Abstract
Objective
To determine the association between symptomatic central venous line (CVL) related deep venous thrombosis (DVT) and a mortality-adjusted measure of duration of mechanical ventilation (MV) in critically ill children with CVL.
Design
Retrospective matched cohort study.
Setting
11 pediatric ICUs across the United States.
Patients
Twenty-nine index critically ill children with CVL-related DVT from a previous prospective observational study on symptomatic venous thromboembolism (VTE) were compared to 116 control children with CVL without VTE. Each index patient was matched to 4 control patients based on age group, disease category, severity of illness score and number of days in the ICU prior to CVL insertion.
Interventions
None.
Measurements and Main Results
Index patients were appropriately matched to control patients, with similar characteristics between the 2 groups. Index patients had fewer ventilator free days (VFD i.e. days alive and breathing unassisted within 28 days after CVL insertion) compared to matched control patients (16.8±11.5 days versus 22.3±4.9 days, P=.040). Index patients also had less ICU free days (ICUFD i.e. days alive and discharged from the ICU within 28 days after CVL insertion) (9.8±9.9 days versus 17.9±5.7 days, P<.001). Durations of MV (17.6±40.6 days versus 5.2±5.5 days, P=.236) and ICU stay (38.1±61.7 days versus 11.9±10.9 days, P=.011) were longer in index patients. The mortality rate was statistically similar between the 2 groups.
Conclusions
The presence of symptomatic CVL-related DVT is associated with worse outcomes, particularly fewer VFD, in critically ill children. The causal relationship that DVT leads to impairment in lung function and delays weaning from MV and discharge from the ICU needs to be proven prospectively. VFD is a possible alternative outcome measure for future DVT studies.
Keywords: Venous thromboembolism, mortality, intensive care unit, catheter, pediatrics, outcome, critical care
INTRODUCTION
Venous thromboembolism (VTE), which includes deep venous thrombosis (DVT) and pulmonary embolism (PE), is a significant problem in adults (1–3). It is the leading cause of preventable excess mortality and morbidity in hospitalized adult patients (1). VTE is also a growing concern in children. The incidence of VTE in children is increasing (4–6) mostly due to the prevalent use of central venous lines (CVL) (6–9). CVL is currently the most significant risk factor for the development of DVT in children (6–11).
The outcomes of DVT in children are less studied compared to adults (12). PE and PE-related deaths, which are the most significant acute outcomes of DVT, also occur in children though less commonly than in adults (12–15). PE is difficult to diagnose in children, particularly in those who are critically ill, due to the non-specificity of its signs and symptoms (2, 9, 12–16). The reported incidences of PE and PE-related deaths are based on registries of symptomatic cases that may underestimate the true incidences of the disease (5, 7, 9, 12).
PE can lead to ventilation and perfusion mismatch in the lungs that may be more pronounced in critically ill patients (8, 9). The impairment in lung function has been associated with difficulty in weaning patients from mechanical ventilation (17). Conceivably, critically ill patients who are breathing unassisted may require mechanical ventilation with PE. Increased duration of mechanical ventilation is associated with morbidities such as pneumonia that may lead to prolonged stay in the intensive care unit (ICU) or even death (18).
In this study, we aim to determine the association between symptomatic CVL-related DVT and a mortality-adjusted measure of duration of mechanical ventilation in critically ill children with CVL. We hypothesize that in this population, mortality-adjusted duration of mechanical ventilation is significantly associated with having DVT.
METHODS
Study Design
We performed a retrospective matched cohort study on critically ill children with CVL. The study sample came from a multicenter prospective observational study on symptomatic VTE, which was conducted between April 2006 and December 2007. After obtaining local institutional review board approval or exemption, 11 pediatric ICUs in the United States collected data on symptomatic VTE under the auspices of the National Association of Children’s Hospitals and Related Institutions (NACHRI) PICU Focus Group. The centers represented a variety of ICU types across different geographic regions in the country. Each center enrolled sequential admissions over a continuous 6 month period. Patients with clinically suspected VTE were evaluated and treated per each clinician’s usual practice. VTE were detected after patients presented with systemic or local symptoms. All VTE were confirmed radiographically. No specific screening procedures were conducted to diagnose asymptomatic VTE.
