Abstract
Background
Lumenal glucose initiates changes in gastrointestinal (GI) function, including inhibition of gastric emptying, stimulation of pancreatic exocrine and endocrine secretion, and intestinal fluid secretion. Glucose stimulates the release of gastrointestinal hormones and 5-hydroxytryptamine (5HT), and activates intrinsic and extrinsic neuronal pathways to initiate change GI function. The precise mechanisms involved in luminal glucose sensing are not clear; studying gut endocrine cells is difficult due to their sparse and irregular localization within the epithelium.
Methods
Here we show a technique to determine activation of gut epithelial cells and the gut-brain pathway in vivo in rats using immunohistochemical detection of the activated, phosphorylated, form of calcium-calmodulin kinase II (pCaMKII).
Key Results
Perfusion of the gut with glucose (60 mg) increased pCaMKII immunoreactivity in 5HT-expressing enterochromaffin (EC) cells, cytokeratin-18 immunopositive brush cells, but not in enterocytes or cholecystokinin (CCK)-expressing cells. Lumenal glucose increased pCaMKII in neurons in the myenteric plexus and nodose ganglion, nucleus of the solitary tract, dorsal motor nucleus of the vagus and the arcuate nucleus. pCaMKII expression in neurons, but not in EC cells, was significantly attenuated by pretreatment with the 5HT3R antagonist ondansetron. Deoxynojirimycin (DNJ) a selective agonist for the putative glucose sensor, sodium-glucose cotransporter- 3 (SGLT-3), mimicked the effects of glucose with increased pCaMKII in ECs and neurons; galactose had no effect.
Conclusions & Inferences
The data suggest that native EC cells in situ respond to glucose, possibly via SGLT-3, to activate intrinsic and extrinsic neurons and thereby regulate GI function.
Keywords: enterochromaffin cell, sodium-glucose cotransporter 3, brush cell, 5-HT3 receptor
Introduction
In the GI tract, glucose serves as a signal for activation of numerous regulatory events. The presence of glucose in the intestinal lumen elicits a number of changes in GI function including stimulation of pancreatic exocrine and endocrine secretion, inhibition of gastric emptying, and reduction of food intake (1). In addition, the presence of glucose has effects on epithelial cell function, such as upregulation of nutrient transporter protein expression by enterocytes (2). Glucose in the lumen of the GI tract is also involved in the regulation of glucose homeostasis (3); glucose releases the incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptides (GLP-1, GLP-2) (1, 3). GIP and GLP-1 increase the amount of insulin released from the islet β-cells and also inhibit glucagon secretion contributing to postprandial regulation of plasma levels of glucose (3).
Glucose in the intestine releases 5-HT from EC cells (4) and 5-HT has many effects on both secretory and motor functions in the gut (5). 5-HT acts via a vagal afferent, 5-HT3R-mediated pathway to inhibit gastric motility and emptying, and stimulate pancreatic exocrine secretion (6-7). Glucose-stimulated intestinal fluid secretion is mediated in part via an action of 5-HT on intrinsic neurons (8). We are interested in understanding the mechanism by which glucose is detected in situ in the GI tract, resulting in release of 5-HT, and the role of the 5-HT3R in subsequent activation of the gut-brain pathway. Our previous published work using a model of EC cells, the BON cell line, has suggested that glucose is detected by gut EC cells, releasing 5-HT via a process involving a member of the sodium-dependent glucose cotransporter (SGLT) protein family (9). Indirect evidence from monitoring changes in gut function and by measurement of 5-HT levels in mesenteric lymph supports a role for a member of the SGLT family of proteins in glucose-sensing by EC cells (10). Glucose and galactose are transported across enterocytes via SGLT-1 located on the apical surface of enterocytes; glucose and galactose have equal affinity for SGLT-1. However, we found that while glucose induced release of 5-HT, galactose was without effect, a result inconsistent with a role for SGLT-1. Another member of this protein family, SGLT-3, has been proposed as a glucose-sensor (11). SGLT-3 binds but does not transport glucose and binds galactose with much lower affinity than glucose. Importantly, when expressed in oocytes, SGLT-3 does not transport glucose but induces a depolarization of the plasma membrane, suggesting that SGLT-3 may act as a glucose sensor.
