Abstract
Antidepressant medications are effective only in a subpopulation of patients with depression, and some patients respond to certain drugs but not others. The biological bases for these clinical observations remain unexplained. To investigate individual differences in response to antidepressants, we have examined the effects of the norepinephrine reuptake inhibitor desipramine (DMI) and the selective serotonin reutake inhibitor fluoxetine (FLU) in the forced swim test (FST) in rats that differ for their emotional behavior. After FST, animals with higher locomotor activity in a novel environment (HR) showed higher c-fos mRNA levels than their counterpart (LR) in the locus coeruleus (LC). No group differences in c-fos mRNA expression were present in the dorsal raphe nucleus or nucleus of the solitary tract (NTS). When behavioral effects of DMI or FLU were compared in HR vs. LR animals in the FST, DMI caused a significant decrease in immobility in LR animals only, while FLU caused a significant reduction in immobility regardless of the animals’ phenotype. Moreover, our results showed a decrease in FST-induced c-fos mRNA levels in prefrontal cortex (PFC) and paraventricular nucleus of the hypothalamus (PVN) in LR but not HR animals after DMI treatment, and a significant decrease in FST-induced corticosterone secretion in DMI-treated LR but not HR rats. Taken together, our results suggest that the HR-LR model is a useful tool to investigate individual differences in responses to norepinephrine reutake inhibitors (NRIs) and that a diffential activation of PFC and/or PVN could underlie some of the inter-individual differences in NRIs efficacy.
Introduction
Partial response and nonresponse to antidepressant medications are common (Joffe RT et al. J Clin Psychiatry 1996:57(Suppl 7):25–31). Clinical studies and animal models of inter-individual differences in sensitivity to antidepressant have been useful in uncovering some the possible mechanism underlying differences in responsiveness to antidepressants. However, the reasons why a percentage of depressed patients taking antidepressants are partially or totally resistant to the treatment are still not well understood, and further studies are necessary to better uncover the biological correlates responsible for differences in response to antidepressant treatments.
Depression disorders are accompanied by dysregulation of noradrenergic and serotonergic systems. Abnormalities in these systems are thought to mediate many of the symptoms of depression, and correction or compensation for such abnormalities may be necessary for antidepressant efficacy (for review see Ressler and Nemeroff Dep and anxiety 2000). Indeed, most of the antidepressants in clinical practice today act upon one or both the noradrenergic and serotonergic systems.
In this study, we compared responses to the selective norepinephrine reuptake inhibitor (NRI) desipramine (DMI), or the selective serotonin reuptake inhibitor (SSRI) fluoxetine (FLU) in the forced swim test (FST) in animals with high (HR) and low (LR) locomotor activity in a novel environment. This model of between-subject variability in rodents has been introduced as a tool to investigate individual differences in addiction-related behaviors (Piazza et al 1988). During the years though, it has become apparent that the differences between these two subgroups of animals extend beyond drug addiction and into affective behavior. For example, HR rats exhibit a prolonged corticosterone (CORT) response after exposure to a mild stress (Piazza 1989, Dellu 1996, Kabbaj 2000), and lower levels of anxiety-like behavior in the light-dark box and elevated plus-maze anxiety tests than their counterpart (Kabbaj 2000).
In order to identify possible differences in FST-induced activation of the noradrenergic and serotonergic systems in HR vs. LR animals, in experiment 1 we compared c-fos mRNA levels in the dorsal raphe (DR), locus coeruleus (LC) and nucleus of the solitary tract (NTS) in these two groups of animals after FST.
We then compared behavioral responses in the FST after DMI or FLU administration in HR vs. LR animals in experiments 2 and 3 respectively to compare climbing, immobility and swimming behavior between these two groups of animals.