Subjects
Patients who were less than 18 years old and had a CVL placed during the ICU admission, were included in the current analysis. All types of CVL (i.e. tunneled, untunneled and peripherally inserted central catheters) were included. We used the first ICU admission of each patient to eliminate the effect of multiple admissions on the outcome measures. Patients were categorized into index or control with respect to having exposure to symptomatic CVL-related DVT during the study period. The relationship between the DVT and the CVL was determined by the local clinician based on the location and timing of the DVT and the location of the CVL. Patients with a CVL-related DVT present on admission were excluded in the current study because the dates of CVL insertion and initial diagnosis of the DVT were not available for these patients. Control patients did not have any documented VTE before or during the study period.
Data Collection
Data from the multicenter prospective study were collected and entered into the Virtual PICU System, LLC (VPS, LLC), a clinical database that connects a global network of children’s hospitals and standardizes data sharing and benchmarking between pediatric ICUs. We obtained the following data from VPS, LLC: age, gender, race and ethnicity, severity of illness score (i.e. Pediatric Index of Mortality 2 [PIM2]) (19), surgical status, diagnosis, date and time of CVL insertion and removal, date and time of commencement and termination of invasive mechanical ventilation, date and time of admission and medical discharge from the ICU, and mortality. Patients’ ages were divided into 3 groups – < 1 year old, 1–13 years old and > 13 years old – based on the known age-related epidemiology of VTE in children (4, 7, 20). Diagnoses were categorized to reflect the association between development of VTE and specific disease groups (2, 7, 10, 11, 16, 21–24).
Outcome Measures
The primary outcome measure was ventilator free days (VFD) at 28 days after CVL insertion. VFD was defined as the number of days within a preset time period (e.g. 28 days after CVL insertion) that a patient was alive and breathing completely unassisted (25). It incorporated mortality, which may falsely decrease the duration of mechanical ventilation without improving clinical outcome, and the duration of mechanical ventilation into a single outcome measure. Patients who died or remained on mechanical ventilation 28 days after CVL insertion were assigned 0 VFD (25). The number of VFD assigned to patients who were alive and breathing unassisted within 28 days after CVL insertion was equal to 28 minus the duration of mechanical ventilation. Secondary outcome measures were ICU free days (ICUFD) at 28 days after CVL insertion, duration of mechanical ventilation, duration of ICU stay and mortality. ICUFD was the number of days within 28 days after CVL insertion that a patient was alive and medically discharged from the ICU. The number of ICUFD assigned to each patient was similar to VFD.
The date and time of CVL insertion were used as a reference point to determine the durations of mechanical ventilation and ICU stay for each patient. Successful termination of mechanical ventilation was defined as more than 48 hours of breathing unassisted (26). Successful discharge from the ICU was defined as more than 48 hours after being discharged from the ICU service. For patients who died, the durations of mechanical ventilation and ICU stay were equal to the days that the patient was on mechanical ventilation and in the ICU, respectively, prior to death. The decisions to commence and terminate mechanical ventilation, as well as to discharge the patient from the ICU, were not protocolized, but were determined by the clinical team. None of the participating ICUs kept patients in the unit for heparin infusion as treatment for VTE.
Statistical Analysis
Matching was used to adjust for variables potentially confounding the association between outcome measures and DVT. We matched each index patient to 4 control patients based on age group, disease category, severity of illness score and number of ICU days prior to CVL insertion. Age group and disease category were consistently reported risk factors for DVT in children (4–7). Severity of illness score is associated with mortality rates (19). Inclusion of the number of ICU days prior to CVL insertion controlled for different clinical care factors that may have affected the development of DVT (27). Matching was performed using Match (28, 29). Match is a non-commercial software program that matches subjects based on 1 or more variables. The program produces near-optimal solutions that are typically superior to those obtained using hand matching or other semi-automated processes.
Patient characteristics expressed as categorical variables were compared using Cochran Q test. Patient characteristics and outcomes of interest expressed as continuous variable were averaged across the 4 control patients matched to every index patient, before comparing the 2 groups using Wilcoxon signed rank test. Mortality rates were compared using rate ratios.
