | 583 | 291 |
|
| ||||||
| Chang | 702 | 351 | 175 | 1053 | 526 | 263 |
2) AUC24/MIC using vancomycin CL estimates from Predictor Models
Using predictor models of vancomycin CL, estimated AUC24/MIC for vancomycin when MIC was 0.5 μg/ml was always > 400 for each model at both 40 and 60 mg/kg/day (data not shown). The predicted AUC24/MIC for each model at MIC of 1.0 and 2.0 μg/ml are displayed in Figure 1. When MIC was 1.0 μg/ml, estimates for AUC24/MIC at a dose of 40 mg/kg/day were always <400 (Figure 1A). In contrast, when MIC was 1.0 μg/ml, a dose of 60 mg/kg/day produced an AUC24/MIC >400 for four of the seven models across all of the ages. For the other three models, achieving AUC24/MIC >400 was only seen at older ages (Figure 1B). When MIC was 2 μg/ml, AUC24/MIC was consistently <400 in each model at all ages (Figure 1C and D).
Figure 1.
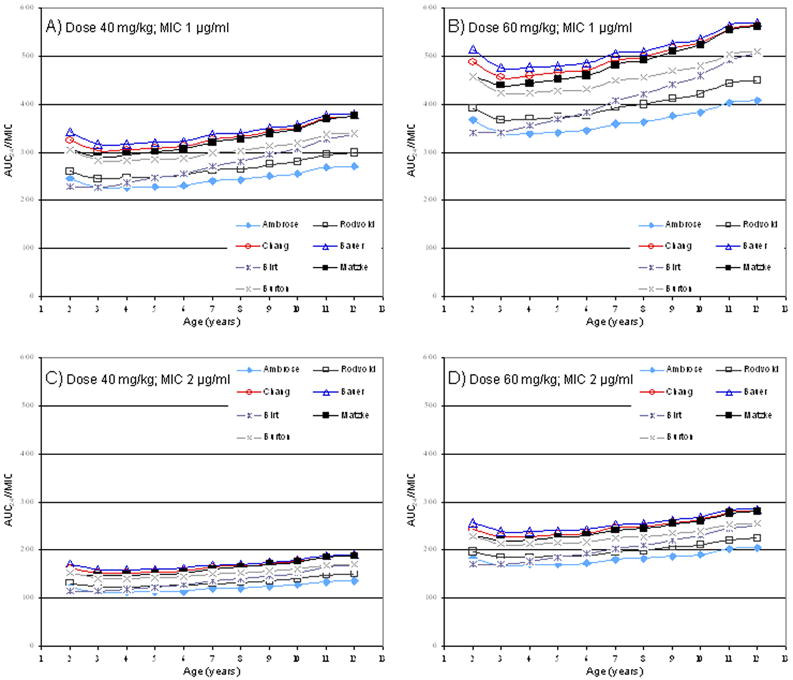
Predicted AUC24/MIC in children (2-12 years old) receiving vancomycin using reported prediction models of vancomycin clearance. Doses of 40 mg/kg/day and 60 mg/kg/day were evaluated at MIC of 1.0 and 2.0 μg/ml. See appendix for each model’s vancomycin clearance prediction equation.
DISCUSSION
In this study, vancomycin AUC24/MIC was modeled in children receiving the recommended pediatric dosages of 40 mg/kg/day or 60 mg/kg/day across a range of MIC values. Two separate methods of calculation were used in the analysis. With a dose of 40 mg/kg/day, the AUC24/MIC target of >400 was achieved only when the MIC of MRSA isolates was 0.5 μg/ml. For MRSA isolates with MIC ≥ 1.0, the AUC24/MIC was always <400 at this dose. At a dose of 60 mg/kg/day, the ability to achieve AUC24/MIC >400 was greatly improved if the MIC was 1.0 μg/ml. But, if the MIC was 2.0 μg/ml, the 60 mg/kg/day dose still predicted AUC24/MIC well below 400. The general agreement of both methods in this study lends validity to the results.
Ours is the first study to examine vancomycin AUC24/MIC in the treatment of MRSA infection in children. Expanding animal and adult literature strongly suggests that AUC24/MIC is the best measure of vancomycin activity and achieving an AUC24/MIC > 400 results in optimal outcomes for the treatment of invasive MRSA infections(9, 11). Whether this relationship applies to children is yet to be determined. Clinical studies evaluating AUC24/MIC and clinical outcome in children will be important as we attempt to optimize vancomycin therapy in this population.