Group differences emerged in c-fos mRNA expression in LC after FST, and HR rats showed a different behavioral response than LR in the FST after DMI treatment. Therefore, in experiment 4 we compared c-fos mRNA levels in LC, NTS and NE projections in the forebrain after FST in vehicle- and DMI-treated HR and LR rats to possibly uncover some of the biological mechanisms responsible for individual differences in responsiveness to DMI in the FST. Finally, since CORT secretion is thought to play an active role in mediating symptoms of depression (for review see Ressler and Nemeroff Dep and anxiety 2000), we also compared FST-induced CORT secretion in HR vs. LR animals in the same experiment. (492 words; limit usually at 500).
Materials and Methods
Animals
For all experiments, a total of 131 adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing approximately 225–250 g upon arrival were used. Animals were housed three per cage in a room adjacent to the testing room, and maintained on a 12/12 h light/dark cycle (lights on at 0700 hours). Rats were acclimated to the animal quarters for 1 week before any experimental procedure. Experiments were conducted between 1300 and 1700 hours, during the light portion of the cycle. Food and water were available ad libitum. Animals were treated in accordance with National Institutes of Health guidelines on laboratory animal use and care.
Experiment 1: comparison of c-fos mRNA levels in DR, LC and NTS in HR vs. LR rats after FST
Locomotor activity test
After 7 days of habituation to the housing conditions, 15 rats were tested for locomotor activity during a 60 min exposure to the mild stress of a novel environment. Each rat was placed in a 43L × 21.5W × 24.5H (in cm) clear acrylic activity monitor, and locomotor activity was monitored by means of two banks of photocells connected to a microprocessor. Rats that exhibited locomotor counts in the highest third of the sample were classified as HR (n=5), whereas rats that exhibited locomotor counts in the lowest third of the sample were classified as LR (n=5) (Cecchi et al).
Forced swim test
The procedure used was that of Porsolt et al. (1978), slightly modified by Lucki and collaborators (cit). The animals were placed in vertical Plexiglas cylinders 60H × 40D (in cm) containing water (25°C) at a depth of 30 cm. On the first day of the experiment, rats were placed in the water for a 15-min period (pretest phase). Twenty-four hours later, the animals were exposed again to the above experimental conditions for a 5-min period (test phase). Animals were then euthanized 30 min after the test phase of the FST, their brain immediately removed, frozen in isopentane cooled to −30°C, and stored at −80°C.
In situ hybridization
The in situ hybridization method used in this study is described in detail by Isgor et al (2003). Briefly, tissue was sectioned at −20°C at a thickness of 12 μm, mounted onto poly(L-lysine)-coated slides, and stored at −80°C until use. Before probe hybridization, tissue was fixed in 4% paraformaldehyde at room temperature, rinsed with aqueous buffers, and dehydrated with graded alcohols. A sense and an antisense riboprobe were synthesized with incorporation of 35S-UTP and 35S-CTP from a cDNA fragment for c-fos generously donated by Dr. T. Curran, and hybridized to tissue overnight at 55°C. Sections were then washed with increasing stringency, dehydrated with graded alcohols, air-dried, and exposed to film. The sense probe showed no detectable signal in the brain. Exposure time was chosen to maximize signal of the antisense probe. After x-ray film exposure, sections were finally stained with cresyl violet, dehydrated, and coverslipped with a xylene-based mounting medium(Permount).
Image analysis
Six tissue sections were selected for each brain region (bregma −7.64 to −8.00 mm, bregma −9.68 to −10.04 mm and bregma −13.68 to −14.08 mm for DR, LC and NTS respectively) from each animal. Digital images of the brain sections were captured from X-ray films in the linear range of the gray levels using a CCD camera (TM-745, Pulnix, USA). Optical density for c-fos mRNA was determined for each section using the Micro Computer Imaging Device (Ontario, Canada) image analysis system. For each animal, data from multiple sections were averaged to obtain a representative value (mean optical density).