Data are presented as mean±SD, number (%) and rate (95% confidence interval [CI]). P<.05 was considered statistically significant for all tests. Statistical tests were performed using SPSS 16.0 for Windows (SPSS, Inc., Chicago, Ill).
RESULTS
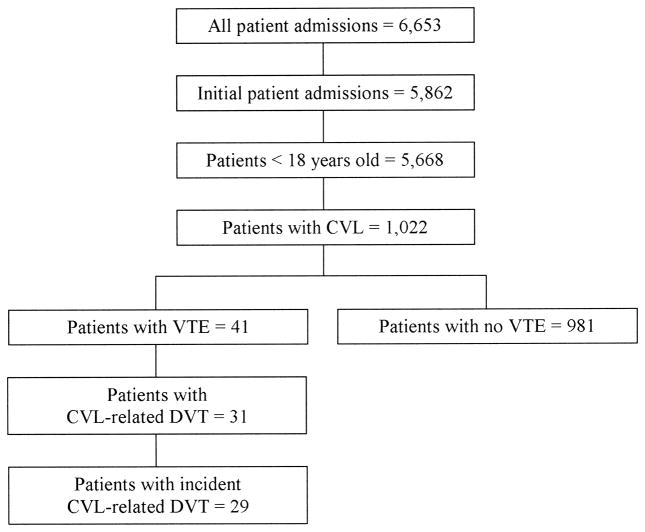
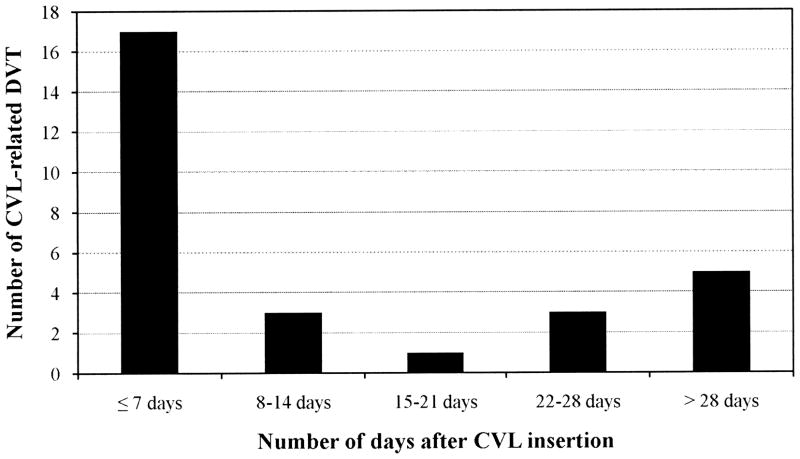
Prospective data were collected from 6,653 patient admissions (Figure 1). We identified 29 index patients with symptomatic CVL-related DVT and 981 control patients without VTE. The incidence of symptomatic CVL-related DVT was 2.9 per 100 patients or 5.2 per 1000 CVL days. DVT was detected in 17 out of 29 (58.6%) cases within 7 days of CVL insertion while 5 cases (17.2%) were diagnosed beyond 28 days of CVL insertion (Figure 2).
Figure 1.
Study patient selection. CVL – central venous line; VTE – venous thromboembolism; DVT – deep venous thrombosis.
Figure 2.
Distribution of central venous line (CVL) related deep venous thrombosis (DVT) based on the day of detection.
Index patients were appropriately matched to control patients with similar distributions of age groups and disease categories, as well as severity of illness scores and number of ICU days prior to CVL insertion between the 2 groups (Table 1). The patients were also comparable in gender, ethnicity, surgical status and duration of mechanical ventilation prior to CVL insertion.
Table 1.
Patient characteristics.
