Abstract
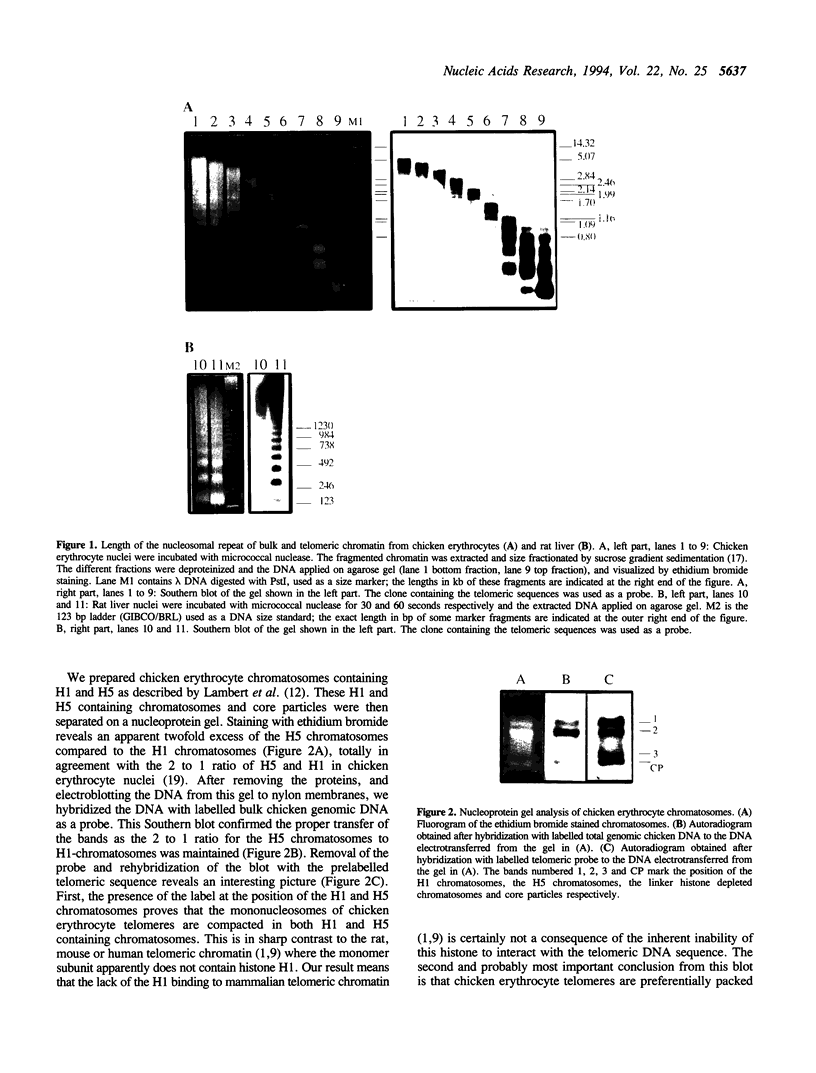
Rat liver telomeric DNA is organised into nucleosomes characterised by a shorter and more homogeneous average nucleosomal repeat than bulk chromatin as shown by Makarov et al. (1). The latter authors were unable to detect the association of any linker histone with the telomeric DNA. We have confirmed these observations but show that in sharp contrast chicken erythrocyte telomeric DNA is organised into nucleosomes whose spacing length and heterogeneity are indistinguishable from those of bulk chromatin. We further show that chicken erythrocyte telomeric chromatin contains chromatosomes which are preferentially associated with histone H1 relative to histone H5. This contrasts with bulk chromatin where histone H5 is the more abundant species. This observation strongly suggests that telomeric DNA condensed into nucleosome core particles has a higher affinity for H1 than H5. We discuss the origin of the discrimination of the lysine rich histones in terms of DNA sequence preferences, telomere nucleosome preferences and particular constraints of the higher order chromatin structure of telomeres.