Experiment 2: comparison of the behavioral effects of DMI during FST in HR vs. LR animals
Forty-five rats were habituated to the housing conditions and screened for their locomotor activity as above, generating HR and LR groups (n=15 per group). After the pretest phase of the FST, HR and LR animals were randomly assigned to two different treatment groups: vehicle (VEH) or 10mg/kg DMI (DMI) to be administered subcutaneously 5 min, 6 hours, and 23 hours after the end of the pretest phase (taghzouti et al). The experimental design yielded 4 groups: HR-VEH, LR-VEH, HR-DMI and LR-DMI (n=7–8 per group). The selected dose of DMI has been shown to be effective but sub-maximal in affecting rats behavior in the FST (Detke and Lucki psychopharmacology 1995); it has therefore been chosen in order to highlight possible differences in response between HR and LR animals. Twenty-four hours after pretest the animals were tested on the FST for a 5-min period as above. Rats behavior during pretest and test was recorded to allow later scoring.
Behavior scoring
The time each rat spent climbing, lying immobile and swimming was measured for each rat during pretest and test. Climbing was defined as the behavior during which a rat makes vigorous movements with its forepaws in and out of the water, usually in contact with the walls; immobility was defined as the behavior during which a rat is only making those movements required to keep its head above the water; finally, swimming was defined as the behavior during which a rat is actively moving around in the cylinder (cit Lucki).
Experiment 3: comparison of the effects of FLU on climbing, immobility and swimming during FST in HR vs. LR animals
Animals were sorted in HR and LR and tested in the FST as above. Vehicle or fluoxetine (20 mg/kg) was administered subcutaneously 5 min, 6 hours, and 23 hours after the end of the pretest phase (n=7–8 per group). This dose of fluoxetine was chosen because it had been shown to be differentially effective in HR vs. LR animals (Taghzouti et al). The time spent by each rat climbing, laying immobile and swimming during pretest and test was measured as above.
Experiment 4: effects of DMI on FST-induced c-fos mRNA levels in NE nuclei and projections and FST-induced CORT secretion in HR vs LR rats
Animals were sorted in HR and LR according to their locomotor activity, and tested in the FST. HR and LR rats received a subcutaneous vehicle or DMI injection (10mg/kg) 5 min, 6 hours, and 23 hours after the end of the pretest phase according to their treatment group as in experiment 2 (n=6 per group). Animals were euthanized 30 min after the test phase of the FST and their brain and blood collected. Brains were immediately frozen in isopentane cooled to −30°C, and stored at −80°C. Blood samples were separated by centrifugation (3000 r.p.m. for 10 min at 4 °C), and plasma was removed, frozen and stored at −80 °C.
In situ hybridization
Brains were processed for in situ hybridization, and c-fos mRNA levels compared in HR vs. LR animals in bed nucleus of stria terminalis (BST; bregma −0.26 to −0.80 mm), hippocampus (HIPP; bregma −3.14 to −3.80 mm), paraventricular nucleus of the hypothalamus (PVN; bregma −1.60 to −2.12 mm), prefrontal cortex (PFC; bregma +3.20 to +3.70), LC and NTS. PFC and PVN have been selected because in a previous study DMI has been shown to affect FST-induced fos-like immunoreactivity in these nuclei (Duncan Ge et al JPET 1996). BST receives one of the densest NE fiber inputs in the brain (Moore et al), and have been shown to play a key role in NE modulation of stress and anxiety (cecchi et al). Finally, functional and structural abnormalities in the HIPP correlate with the presence and severity of affective disorders (Benedetti F et al Curr op psychiatry 2006). Six tissue sections were selected for each brain region and, for each animal, data from multiple sections were averaged to obtain a representative value (mean optical density).
CORT essay
We compared FST-induced CORT secretion in vehicle- and DMI-injected HR vs. LR animals 30 min after the end of the test, since it has been shown that this time corresponds to the peak of FST-induced CORT secretion (connor tj, Kelly jp Leonard be PBB 1997). Plasma CORT levels were determined by radioimmunoassay using a highly specific antibody developed in our laboratory and characterized by Dr. D. L. Helmreich. Specific details of the antibody and the radioimmunoassay procedure have been published previously (Campeau et al 1997). Intra- and inter-assay coefficients of variation were less than 10%.