Vancomycin is commonly used for the treatment of suspected or proven invasive MRSA infections in children, yet studies examining its pharmacokinetics in this population are limited. In our first approach for the prediction of AUC24/MIC in children, we used vancomycin clearance estimates from four pediatric studies(15-18). All four predicted similar vancomycin AUC24/MIC values but those predicted by Schaad, et al. were slightly lower for each prediction given the greater clearance estimate. Of note, two of the other studies included infants(15, 16). Considering that kidney function in infants is reduced compared to children and the major route of elimination for vancomycin is the kidney, the vancomycin clearance reported from these two studies likely underestimates the true vancomycin clearance of children. Therefore, the predictions from these two studies possibly overestimate AUC24/MIC. Our second approach utilizing predictor models of vancomycin clearance were generally in agreement with AUC24/MIC values calculated using vancomycin clearance estimates from the pediatric literature. The only exception was for three of the prediction models that did not achieve AUC24/MIC >400 specifically in younger children at a dose of 60 mg/kg/day and MIC of 1.0 μg/ml (Figure 1B). Of these three, the Birt and Rodvold model calculated very high non-renal clearance components unlikely to be seen in young children (23, 25) while the Ambrose model expressed vancomycin clearance as equal to the creatinine clearance(24) (see Appendix). These approaches would be expected to bias the results toward underestimating AUC24/MIC.
Our analysis represents basic modeling work and not patient data. Modeling allows for initial insight without the cost, time and possible safety concerns associated with additional pediatric clinical trials. Actual reported study data from pediatric patients was used for the majority of calculations. Unfortunately, the pharmacokinetic and pharmacodynamic literature on vancomycin use in children is limited, and much of the reported data originated from combined study populations of infants and children. Furthermore, assumptions were made for the predictor models of vancomycin CL, as most predictor models were derived from adult data. However, these two potential population biases would be expected to skew our results towards greater AUC24/MIC values as both infants and adults generally have decreased renal function when compared to children. Nonetheless, it will be important to validate AUC24/MIC levels in children.
Vancomycin serum trough levels are routinely recommended to optimize dosing and to monitor for toxicity. Goal troughs of 5-15 μg/ml for non-CNS MRSA infections have been suggested(24). However, with the emergence of MIC creep and in the light of increasing reports of treatment failures, recommendations for achieving vancomycin serum troughs of 15-20 μg/ml have been made for certain adult populations(10). Reports of serum vancomycin trough levels with standard dosing in children suggest that substantially lower trough levels are common. In a study of infants and children treated for suspected staphylococcal infections, vancomycin 40mg/kg/day divided every 6 hours achieved goal troughs (5-15 μg/ml) in only 45% of control patients(15). In those patients with malignancy, 88% of patients required >60mg/kg/day to achieve these goals. Similarly, in a study by Glover et al.(30), pediatric intensive care unit patients with normal renal function required a mean dose of 60 mg/kg/day to achieve a mean trough level of 7.8 μg/ml. These data support the notion that a vancomycin starting dose of 40mg/kg/day is unlikely to achieve significantly increased vancomycin troughs levels in children.
While increased serum troughs are generally associated with greater AUC, serum trough does not directly reflect the AUC. The trough is highly dependent on the dosing interval (i.e. for given daily dose a longer dosing interval results in a lower trough). Therefore, dose adjustments based on trough alone may be clinically misleading. If vancomycin exposure (i.e. AUC) in children is shown to be important, then clinically practical methods of measuring AUC in children will need to be developed.
Recent reports of MRSA treatment failures can be understood from pharmacodynamic considerations. At the present time, an MIC of 2.0 μg/ml is considered susceptible. But, our modeling work shows it is not possible to achieve AUC24/MIC >400 when the MIC is 2.0 μg/ml even at 60 mg/kg/day. This finding has similarly been shown in adults(9, 31). Consequently, treatment strategies should strongly consider alternative agents, such as linezolid or daptomycin, for optimal treatment of invasive MRSA infections with MIC ≥2.0 μg/ml. Our work has also shown that using a vancomycin dose of 40 mg/kg/day in children even when the MIC is 1.0 μg/ml does not achieve AUC24/MIC >400, suggesting this dose might not be the most appropriate choice if an MIC of 1.0 μg/ml is commonly observed at the given institution. Additionally, treatment failure with vancomycin in supposedly susceptible strains (especially when MIC is ≤ 1.0 μg/ml) should raise the suspicion for heteroresistance.
Because of concerns of increasing vancomycin treatment failures, an MIC50/90 of 1.0 μg/ml and our modeling work described here, in July 2008 a new vancomycin dosing strategy was adopted at our institution. For children with normal renal function and suspected or proven invasive MRSA infections, our initial starting dose is 60 mg/kg/day (15 mg/kg IV q6h). Based on the results of our study, it is likely that this dosing strategy would be appropriate for other pediatric institutions that commonly treat staphylococcal isolates with MIC of 1.0 μg/ml.
In summary, the current empiric vancomycin dose recommendation in children of 40 mg/kg/day is unlikely to achieve the recommended pharmacodynamic target of AUC24/MIC >400 in MRSA with MIC of 1.0 μg/ml or greater. Implications of these findings are: 1) empiric treatment of invasive MRSA infections in children should consider a vancomycin dose of 60 mg/kg/day; 2) active surveillance of MIC trends is important in starting dose considerations; 3) further pharmacokinetic and pharmacodynamic studies are needed in children to optimize vancomycin dosing.