| Index |
Matched Control |
||
|---|---|---|---|
| N=29 | N=116 | P value | |
| Age group, no. (%) | .218 | ||
| < 1 year old | 21 (72.4) | 80 (69.0) | |
| 1–13 years old | 6 (20.7) | 25 (21.6) | |
| > 13 years old | 2 (6.9) | 11 (9.5) | |
| Gender, no. (% male) | 19(65.5) | 65 (56.0) | .570 |
| Race and ethnicity, no. (%) | .259 | ||
| Non-Hispanic white | 17 (58.6) | 56 (48.3) | |
| African-American | 5 (17.2) | 15 (12.9) | |
| Hispanic white | 1 (3.4) | 11 (9.5) | |
| Others | 6 (20.7) | 34 (29.3) | |
| PIM2, mean±SD | 0.09±0.14 | 0.07±0.10 | .859 |
| Surgical, no. (%) | 23 (79.3) | 82 (70.7) | .465 |
| Disease category, no. (%) | .615 | ||
| CHD | 13 (44.8) | 54 (46.6) | |
| Cancer | 1 (3.4) | 6 (5.2) | |
| Trauma | 4 (13.8) | 18 (15.5) | |
| Sepsis | 1 (3.4) | 2 (1.7) | |
| Others | 10 (34.5) | 36 (31.0) | |
| ICU days prior to CVL insertion, mean±SD | 0.7±2.0 | 0.5±0.8 | .736 |
| MV days prior to CVL insertion, mean±SD | 0.4±1.9 | 0.1±0.2 | .720 |
Index patients with central venous line (CVL)-related deep venous thrombosis were matched to control patients without venous thromboembolism based on age group, disease category, severity of illness score (Pediatric Index of Mortality 2 [PIM2]) and number of days in the intensive care unit (ICU) prior to CVL insertion. CHD – congenital heart disease; MV – mechanical ventilation.
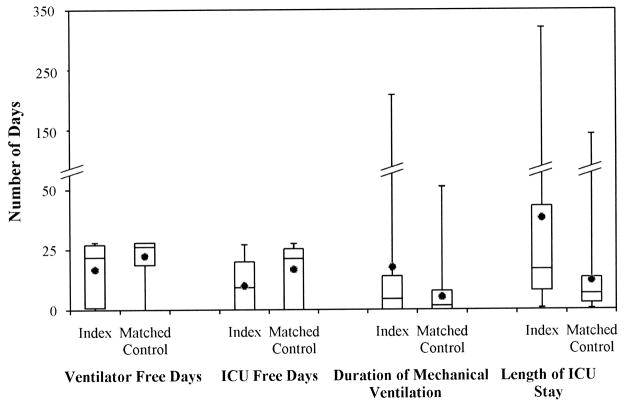
The mean VFD was 5.5 days (P=.040) less in the index patients compared to the matched controls (Table 2 and Figure 3). Similarly, the mean ICUFD was 8.1 days (P<.001) less in the index patients. Duration of mechanical ventilation was longer in the index patients by 12.4 days. This did not reach statistical significance (P=.236). Index patients, on average, stayed in the ICU 26.2 days longer than control patients (P=.011). DVT was not associated with mortality with a rate ratio of 1.50 (95% CI: 0.42, 5.30; P=.536).
Table 2.
Patient outcomes.
| Index |
Matched Control |
||
|---|---|---|---|
| N=29 | N=116 | P value | |
| Ventilator free days, mean±SD | 16.8±11.5 | 22.3±4.9 | .040 |
| ICU free days, mean±SD | 9.8±9.9 | 17.9±5.7 | <.001 |
| Duration of mechanical ventilation, mean±SD | 17.6±40.6 | 5.2±5.5 | .236 |
| ICU length of stay, mean±SD | 38.1±61.7 | 11.9±10.9 | .011 |
| Mortality rate per 100 patients, no. (rate; 95% CI) | 3 (10.3; 2.8–27.2) | 8 (6.9; 3.3–13.2) | .536 |
Index patients with central venous line (CVL)-related deep venous thrombosis were matched to control patients without venous thromboembolism based on age group, disease category, severity of illness score (Pediatric Index of Mortality 2 [PIM2]) and number of days in the intensive care unit (ICU) prior to CVL insertion. CI – confidence interval.
Figure 3.
Box plot comparing outcomes between index patients with central venous line (CVL) related deep venous thrombosis and control patients without venous thromboembolism matched based on age group, disease category, severity of illness score and days in the intensive care unit (ICU) prior to CVL insertion.
DISCUSSION
In this retrospective analysis, we report that patients with CVL-related DVT had worse outcomes with fewer VFD and ICUFD compared to patients without VTE, after adjusting for age, disease category, severity of illness and number of days in the ICU prior to CVL insertion. This was most likely due to prolongation in the durations of mechanical ventilation and ICU stay, respectively, and not due to increased mortality. Mortality was statistically similar between the 2 groups.