Statistical analysis
Statistical comparisons were done by Student’s t test or ANOVA when more than two groups were compared. Where ANOVA indicated significant main effects or significant interactions, post-hoc comparisons were conducted using the Newman-Keuls test.
Results
Experiment 1: HR animals show higher c-Fos mRNA levels than LR in LC after FST
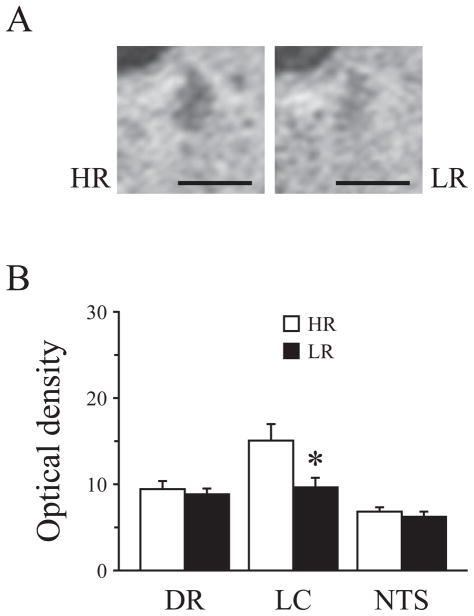
Levels of c-fos mRNA expression in the LC were significantly higher in HR than LR animals after FST (t=3.75). By contrast, there was no significant difference in c-fos mRNA levels in DR and NTS between the two groups of animals (see figure 1).
Figure 1.
Comparison of c-fos mRNA levels in DR, LC and NTS after FST in HR vs. LR rats. A) Representative coronal sections showing mRNA signal for c-fos after FST in the LC of HR and LR animals as measured by in situ hybridization. B) Optical density for c-fos mRNA in the DR, LC and NTS after FST in HR vs. LR animals. All values are mean ± SEM (n=5 per group) *=p< 0.05 compared to HR animals. Student’s t test.
Experiment 2: DMI significantly decreases immobility in FST in LR but not HR animals
HR and LR animals behaved similarly in during pretest, with no significant differences between groups in climbing, immobility or swimming time (data not shown).
During test, DMI treatment had a significant effect on climbing time (F1,25=10.65, p<0.01), with no significant phenotype effect or significant phenotype x treatment interaction (see figure 2a). Statistical analyses for immobility time showed a significant group x treatment interaction (F1,25=4.10, p<0.05), with no significant phenotype or treatment effects. Subsequent post-hoc analyses indicated that DMI significantly decreased immobility time in LR but not HR animals (see figure 2b). Finally, two-way ANOVA for swimming time revealed a significant treatment effect (F1,25=5.14, p<0.05) and a significant phenotype x treatment interaction (F1,25=4.36, p<0.05) with no significant phenotype effect. Subsequent post-hoc analyses showed a significant DMI-induced increase in swimming time in HR but not LR animals (see figure 2c).
Figure 2.
Behavioral effects of DMI treatment (10 mg/kg) on the FST. A) Effects of DMI on climbing (A), immobility (B) and swimming time (C) in HR and LR animals during the test phase of the FST. All values are mean ± SEM (n=7–8 per group) *=p< 0.05, **=p<0.01 compared to vehicle-treated rats in the same phenotype group. Two-way ANOVA followed by Newman-Keuls test for post-hoc comparisons.
Experiment 3: FLU similarly affects HR and LR animals behavior in the FST
Like in experiment 2, HR and LR animals behaved similarly during pretest (data not shown).
During test, there were no significant effects of FLU treatment on climbing time (see figure 3a). Statistical analyses for immobility time showed a significant effect of treatment (F1,26=9.071, p<0.01), with no significant phenotype effect or significant phenotype × treatment interaction (see figure 3b). Finally, statistical analyses for swimming time revealed a significant increase in FLU treated animals (F1,26=5.187, p<0.05) with no significant phenotype effect or significant phenotype × treatment interaction (see figure 3c).