Acknowledgments
Sources of Support Supported by Grant T32 GM07546 from the National Institute of General Medical Sciences
Supported by Grant T32 HD044331 from the National Institute of Child Health and Human Development.
Appendix – Vancomycin clearance prediction equations
Ambrose method(24): CLvanco (mL/min) = CrCL
Chang method(15): CLvanco (ml/min) = (CrCL × 0.7099) + 1.4084
Birt method(25): CLvanco (mL/min) = (0.674 × CrCL) + 13.45
Burton revised method(21): CLvanco (L/hr) = CrCL (mL/min) × 0.048
Rodvold method(23): CLvanco (mL/min per 1.73 m2) = (CrCL [mL/min per 1.73 m2] × 0.79) + 15.7
Bauer method(20): CLvanco (mL/min/kg) = (0.695 × CrCL [mL/min/kg]) + 0.05
Matzke method(22): CLvanco (mL/min) = (CrCL × 0.689) + 3.66
(CLvanco = vancomycin clearance. Creatinine clearance (CrCl) units are in mL/min unless otherwise noted.)
References
- 1.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: a 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159:980–985. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 3.Moise PA, Sakoulas G, Forrest A, Schentag JJ. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2007;51:2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakoulas G, Moise-Broder PA, Schentag J, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 6.Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360–367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 7.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-VISA, vancomycin susceptible clinical MRSA blood isolates from 2001-2005 [abstract A-084]. Program and Abstracts of the 106th Annual Meeting of the American Society for Microbiology; Orlando, FL. May 21 to 25, 2006; Washington, DC: American Society for Microbiology; 2006. [Google Scholar]
- 8.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohr JF, Murray BE. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:1536–1542. doi: 10.1086/518451. [DOI] [PubMed] [Google Scholar]
- 10.Society AT, America IDSo. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 11.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 12.Vancocin(R), vancomycin injection USP. Deerfield, IL: Baxter Healthcare Corporation; 2003. package insert. [Google Scholar]
- 13.Robertson J, Shilkofski N. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 17. Philadelphia, Pa: Elsevier Mosby; 2005. [Google Scholar]
- 14.Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 14. Hudson, Ohio: Lexi-Comp; 2007. [Google Scholar]
- 15.Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. Pediatr Infect Dis J. 1995;14:667–673. doi: 10.1097/00006454-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lamarre P, Lebel D, Ducharme MP. A population pharmacokinetic model for vancomycin in pediatric patients and its predictive value in a naive population. Antimicrob Agents Chemother. 2000;44:278–282. doi: 10.1128/aac.44.2.278-282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaad UB, McCracken GH, Jr, Nelson JD. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 18.Wrishko RE, Levine M, Khoo D, Abbott P, Hamilton D. Vancomycin pharmacokinetics and Bayesian estimation in pediatric patients. Ther Drug Monit. 2000;22:522–531. doi: 10.1097/00007691-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Baker D, Rotschafer JC, Sawchuk R, Crossley KB, Solem LD. Vancomycin pharmacokinetics. J Pediatr. 1980;97:502–503. doi: 10.1016/s0022-3476(80)80220-4. [DOI] [PubMed] [Google Scholar]
- 20.Bauer LA. Applied clinical pharmacokinetics. New York: McGraw-Hill; 2001. [Google Scholar]
- 21.Burton ME, Gentle DL, Vasko MR. Evaluation of a Bayesian method for predicting vancomycin dosing. DICP. 1989;23:294–300. doi: 10.1177/106002808902300404. [DOI] [PubMed] [Google Scholar]
- 22.Matzke GR, McGory RW, Halstenson CE, Keane WF. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984;25:433–437. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodvold KA, Blum RA, Fischer JH, et al. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988;32:848–852. doi: 10.1128/aac.32.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrose P, Winter ME. Vancomycin. In: Winter ME, editor. Basic clinical pharmacokinetics. 4. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 451–476. [Google Scholar]
- 25.Birt JK, Chandler MH. Using clinical data to determine vancomycin dosing parameters. Ther Drug Monit. 1990;12:206–209. doi: 10.1097/00007691-199003000-00017. [DOI] [PubMed] [Google Scholar]
- 26.CDC. National Health and Nutrition Examination Survey 1999-2000. [June 15, 2008]; CDC web site Available at: http://www.cdc.gov/nchs/about/major/nhanes/nhanes99_00.htm.
- 27.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 28.Schwartz GJ, Haycock GB, Spitzer A. Plasma creatinine and urea concentration in children: normal values for age and sex. J Pediatr. 1976;88:828–830. doi: 10.1016/s0022-3476(76)81125-0. [DOI] [PubMed] [Google Scholar]
- 29.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 30.Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care. 2000;15:1–4. doi: 10.1053/jcrc.2000.0150001. [DOI] [PubMed] [Google Scholar]
- 31.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130:947–955. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]