CVL is the most significant risk factor for the development of DVT in children (6–8, 10). Therefore, efforts to decrease the incidence of DVT in children have focused on this subset of patients (21, 23, 24, 30). CVL insertion damages the endothelium, initiating thrombus formation (31). Patients requiring CVL are also likely to have hypercoagulable states due to their underlying disease process (2, 3, 11, 16, 23, 24), to be immobile from their illness (10) or surgical status (32), or to be provided with interventions that further increase the risk for developing DVT (2, 3, 10, 32).
Nearly 20% of the DVT in our study were diagnosed beyond 28 days after CVL insertion, which was longer than our threshold for VFD and ICUFD. It is possible that these patients developed DVT earlier but it was not detected in the 28 day study window. In the study by Beck et al, where critically ill children with CVL were prospectively screened for symptomatic and asymptomatic DVT by ultrasonography, 88% of the DVT were detected within 4 days of CVL insertion and all DVT were diagnosed by 21 days after CVL insertion (33). Symptoms associated with DVT were noted in the regular structured physical examination within a day of DVT diagnosis by ultrasonography. In the absence of regular structured physical examinations in our study, it is possible that DVT symptoms were missed, delaying the diagnosis beyond 28 days after CVL insertion (8).
VFD and ICUFD are novel outcome measures for DVT studies both in adults and children. It is logical to incorporate duration of mechanical ventilation and mortality into a single measure. PE, which is the most significant complication of DVT, primarily affects lung function (8, 9, 17). When severe or in the presence of other co-morbid conditions, PE can lead to delays in weaning from mechanical ventilation, prolonged ICU stay and even death (2, 9, 17). VFD is a standard outcome measure in trials on acute respiratory failure in adults and children (25, 34, 35).
The duration of mechanical ventilation in patients with CVL-related DVT was longer compared to patients without VTE. The difference was not statistically significant in our analysis. However, we are underpowered to make any conclusions regarding this association. The association of duration of mechanical ventilation with DVT in children has not been investigated. In adults, two studies that prospectively screened critically ill patients for DVT reported contrasting results (2, 3). Prolonged duration of mechanical ventilation in patients with DVT was reported by Cook et al (2) but not by Ibrahim et al (3). Differences in sample size and in the patient populations among our study and the 2 adult studies may partly explain the disparity in results.
Patients with CVL-related DVT stayed in the ICU longer than children without VTE which is consistent with results from other pediatric populations. Hanson et al (10) and Candrilli et al (36) reported an independent association between DVT and prolonged ICU and hospital stay in children with trauma. In critically ill adults with DVT, Cook et al reported longer ICU and hospital lengths of stay (2). On the contrary, Ibrahim et al did not find any difference in lengths of ICU and hospital stay (3). The small sample size and more complex patient selection methods may have contributed to the lack of a significant association in the latter study.
Similar to findings in critically ill adults (2, 3), we did not find any statistically significant association between CVL-related DVT and mortality. While none of these studies, including ours, were adequately powered to detect a difference in mortality, it is possible that DVT is not associated with increased mortality in critically ill patients.
Decreased VFD and ICUFD in patients with CVL-related DVT are consistent with our hypothesis that DVT impairs lung function and prolongs duration of mechanical ventilation and ICU stay. Alternatively, prolonged mechanical ventilation and ICU stay may have predisposed the patients to the development of DVT. However, with DVT being an early event in the CVL course, it seems more likely that the differences in the outcome measures are due to thrombus formation. We cannot prove causality between CVL-related DVT and our outcome measures based on our study design. A randomized trial aimed at decreasing the incidence of DVT with a prophylactic intervention may be able to determine causality.
Our study has several strengths. Our patients were enrolled from 11 pediatric ICUs across the United States representing a variety of cases, ICU types and geographical distribution. The database from which our data were derived currently represents the largest collection of prospectively collected data on symptomatic VTE in critically ill children. The outcome measures used in the study, particularly VFD and ICUFD, are novel and have not been used in DVT studies in children or adults. Lastly, the decisions to commence and terminate mechanical ventilation and discharge patients from the ICU were not protocolized and were subjected to variations in physician practice. This increases the generalizability of our results.