Figure 3.
Behavioral effects of FLU treatment (20 mg/kg) on the FST. Climbing (A), immobility (B) and swimming time (C) during the test phase of the FST in HR and LR animals in response to saline or FLU treatment. All values are mean ± SEM (n=7–8 per group). *=p< 0.05, **=p<0.01 compared to HR animals. Two-way ANOVA.
Experiment 4: DMI differentially affects FST-induced c-fos mRNA expression in NTS, PFC and PVN, and FST-induced CORT secretion in HR vs. LR animals
When results for DMI effects of FST-induced c-fos expression in LC where analyzed, two-way ANOVA showed significant phenotype and treatment effects (F1,16=37.950, p<0.01; and F1,16=53.895, p<0.01 respectively), with also a significant phenotype × treatment interaction (F1,16=10.803, p<0.01). Subsequent post-hoc analyses confirmed results in experiment 1 indicating higher FST-induced c-fos mRNA levels in HR than in LR animals, and showed a significant decrease in c-fos mRNA expression after DMI treatment regardless of the rats phenotype (see figure 4a).
Figure 4.
Effects of DMI treatment (10 mg/kg) on FST-induced c-fos mRNA levels. Messenger RNA levels for c-fos after FST in the LC (A), NTS (B), PFC (C) and PVN (D) of HR and LR animals treated with either saline or DMI as measured by in situ hybridization. All values are mean ± SEM (n=5–6 per group). *=p< 0.05, **=p<0.01 compared to vehicle-treated rats in the same phenotype group. #=p< 0.05, ##=p<0.01 compared to HR animals in the same treatment group. Two-way ANOVA followed by Newman-Keuls test for post-hoc comparisons.
By contrast, statistical analyses for DMI effects of FST-induced c-fos expression in NTS showed a significant decrease in FST-induced c-fos mRNA expression with no significant phenotype effect or phenotype × treatment interaction (see figure 4b).
When data for the brain regions in the forebrain were analyzed, DMI showed no phenotype-dependent effects in cingulate cortex, BST and CA1, CA2 and CA3 subregions of the HIPP (data not shown). Statistical analyses for PFC and PVN revealed instead a significant treatment effect for PFC (F1,20=15.748, p<0.01) and a significant phenotype × treatment interaction for both PFC and PVN (F1,20=19.200, p<0.01; and F1,20=7.200, p<0.05 respectively). Subsequent post-hoc analyses showed a significant decrease in c-fos mRNA expression after DMI treatment only in LR animals (see figures 4c and 4d).
Finally, statistical analyses for FST-induced CORT secretion indicated a significant effect for DMI treatment (F1,18=11.944, p<0.01) with also phenotype × treatment interaction very close to significance (F1,18=3.861, p<0.066). The post-hoc analyses following two-way ANOVA showed a significant decrease in FST-induced CORT secretion in LR but not HR animals (see figure 5).
Figure 5.
Effects of DMI on FST-induced CORT levels. CORT levels for HR and LR animals treated with either saline or DMI 30 min after the test phase of the FST. All values are mean ± SEM (n=5–6 per group). *=p< 0.05 compared to vehicle-treated rats in the same phenotype group. Two-way ANOVA followed by Newman-Keuls test for post-hoc comparisons.
Discussion
Our results show higher levels of LC activation, as measured by c-fos mRNA expression, in HR than in LR animals after FST. These results are consistent with previous reports of higher NE release in HIPP in HR than in LR rats during restraint stress (Rosario and Abercrombie, Brain res bull 1999): HIPP receives NE afferents exclusively from the LC through the dorsal NE bundle (Loy R et al 1980 jcn), and our data add further evidence of higher activation of this NE pathway in HR animals during inescapable stress.