In the present study, we sought to validate a method for determining activation of epithelial cells and the gut-brain pathway in response to intestinal glucose and further, to determine the role of 5-HT3Rs and SGLT-3 in this activation. Our understanding of glucose-sensing in the gut derives largely from indirect measures of functional changes in response to glucose perfusion (6, 10, 12-13) or using isolated gut endocrine cells or cell lines that secrete GI hormones or 5-HT (9, 14). However, these preparations do not account for the complex structure of the gut epithelium and moreover, cannot trace pathways activated secondary to glucose-sensing cells in the gut wall. We used a model of luminal infusion of glucose in awake rats followed by immunochemical detection of the activated, phosphorylated form of Ca2+/calmodulin-dependent protein kinase II (CaMKII). CaMKII is a protein kinase that is widely expressed in a variety of tissues; it autophosphorylates in response to elevated intracellular Ca2+ and functions as an intracellular signaling protein (15). Phosphorylated CaMKII (pCaMKII) has a relatively unique property that allows prolonged phosphorylation to transient Ca2+ signals making it a good marker for cellular activation. We compared levels of immunoreactivity in gut epithelial cells, myenteric neurons and in central neurons that are activated in response to visceral stimuli including the nucleus of the solitary tract (NTS), the location of the termination of vagal afferent fibers, dorsal motor nucleus (DMV) that contain the cell bodies of the preganglionic vagal efferent neurons, and also the arcuate nucleus of the hypothalamus (ARC), an area important for the integration of visceral afferent information (16), following duodenal infusion of either vehicle or glucose in awake, chronically cannulated rats. To investigate the role of 5-HT3Rs in glucose-induced activation of gut EC cells and the gut-brain pathway, we infused glucose in the absence or presence of the 5-HT3R antagonist ondansetron. To investigate the role of SGLT-3, we compared the effects of duodenal infusion with glucose with that of galactose, and also infused deoxynojirimycin (DNJ), an imino sugar shown to be a potent agonist for SGLT-3, but not SGLT-1 (11, 17-18).
Methods
Animals Care and Procedures
Animals were maintained and handled in accordance with policies of the Institutional Animal Care and Use Committee, University of California at Davis. Male Sprague-Dawley rats (Harlan, San Diego, CA, initial weight 240-280 g) were given ad libitum access to standard chow and water and housed in a controlled environment (relative humidity (99%), temperature (20 ± 2 °C), 12 hr light/dark cycle). Rats were fasted overnight with free access to water prior to surgical procedures, duodenal perfusions, or tissue procurement.
Rats were anesthetized with sodium methohexital (4.5mg/100gm body weight) and an indwelling cannulas were placed into the proximal duodenum approximately 1 cm distal to the gastric-duodenal junction using sterile surgical technique; rats were allowed to recover for 4 days before being used in experiments. The duodenum of rats was infused with water (0.5ml vehicle), glucose (60 mg; 0.5 ml), galactose (60 mg) or DNJ (0.54mg:0.5ml). Five minutes after the start of perfusion, rats were deeply anesthetized (sodium pentobarbital; 100 mg/kg IP) anesthetized and transcardially perfused with 60 ml of 0.9% NaCl with 0.1% heparin at 4°C followed by 4% paraformaldehyde (1ml/gm body weight) in phosphate-buffered saline (PFA-PBS, pH 7.4) at 4°C. Tissue was rapidly removed and post-fixed for 1hr at 4°C in 4% PFA-PBS. Tissue was then stored at 4°C in 25% sucrose with 0.01% sodium azide dissolved in PBS until sectioned. To determine the role of serotonin in glucose signaling, one group of rats was pretreated (30 min) with the 5HT3R antagonist ondansetron (0.5mg:0.5ml in 0.9% saline IP; Glaxo Group Research, Greenford, UK) followed by intraduodenal infusion with glucose.