Our study also has limitations that need to be considered. The study design precludes us from proving causality. The results may not be applicable to asymptomatic DVT, which is more common than symptomatic DVT (22, 23, 30, 33, 37–39). The diagnosis of CVL-related DVT was not centrally adjudicated. Finally, indication for mechanical ventilation and information on other risk factors for DVT were not collected and, therefore, not available for analysis (2, 3, 5–7, 10, 32).
Efforts to study DVT prevention in children is hindered by the absence of an acceptable outcome measure (40). In adults, due to the strong concordance between symptomatic and asymptomatic DVT, PE and PE-related death, the detection of DVT by a sensitive screening procedure such as ultrasonography is an accepted outcome in DVT prevention trials (1). Despite data to show that asymptomatic DVT leads to similar outcomes, such as post-thrombotic syndrome (41), a survey of pediatric critical care physicians reported that a majority of practitioners believe that asymptomatic DVT is not clinically significant and does not require treatment (42). Our study offers potential additional outcome measures in studying DVT. VFD, in particular, is clinically significant and accepted by pediatric intensivists as it has been used in prior studies on acute respiratory failure in children (26, 34).
Clinically, unsuspected PE from DVT should be considered in patients presenting with difficulty in weaning from mechanical ventilation. Prophylactic strategies against DVT may potentially decrease duration of mechanical ventilation. Alternatively, early extubation may minimize the development of DVT.
CONCLUSION
In critically ill children with CVL, symptomatic CVL-related DVT is associated with worse outcome, particularly fewer VFD. The decrease in VFD is more likely due to prolonged duration of mechanical ventilation and not due to an increase in mortality. The causal relationship between CVL-related DVT and the different outcome measures needs to be investigated prospectively. VFD is a possible alternative outcome measure that may be used in future DVT studies. The results of our study require validation in patients with the more common asymptomatic CVL-related DVT.
Acknowledgments
The authors wish to acknowledge gratefully the support of the NACHRI PICU Focus Group directed by Lynne Lostocco and the VPS, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated. The following members of the NACHRI PICU FOCUS Group contributed to the data collection necessary for the completion of this study: Children’s Hospital Medical Center of Akron, Akron, OH: Ann-Marie Brown and Kathleen Taylor; Children’s Hospital of Illinois, Peoria, IL: Al Torres and Paula LaFond; Children’s Hospital of Miami, Miami, Florida: Balagangadhar Totapally; Children’s Hospital of Wisconsin, Milwaukee, WI: Sheila Hanson and Jennifer McArthur; Cook Children’s Medical Center, Ft. Worth, TX: Cheryl Peterson; C.S. Mott Children’s Hospital, Ann Arbor, MI: Ron Dechert; Dell Children’s Medical Center of Central Texas, Austin, TX: LeeAnn Chrisite and Mari-Ann Alexander; Inova Fairfax Hospital for Children, Fairfax, VA: Peter Grundl, Steve Keller and Elizabeth Suddaby; The Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack, NJ: Stephen Percy, Jr.; Kosair Children’s Hospital, Louisville, KY: Deborah Campbell and Justine O’Flynn; and, University of Minnesota Medical Center, Fairview, MN: Sandra Hangstrom, Nancy Kase and Heidi Linhoff.
This publication was made possible in part by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH website.
The authors would also like to thank Drs. Jacques Lacroix and Sheila Hanson for their insightful reviews of the manuscript.
Financial support: Biostatistical support provided by Veronika Northrup was funded through CTSA Grant Number UL1 RR024139.
Footnotes
Institution where work was performed: Yale University School of Medicine
No reprints will be ordered.
Financial disclosure: Dr. Northrup received funding from NIH. The other authors have not disclosed any potential conflicts of interest.
Contributor Information
Edward Vincent S. Faustino, Department of Pediatrics, Yale University School of Medicine.
Karla A. Lawson, Trauma Services Department, Dell Children’s Medical Center of Central Texas.
Veronika Northrup, Yale Center for Clinical Investigation, Yale University School of Medicine.
Renee A. Higgerson, Pediatric Intensive Care Unit, Dell Children’s Medical Center of Central Texas.