When we investigated the effects of DMI in the FST in HR vs. LR animals, DMI produced a significant increase in climbing regardless of the rats phenotype, and a decrease in swimming only in HR rats. The significance of climbing and swimming behaviors in the FST is unclear. It has been suggested that they represent active coping strategies aimed at escaping and removal of stress (Thierry b et al behav. Neural boil 1984). On the other hand, a decrease in immobility during FST is believed to be foretelling of a compound antidepressant effect (porsolt et al Nature 1977). Our data indicate that novelty-seeking behavior in rats is predictive of DMI efficacy in reducing immobility time in the FST: in our study, DMI was effective in decreasing immobility only in LR rats. The decrease in immobility in this group of animals was accompanied by significantly lower FST-induced c-fos mRNA levels in PFC and PVN, and significantly lower CORT levels compared to vehicle injected LR rats. Our data are consistent with previous reports of decrease in fos–like immunoreactivity in the PFC and PVN but dorsal-lateral BST after FST in animals treated with NRIs at an effective dose (Duncan et al Jpet 1996). Moreover, Connor and collaborators have shown an attenuation of FST-associated elevation in serum corticosterone after DMI treatment (Connor TJ et al PBB 2000).
In agreement with our findings, inhibition of PFC seems to facilitate NRIs behavioral effects in the FST. For example, lesions of the NA dorsal bundle facilitate the increase in climbing and decrease in immobility induced by the NRI reboxetine in the FST (Cryan JF et al eur j pharm 2002). Moreover, in a genetic model of depression, catecholamine levels are increased in the PFC of Flinders sensitive rats when compared to normal Sprague-Dawley controls; catecholamine levels and immobility time in the FST become normalized in these rats after chronic treatment with DMI (Zangen A et al Brain res 1999).
A decrease in FST-induced fos-like immunoreactivity in the PVN has been previously reported after DMI administration (Duncan et al Jpet 1996). Levels of PVN activation and CORT secretion regulate immobility in the FST. Adrenalectomized animals show lower immobility in the FST, and CORT administration reverses the effects of adrenalectomy (Mitchell and Meaney behav neurosci 1991). Moreover, administration of metyrapone, a blocker of CORT synthesis, before the test phase of the FST significantly reduces immobility time and its effect is partially reverted by CORT injection (Baez and Volosin PBB 1994). It is not known exactly how CORT affects immobility time in the FST. Microinjection of the glucocorticoid receptor antagonist RU38486 in the dentate gyrus of the HIPP diminishes immobility time in the test (De Kloet, De Cock neuroendocrinology 1988). It has therefore been proposed that this brain region is at least in part responsible for behavioral effects of CORT in the FST. Interestingly, anti-glucocorticoid treatment seems to be beneficial to patients with major depression (for review see Wolkowitz and Reus psychosom med 1999), and has been shown to have synergistic effects when administered with the NRI imipramine in patients with treatment-resistant depression (Rogoz Z et al Pol J Pharmacol 2004).
Even though direct projections from the PFC to the PVN are limited, the PFC has been shown to regulate PVN activation and ACTH secretion during stress (Figueiredo HF et al E J Neurosci 2003). It is therefore possible that changes in FST-induced c-fos expression in the PVN and CORT secretion are the consequence of a direct action of DMI on the PFC that would in turn affect PVN and HPA-axis activation. However, PFC seems to exert an inhibitory effect on HPA axis during stress; therefore, the apparent decrease in PFC activation during FST after DMI treatment should lead to and increase in CORT secretion and consequent increase in immobility. Instead, lesion of the dorsal NA bundle potentiates the effects of reboxetine in the FST. Moreover, an intact ventral NA bundle is required for reboxetine-induced antidepressant-like behavior in the FST (Cryan et al E J Pharmacol 2002). In our opinion, it is more likely that DMI-induced effects in PVN during FST are not PFC-mediated and that an inhibition of PFC favors the DMI-induced behavioral changes in the FST independent of PVN. For example, it has been recently found that PFC helps detect if a stressor is controllable or incontrollable. If the stressor is deemed controllable DR activation is inhibited by the PFC. As a consequence, behaviors associated with incontrollable stress are blocked (Amat J et al Nat neurosci 2005). Changes in PFC activation could alter the animal perception of stress controllability and, as a consequence, delay the transition between active and passive coping strategies in the FST.