Immunohistochemistry
The duodenum, nodose ganglion, brainstem and hypothalamus were sliced into 8μm sections using a cryostat, melted onto coated slides and washed in 0.1M phosphate buffer (PBS, 3× 10 minutes each). Sections were incubated in 20% goat serum dissolved in PBS for 30min at 37°C. Sections of duodenum were incubated with antibodies directed against pCaMKII and 5HT or cholecystokinin (CCK) at 37°C (anti-pCaMKII pT286, 1:200 rabbit IgG polyclonal antibody, Promega, Madison, WI; anti-5HT, 1:1000 mouse monoclonal antibody, Dako Corp, Carpinteria, CA; anti-CCK, 1:1000 mouse monoclonal antibody CURE/UCLA Digestive Diseases Research Center. Neural tissue labeled for pCaMKII was also co-incubated with an antibody directed against vertebrate neuron-specific nuclear protein (NeuN) (anti-NeuN, a mouse monoclonal, Chemicon, Temecula, CA, 1:100). Neurons in myenteric plexus were identified by labeling with an antibody direct against microtubule associated proteins (anti-MAP2, 1:100 mouse monoclonal antibody, Sigma Chemical, St Louis, MO). Brush cells were identified by characteristic morphology and labeling for cytokeratin 18 (anti-cytokeratin peptide-18, 1:100 mouse monoclonal antibody, Sigma Chemical, St Louis, MO). Slides were incubated with primary antibodies for 2.5 hr at 37°C then wash in PBS 3× 10 min each. Tissue was then incubated with the secondary antibodies; goat anti-rabbit IgG conjugated AlexaFluor 488 and goat anti-mouse AlexaFluor 546 (Molecular Probes, Eugene, OR) both diluted 1:500 in 2% GS-PBS at 37°C for 30min. Slides were washed in 0.1 M PBS 1× 10 min, 1× overnight at 4°C in PBS, and 1× 10 min. Slides were cover-slipped using Gelmount (Biomeda, Foster City, CA).
Images of fluorescent labeling were acquired using a confocal microscope (BioRad, Radiance System 2100, Hercules, CA). All images were collected using the same confocal parameters. Images of individual neurons were analyzed using Scion Image software (based on NIH Image for Macintosh; Beta 4.0.2, Scion Corp., 2000). Quantification of CaMKII phosphorylation in immunohistochemical images is presented as percent labeled pixels. This is a percentage of the total area of the cytoplasm and plasma membrane that shows immunohistochemical labeling for pCaMKII as previously outlined in detail (19).
Western Blots
The technique has been published previously (10). Briefly, 20 μg of protein in NuPAGE LDS sample buffer (Invitrogen Corporation, Carlsbad, CA) was heated for 10 minutes at 70°C and loaded onto a 7% Tris-acetate gel (Invitrogen × Cell SureLock Mini-Cell apparatus). The gel was run for 60 minutes at 125V, 120mA and the proteins transferred (1hr, at 30V, 200mA) to a Fluortrans PVDF membrane (Pall Corporation, East Hills, NY). Membrane blots were washed in Tris-buffered saline containing Tween-20 (TBST) and then were blocked in 5% milk-TBST for 1hr at room temperature. Blots were incubated with pCaMKII primary antibody (1:500, a rabbit IgG polyclonal antibody from Promega, Madison, WI) for 1 hr at room temperature then overnight at 4°C. Membranes were then washed in 5% milk-TBST (3× 15min each) and incubated for 1 hr at room temperature with an anti-rabbit secondary antibody conjugated with HRP at a 1:1000 dilution in 5% milk TBST (Cell Signaling, Danvers, MA). The blots were then incubated in 1× LumiGlo for 1 min and exposed for film development using a 100 Plus automatic X-ray film Processor (All Pro Imaging, Hicksville, NJ). The intensities of the bands within blots were quantified using ImageQuant v 5.1 software (Amersham Biosciences, Amersham, UK). Membranes were then stripped and re-incubated with CaMKII primary antibody (1:500, a rabbit IgG polyclonal antibody from Santa Cruz Antibodies, Santa Cruz, CA) and quantified as above for CaMKII expression.
Statistical Analysis
Data was analyzed with ANOVA to determine whether any of the means for treatment groups within a specific experiment were significantly different (p<0.05). If significance was determined, then a Tukey’s test was employed to compare all possible pairs of means. If two specific means were analyzed for significance then a Student’s t-test was employed. Significance was assumed with a probability level < 0.05.