Full text
PDF



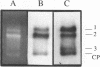
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Bates D. L., Thomas J. O. Histones H1 and H5: one or two molecules per nucleosome? Nucleic Acids Res. 1981 Nov 25;9(22):5883–5894. doi: 10.1093/nar/9.22.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Chiou S. S. Non-nucleosomal packaging of a tandemly repeated DNA sequence at termini of extrachromosomal DNA coding for rRNA in Tetrahymena. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2263–2267. doi: 10.1073/pnas.78.4.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: no end in sight. Cell. 1994 Jun 3;77(5):621–623. doi: 10.1016/0092-8674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Buckle R. S., Maman J. D., Allan J. Site-directed mutagenesis studies on the binding of the globular domain of linker histone H5 to the nucleosome. J Mol Biol. 1992 Feb 5;223(3):651–659. doi: 10.1016/0022-2836(92)90981-o. [DOI] [PubMed] [Google Scholar]
- Caron F., Thomas J. O. Exchange of histone H1 between segments of chromatin. J Mol Biol. 1981 Mar 15;146(4):513–537. doi: 10.1016/0022-2836(81)90045-0. [DOI] [PubMed] [Google Scholar]
- Cerf C., Lippens G., Ramakrishnan V., Muyldermans S., Segers A., Wyns L., Wodak S. J., Hallenga K. Homo- and heteronuclear two-dimensional NMR studies of the globular domain of histone H1: full assignment, tertiary structure, and comparison with the globular domain of histone H5. Biochemistry. 1994 Sep 20;33(37):11079–11086. doi: 10.1021/bi00203a004. [DOI] [PubMed] [Google Scholar]
- Fang G., Cech T. R. The beta subunit of Oxytricha telomere-binding protein promotes G-quartet formation by telomeric DNA. Cell. 1993 Sep 10;74(5):875–885. doi: 10.1016/0092-8674(93)90467-5. [DOI] [PubMed] [Google Scholar]
- Giraldo R., Rhodes D. The yeast telomere-binding protein RAP1 binds to and promotes the formation of DNA quadruplexes in telomeric DNA. EMBO J. 1994 May 15;13(10):2411–2420. doi: 10.1002/j.1460-2075.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C. W. Mammalian telomere dynamics: healing, fragmentation shortening and stabilization. Curr Opin Genet Dev. 1994 Apr;4(2):203–211. doi: 10.1016/s0959-437x(05)80046-2. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hicke B. J., Celander D. W., MacDonald G. H., Price C. M., Cech T. R. Two versions of the gene encoding the 41-kilodalton subunit of the telomere binding protein of Oxytricha nova. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1481–1485. doi: 10.1073/pnas.87.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. Y., Garrard W. T. Electrophoretic analyses of nucleosomes and other protein-DNA complexes. Methods Enzymol. 1989;170:116–142. doi: 10.1016/0076-6879(89)70044-6. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Käs E., Laemmli U. K. Highly preferential nucleation of histone H1 assembly on scaffold-associated regions. J Mol Biol. 1989 Dec 5;210(3):573–585. doi: 10.1016/0022-2836(89)90133-2. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Hofsteenge J. The repressor MDBP-2 is a member of the histone H1 family that binds preferentially in vitro and in vivo to methylated nonspecific DNA sequences. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9499–9503. doi: 10.1073/pnas.89.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S., Muyldermans S., Baldwin J., Kilner J., Ibel K., Wijns L. Neutron scattering studies of chromatosomes. Biochem Biophys Res Commun. 1991 Sep 16;179(2):810–816. doi: 10.1016/0006-291x(91)91889-k. [DOI] [PubMed] [Google Scholar]
- Lauderdale J. D., Stein A. Effects of plasmid length and positioned nucleosomes on chromatin assembly in vitro. Biochemistry. 1993 Jan 19;32(2):489–499. doi: 10.1021/bi00053a013. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Lejnine S., Bedoyan J., Langmore J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell. 1993 May 21;73(4):775–787. doi: 10.1016/0092-8674(93)90256-p. [DOI] [PubMed] [Google Scholar]