DMI treatment decreased FST-induced c-fos in LC in both HR and LR rats, and in NTS in HR rats alone. These findings are consistent with previous studies suggesting that inhibition of NE reuptake by NRIs causes a stimulation of postsynaptic receptors which in turn decreases the activity of the presynaptic neurons by a feed-back mechanism (Nyback and Walters and Roth 1975 e j pharm, Scuvee-Moreau JJ, Dresse AE. Eur j pharm 1979). It has been suggested that such inhibition could be critical in mediating the effects of DMI on immobility during forced swim test in rats (kostowski W et Nowakowska E et al. Pol j PP 1984). However, in our study, FST-induced c-fos expression was similarly reduced in LC in HR and LR animals, regardless of their response to DMI in the FST, and reduced in NTS only in HR animals. Moreover, in LR animals’ c-fos mRNA levels after FST were altered by DMI in the PVN but not the BST, even though both nuclei receive their NE input mainly through the ventral bundle (moore etc). Similarly, FST-induced c-fos mRNA expression was decreased in PFC but not HIPP after DMI administration in LR rats, even though these brain regions both receive NE innervation through the dorsal bundle (moore etc). Our results suggest that the mechanisms through which DMI affects immobility time in the FST are, at least in part, postsynaptic.
In our study, HR and LR animals showed similar c-fos mRNA expression in DR after FST, and a similar decrease in immobility time and increase in swimming after FLU administration in both groups of animals. In a previous study, Taghzouti and collaborators have shown a differential response to FLU in HR vs. LR animals in the FST. In their study, FLU caused a significant decrease in immobility time in LR animals in the FST. By contrast, HR animals showed a significant increase in immobility after FLU administration. Similar results were obtained for swimming time. In the broad population of rats, they observed no effect of FLU on immobility time (taghzouti et al 1999 Biol psychiatry). Most of the published studies report a decrease in immobility time and increase in swimming behavior in the FST after FLU administration (for review see Cryan jf et al Neurosci and biobehavioral reviews 2005). There have been however reports of a lack of effect of SSRIs in the FST in rats (Borsini Neurosci biobehav rev 1995). It is possible that small differences in experimental protocol and/or scoring of the results might be behind the heterogeneity of results obtained in the FST after SSRIs administration.
Conclusion
In conclusion, our results show that differences in novelty-seeking behavior can predict inter-individual variability in responses to DMI in the FST in rats. In our study DMI was selectively effective in decreasing immobility only in LR animals. This behavioral effect was accompanied by a decrease in FST-induced c-fos mRNA expression in the PFC and PVN, and by lower FST-induced CORT secretion. Our study proposes the HR-LR model as a valuable tool to investigate between-subject variability in responsiveness to DMI in the FST, and suggest that further studies based on this model of inter-individual variability in rats could possibly lead to a better understanding of the biological mechanisms underlying individual differences in behavioral and physiological responses to NRIs in humans.