References
- 1.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33(7):1565–1571. doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim EH, Iregui M, Prentice D, et al. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30(4):771–774. doi: 10.1097/00003246-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 5.Newall F, Wallace T, Crock C, et al. Venous thromboembolic disease: a single-centre case series study. J Paediatr Child Health. 2006;42(12):803–807. doi: 10.1111/j.1440-1754.2006.00981.x. [DOI] [PubMed] [Google Scholar]
- 6.Sandoval JA, Sheehan MP, Stonerock CE, et al. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. J Vasc Surg. 2008;47(4):837–843. doi: 10.1016/j.jvs.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 7.Monagle P, Adams M, Mahoney M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47(6):763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg NA, Bernard TJ. Venous thromboembolism in children. Pediatr Clin North Am. 2008;55(2):305–322. vii. doi: 10.1016/j.pcl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Van Ommen CH, Heijboer H, Buller HR, et al. Venous thromboembolism in childhood: a prospective two-year registry in The Netherlands. J Pediatr. 2001;139(5):676–681. doi: 10.1067/mpd.2001.118192. [DOI] [PubMed] [Google Scholar]
- 10.Hanson SJ, Punzalan RC, Greenup RA, et al. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma. 2010;68(1):52–56. doi: 10.1097/TA.0b013e3181a74652. [DOI] [PubMed] [Google Scholar]
- 11.McCrory MC, Brady KM, Takemoto C, et al. Thrombotic disease in critically ill children. Pediatr Crit Care Med. 2010 doi: 10.1097/PCC.0b013e3181ce7644. [DOI] [PubMed] [Google Scholar]
- 12.Van Ommen CH, Peters M. Acute pulmonary embolism in childhood. Thromb Res. 2006;118(1):13–25. doi: 10.1016/j.thromres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Babyn PS, Gahunia HK, Massicotte P. Pulmonary thromboembolism in children. Pediatr Radiol. 2005;35(3):258–274. doi: 10.1007/s00247-004-1353-y. [DOI] [PubMed] [Google Scholar]
- 14.Biss TT, Brandao LR, Kahr WH, et al. Clinical features and outcome of pulmonary embolism in children. Br J Haematol. 2008;142(5):808–818. doi: 10.1111/j.1365-2141.2008.07243.x. [DOI] [PubMed] [Google Scholar]
- 15.Derish MT, Smith DW, Frankel LR. Venous catheter thrombus formation and pulmonary embolism in children. Pediatr Pulmonol. 1995;20(6):349–354. doi: 10.1002/ppul.1950200603. [DOI] [PubMed] [Google Scholar]
- 16.Parker RI. Thrombosis in the pediatric population. Crit Care Med. 2010;38(2 Suppl):S71–75. doi: 10.1097/CCM.0b013e3181c9cce9. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274(4):335–337. [PubMed] [Google Scholar]
- 18.Srinivasan R, Asselin J, Gildengorin G, et al. A prospective study of ventilator-associated pneumonia in children. Pediatrics. 2009;123(4):1108–1115. doi: 10.1542/peds.2008-1211. [DOI] [PubMed] [Google Scholar]
- 19.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(2):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 20.Vu LT, Nobuhara KK, Lee H, et al. Determination of risk factors for deep venous thrombosis in hospitalized children. J Pediatr Surg. 2008;43(6):1095–1099. doi: 10.1016/j.jpedsurg.2008.02.036. [DOI] [PubMed] [Google Scholar]
- 21.Massicotte P, Julian JA, Gent M, et al. An open-label randomized controlled trial of low molecular weight heparin for the prevention of central venous line-related thrombotic complications in children: the PROTEKT trial. Thromb Res. 2003;109(2–3):101–108. doi: 10.1016/s0049-3848(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 22.Dubois J, Rypens F, Garel L, et al. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. CMAJ. 2007;177(10):1185–1190. doi: 10.1503/cmaj.070316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanslik A, Thom K, Haumer M, et al. Incidence and diagnosis of thrombosis in children with short-term central venous lines of the upper venous system. Pediatrics. 2008;122(6):1284–1291. doi: 10.1542/peds.2007-3852. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell LG, Andrew M, Hanna K, et al. A prospective cohort study determining the prevalence of thrombotic events in children with acute lymphoblastic leukemia and a central venous line who are treated with L-asparaginase: results of the Prophylactic Antithrombin Replacement in Kids with Acute Lymphoblastic Leukemia Treated with Asparaginase (PARKAA) Study. Cancer. 2003;97(2):508–516. doi: 10.1002/cncr.11042. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vriesendorp TM, DeVries JH, Rosendaal F, et al. Severe hypoglycemia in critically ill: risk and outcomes. Crit Care Med. 2008;36(4):1390. doi: 10.1097/CCM.0b013e31816a1788. author reply 1390–1391. [DOI] [PubMed] [Google Scholar]
- 28.Van Casteren M, Davis MH. Match: a program to assist in matching the conditions of factorial experiments. Behav Res Methods. 2007;39(4):973–978. doi: 10.3758/bf03192992. [DOI] [PubMed] [Google Scholar]
- 29.MRC Cognition and Brain Sciences Unit. [cited 2010 March 31]Available from: http://www.mrc-cbu.cam.ac.uk/people/maarten.van-casteren/mixandmatch.html.