Results
Activation of Gut Epithelial Cells by Glucose
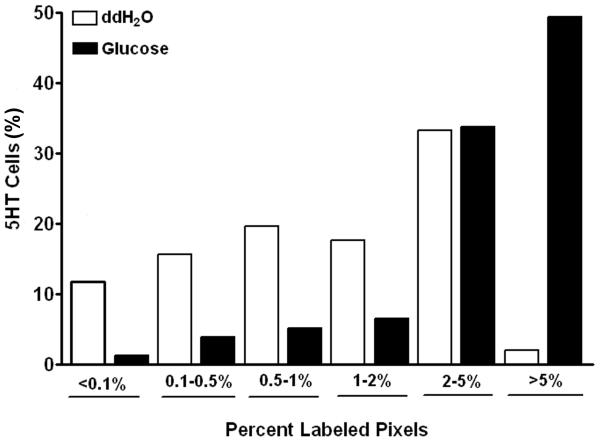
There was little or no immunoreactivity for pCaMKII in epithelial cells of the duodenum of rats treated with water (Fig 1A). Infusion of glucose induced immunohistochemical labeling for pCaMKII in epithelial cells; these cells had the characteristics of ECs and EECs with a flask-like shape and basally-located nucleus. Double-labeling for 5-HT identified many of these pCaMKII immunopositive cells as 5-HT-expressing EC cells (Fig 1B). There was no evidence of immunohistochemical labeling for pCaMKII in enterocytes. Lumenal glucose infusion produced a 4-fold increase (p<0.01) in cellular labeling for pCaMKII in ECs relative to water (Fig 1AB, Table 1). Frequency analysis for levels of pCaMKII expression was performed on 5HT-immunopositive cells. Approximately 98% percent of ECs in rats infused with water had pCaMKII expression levels of 5% or less. In contrast, in rats infused with glucose, 50% of ECs had pCaMKII labeling levels greater than 5% (Fig 2). This suggests that a relatively high percentage of ECs in the duodenum, likely about half of the total EC population, are responding to glucose as determined by an increase in immunoreactivity for pCaMKII.
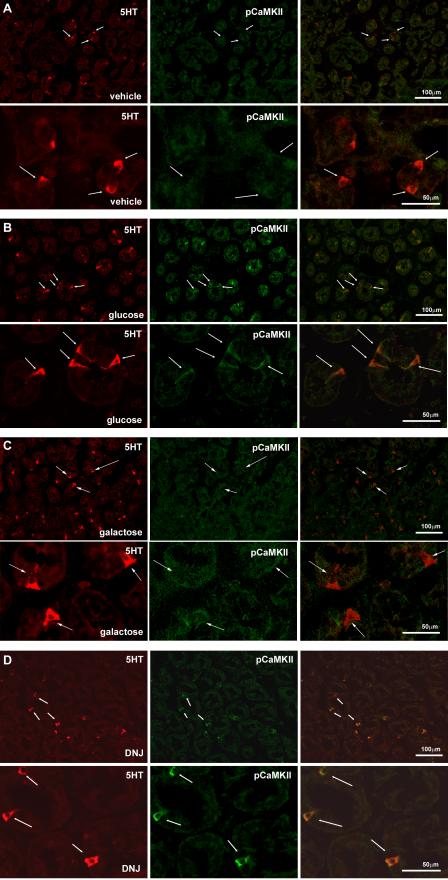
Figure 1.
Photomicrographs of sections of rat duodenum to show pCaMKII immunoreactivity (red; left column) and 5-HT immunoreactivity to identify enterochromaffin cells (green; middle column), together with the composite image (right column) following duodenal infusion with A. vehicle, (0.5ml), B. glucose (60mg), C. galactose (60mg) or D. the SGLT-3 agonist, DNJ (0.54 mg). Perfusion of the duodenum of awake rats with glucose and DNJ, but not galactose, significantly increased pCaMKII immunoreactivity in cells co-labeled for 5-HT and not in the absorptive enterocytes.
Table 1.