- Mohr E., Trieschmann L., Grossbach U. Histone H1 in two subspecies of Chironomus thummi with different genome sizes: homologous chromosome sites differ largely in their content of a specific H1 variant. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9308–9312. doi: 10.1073/pnas.86.23.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S., Lasters I., Hamers R., Wyns L. Assembly of oligonucleosomes into a limit series of multimeric higher-order chromatin structures. Eur J Biochem. 1985 Aug 1;150(3):441–446. doi: 10.1111/j.1432-1033.1985.tb09040.x. [DOI] [PubMed] [Google Scholar]
- Muyldermans S., Lasters I., Wyns L., Hamers R. Protection of discrete DNA fragments by the complex H1-octamerhistones or H5-octamerhistones after micrococcal nuclease digestion. Nucleic Acids Res. 1981 Aug 11;9(15):3671–3680. doi: 10.1093/nar/9.15.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyldermans S., Travers A. A. DNA sequence organization in chromatosomes. J Mol Biol. 1994 Jan 21;235(3):855–870. doi: 10.1006/jmbi.1994.1044. [DOI] [PubMed] [Google Scholar]
- Ner S. S., Travers A. A. HMG-D, the Drosophila melanogaster homologue of HMG 1 protein, is associated with early embryonic chromatin in the absence of histone H1. EMBO J. 1994 Apr 15;13(8):1817–1822. doi: 10.1002/j.1460-2075.1994.tb06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings S., Muyldermans S., Wyns L. Comparative filter binding study of H5 to nucleosome core particles, H1, H5 depleted chromatosomes and DNA fragments. Mol Biol Rep. 1988;13(4):191–196. doi: 10.1007/BF00788170. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan V., Finch J. T., Graziano V., Lee P. L., Sweet R. M. Crystal structure of globular domain of histone H5 and its implications for nucleosome binding. Nature. 1993 Mar 18;362(6417):219–223. doi: 10.1038/362219a0. [DOI] [PubMed] [Google Scholar]
- Roche J., Gorka C., Goeltz P., Lawrence J. J. Association of histone H1(0) with a gene repressed during liver development. Nature. 1985 Mar 14;314(6007):197–198. doi: 10.1038/314197a0. [DOI] [PubMed] [Google Scholar]
- Satchwell S. C., Drew H. R., Travers A. A. Sequence periodicities in chicken nucleosome core DNA. J Mol Biol. 1986 Oct 20;191(4):659–675. doi: 10.1016/0022-2836(86)90452-3. [DOI] [PubMed] [Google Scholar]
- Schultze P., Smith F. W., Feigon J. Refined solution structure of the dimeric quadruplex formed from the Oxytricha telomeric oligonucleotide d(GGGGTTTTGGGG). Structure. 1994 Mar 15;2(3):221–233. doi: 10.1016/s0969-2126(00)00023-x. [DOI] [PubMed] [Google Scholar]
- Sun J. M., Ali Z., Lurz R., Ruiz-Carrillo A. Replacement of histone H1 by H5 in vivo does not change the nucleosome repeat length of chromatin but increases its stability. EMBO J. 1990 May;9(5):1651–1658. doi: 10.1002/j.1460-2075.1990.tb08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. M., Ali Z., Lurz R., Ruiz-Carrillo A. Replacement of histone H1 by H5 in vivo does not change the nucleosome repeat length of chromatin but increases its stability. EMBO J. 1990 May;9(5):1651–1658. doi: 10.1002/j.1460-2075.1990.tb08285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerup H., Dousmanis A., de Lange T. Unusual chromatin in human telomeres. Mol Cell Biol. 1994 Sep;14(9):5777–5785. doi: 10.1128/mcb.14.9.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga-Weisz P., Zlatanova J., Leuba S. H., Schroth G. P., van Holde K. Binding of histones H1 and H5 and their globular domains to four-way junction DNA. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3525–3529. doi: 10.1073/pnas.91.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman S. E., Sittman D. B., Chaires J. B. Preferential binding of H1e histone to GC-rich DNA. Biochemistry. 1994 Jan 11;33(1):384–388. doi: 10.1021/bi00167a049. [DOI] [PubMed] [Google Scholar]
- Wright J. H., Gottschling D. E., Zakian V. A. Saccharomyces telomeres assume a non-nucleosomal chromatin structure. Genes Dev. 1992 Feb;6(2):197–210. doi: 10.1101/gad.6.2.197. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Shiue L., Kaplan S., de Lange T. A mammalian factor that binds telomeric TTAGGG repeats in vitro. Mol Cell Biol. 1992 Nov;12(11):4834–4843. doi: 10.1128/mcb.12.11.4834. [DOI] [PMC free article] [PubMed] [Google Scholar]