- 30.Ruud E, Holmstrom H, De Lange C, et al. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies--a randomized, controlled study. Acta Paediatr. 2006;95(9):1053–1059. doi: 10.1080/08035250600729092. [DOI] [PubMed] [Google Scholar]
- 31.Male C, Chait P, Andrew M, et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood. 2003;101(11):4273–4278. doi: 10.1182/blood-2002-09-2731. [DOI] [PubMed] [Google Scholar]
- 32.Ortel TL. Acquired thrombotic risk factors in the critical care setting. Crit Care Med. 2010;38(2 Suppl):S43–50. doi: 10.1097/CCM.0b013e3181c9ccc8. [DOI] [PubMed] [Google Scholar]
- 33.Beck C, Dubois J, Grignon A, et al. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: a prospective study. J Pediatr. 1998;133(2):237–241. doi: 10.1016/s0022-3476(98)70226-4. [DOI] [PubMed] [Google Scholar]
- 34.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293(4):470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 35.Curley MA, Harris SK, Fraser KA, et al. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7(2):107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candrilli SD, Balkrishnan R, O’Brien SH. Effect of injury severity on the incidence and utilization-related outcomes of venous thromboembolism in pediatric trauma inpatients. Pediatr Crit Care Med. 2009;10(5):554–557. doi: 10.1097/PCC.0b013e3181a705d3. [DOI] [PubMed] [Google Scholar]
- 37.Talbott GA, Winters WD, Bratton SL, et al. A prospective study of femoral catheter-related thrombosis in children. Arch Pediatr Adolesc Med. 1995;149(3):288–291. doi: 10.1001/archpedi.1995.02170150068012. [DOI] [PubMed] [Google Scholar]
- 38.Male C, Julian JA, Massicotte P, et al. Significant association with location of central venous line placement and risk of venous thrombosis in children. Thromb Haemost. 2005;94(3):516–521. doi: 10.1160/TH03-02-0091. [DOI] [PubMed] [Google Scholar]
- 39.Anton N, Cox PN, Massicotte MP, et al. Heparin-bonded central venous catheters do not reduce thrombosis in infants with congenital heart disease: a blinded randomized, controlled trial. Pediatrics. 2009;123(3):e453–458. doi: 10.1542/peds.2008-1508. [DOI] [PubMed] [Google Scholar]
- 40.Massicotte MP, Sofronas M, deVeber G. Difficulties in performing clinical trials of antithrombotic therapy in neonates and children. Thromb Res. 2006;118(1):153–163. doi: 10.1016/j.thromres.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Kuhle S, Spavor M, Massicotte P, et al. Prevalence of post-thrombotic syndrome following asymptomatic thrombosis in survivors of acute lymphoblastic leukemia. J Thromb Haemost. 2008;6(4):589–594. doi: 10.1111/j.1538-7836.2008.02901.x. [DOI] [PubMed] [Google Scholar]
- 42.Kotsakis A, Cook D, Griffith L, et al. Clinically important venous thromboembolism in pediatric critical care: a Canadian survey. J Crit Care. 2005;20(4):373–380. doi: 10.1016/j.jcrc.2005.09.012. [DOI] [PubMed] [Google Scholar]