Acknowledgments
We thank James Stewart for his excellent technical assistance.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Baez M, Volosin M. Corticosterone influences forced swim-induced immobility. Pharmacol Biochem Behav. 1994;49:729–36. doi: 10.1016/0091-3057(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Pontiggia A. Depression and neurological disorders. Curr Opin Psychiatry. 2006;19:14–8. doi: 10.1097/01.yco.0000194147.88647.7f. [DOI] [PubMed] [Google Scholar]
- Borsini F. Role of the serotonergic system in the forced swimming test. Neurosci Biobehav Rev. 1995;19:377–95. doi: 10.1016/0149-7634(94)00050-b. [DOI] [PubMed] [Google Scholar]
- Campeau S, Akil H, Watson SJ. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mRNA in the forebrain associated with audiogenic stress in rats. J Neurosci. 1997;17:5979–92. doi: 10.1523/JNEUROSCI.17-15-05979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Kelliher P, Shen Y, Harkin A, Kelly JP, Leonard BE. Effect of subchronic antidepressant treatments on behavioral, neurochemical, and endocrine changes in the forced-swim test. Pharmacol Biochem Behav. 2000;65:591–7. doi: 10.1016/s0091-3057(99)00192-6. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Kelly JP, Leonard BE. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav. 1997;58:961–7. doi: 10.1016/s0091-3057(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–15. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Vallee M, Maccari S, Piazza PV, Le Moal M, Simon H. Behavioral reactivity to novelty during youth as a predictive factor of stressinduced corticosterone secretion in the elderly--a life-span study in rats. Psychoneuroendocrinology. 1996;21:441–53. doi: 10.1016/0306-4530(96)00017-0. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther. 1996;277:1076–89. [PubMed] [Google Scholar]
- Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- Isgor C, Cecchi M, Kabbaj M, Akil H, Watson SJ. Estrogen receptor beta in the paraventricular nucleus of hypothalamus regulates the neuroendocrine response to stress and is regulated by corticosterone. Neuroscience. 2003;121:837–45. doi: 10.1016/s0306-4522(03)00561-x. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheiner J, Bertilsson L, Bruus H, Wolff A, Roots I, Bauer M. Individualized medicine - implementation of pharmacogenetic diagnostics in antidepressant drug treatment of major depressive disorders. Pharmacopsychiatry. 2003;36(Suppl 3):S235–43. doi: 10.1055/s-2003-45136. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Danysz W, Plaznik A, Nowakowska E. Studies on the locus coeruleus system in an animal model for antidepressive activity. Pol J Pharmacol Pharm. 1984;36:523–30. [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Meaney MJ. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav Neurosci. 1991;105:798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Nyback HV, Walters JR, Aghajanian GK, Roth RH. Tricyclic antidepressants: effects on the firing rate of brain noradrenergic neurons. Eur J Pharmacol. 1975;32:302–12. doi: 10.1016/0014-2999(75)90297-6. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Rogoz Z, Skuza G, Wojcikowski J, Daniel WA, Wrobel A, Dudek D, Zieba A. Effect of metyrapone supplementation on imipramine therapy in patients with treatment-resistant unipolar depression. Pol J Pharmacol. 2004;56:849–55. [PubMed] [Google Scholar]
- Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Res Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- Scuvee-Moreau JJ, Dresse AE. Effect of various antidepressant drugs on the spontaneous firing rate of locus coeruleus and dorsal raphe neurons of the rat. Eur J Pharmacol. 1979;57:219–25. doi: 10.1016/0014-2999(79)90368-6. [DOI] [PubMed] [Google Scholar]
- Stead JD, Clinton S, Neal C, Schneider J, Jama A, Miller S, Vazquez DM, Watson SJ, Akil H. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav Genet. 2006;36:697–712. doi: 10.1007/s10519-006-9058-7. [DOI] [PubMed] [Google Scholar]
- Taghzouti K, Lamarque S, Kharouby M, Simon H. Interindividual differences in active and passive behaviors in the forced-swimming test: implications for animal models of psychopathology. Biol Psychiatry. 1999;45:750–8. doi: 10.1016/s0006-3223(98)00156-5. [DOI] [PubMed] [Google Scholar]
- Thierry B, Steru L, Chermat R, Simon P. Searching-waiting strategy: a candidate for an evolutionary model of depression? Behav Neural Biol. 1984;41:180–9. doi: 10.1016/s0163-1047(84)90555-7. [DOI] [PubMed] [Google Scholar]
- Young AH. Antiglucocoticoid treatments for depression. Aust N Z J Psychiatry. 2006;40:402–5. doi: 10.1080/j.1440-1614.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- Zangen A, Overstreet DH, Yadid G. Increased catecholamine levels in specific brain regions of a rat model of depression: normalization by chronic antidepressant treatment. Brain Res. 1999;824:243–50. doi: 10.1016/s0006-8993(99)01214-7. [DOI] [PubMed] [Google Scholar]