Effect of duodenal lumen perfusion of vehicle (water), glucose, galactose or DNJ on levels of immunoreactivity for pCaMKII in various cell types. Data expressed as percentage labeled pixels and given as mean ± SEM.
| Treatment | vehicle | glucose | galactose | DNJ | |
|---|---|---|---|---|---|
| ECs | 1.5 ± 0.3 | 5.8 ± 0.4* | 1.5 ± 0.4 | 3.8 ± 0.3* | N=29 rats, 512 cells |
| Myenteric neurons | 2.0 ± 0.3 | 5.2 ± 0.5* | 2.9 ± 0.5 | 7.6 ± 0.7** | N=22 rats, 337 ganglia |
| Nodose neurons | 1.9 ± 0.5 | 7.0 ± 1.8* | 1.4 ± 0.5 | 4.7 ± 0.9* | N=20 rats, 314 sections |
| NTS | 0.6 ± 0.2 | 2.9 ± 0.4* | 0.7 ± 0.2 | 3.6 ± 0.9* | N=18 rats, 431 sections |
| DMN | 1.1 ± 0.3 | 3.9 ± 0.4* | 1.6 ± 0.3 | 5.0 ± 1.7* | N=18 rats, 431 sections |
| arcuate | 0.5 ± 0.1 | 1.3 ± 0.2* | 0.5 ± 0.1 | 1.0 ± 0.1* | N=18 rats, 254 sections |
P<0.05 vehicle vs glucose or DNJ;
P<0.01 vehicle vs glucose or DNJ.
Figure 2.
Frequency analysis to show the relative intensity of pCaMKII immunoreactivity in duodenal 5HT immunopositive cells following duodenal infusion with either vehicle or glucose (60mg). In duodenum from rats perfused with water, more than 95% of ECs have pCaMKII labeling levels less than 5%; in contrast, in rats infused with glucose more than 50% of ECs have levels of immunoreactive pCaMKII greater than 5%. Data expressed as mean of % labeled pixels (n=29 rats, n= 512 cells).
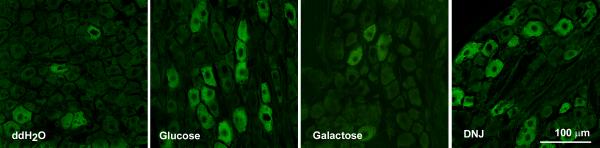
There was no significant expression of pCaMKII in EECs immunopositive for CCK following duodenal glucose infusion (0.63 ± 0.13% vs 0.30 ± 0.08% vehicle vs glucose, NS, n=10 rats, n=215 cells). Glucose infusion increased pCaMKII immunoreactivity in GIP- and GLP-1- immunopositive cells (Supplemental Figure 1). Duodenal brush cells were immunopositive for cytokeratin 18 as previously described (20) and had expressed immunoreactive pCaMKII in response to duodenal infusion of glucose (Supplemental Figure 2). Following glucose infusion, there was a significant increase in pCamKII immunopositive pixels compared to water treatment (2.7 ± 1.3% vs 11.8 ± 1.4% water vs glucose, p<0.01).
Activation of myenteric neurons by glucose
Activation of pCaMKII was also examined in neurons in the myenteric plexus of the duodenum. pCaMKII expression levels in neurons were examined using two criteria. First, the percentage of pixels that showed pCaMKII labeling was determined for an entire ganglion and second, the number of neurons with 20% or more pCaMKII immunopositive pixels was counted. Cells were classified into two groups, those with an area larger or smaller than 150μ2. Labeling of the myenteric plexus with the neuronal marker, MAP2 showed that cells with an area greater than in the myenteric plexus were neurons. The total number of cells meeting this size criterion was counted along with the number of cells showing ≥ 20% immunolabeling; these were considered to be activated neurons. There was a greater than 2-fold increase in the % labeled pixels for pCaMKII in duodenal myenteric neurons taken from rats treated with glucose compared to those treated with vehicle (Fig 3, p<0.01). In the myenteric plexus of rats infused with water, 12.0 ± 1.8 % of neurons had a labeling intensity ≥ 20%; in the myenteric plexus from rats infused with glucose, there was a significant increase in the percentage of neurons (31.4 ± 4.5 %) with labeling intensity ≥ 20% (Table 1, p<0.01).
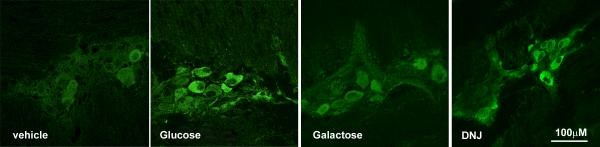
Figure 3.
Photomicrographs of pCaMKII immunoreactivity in myenteric plexus (upper panel) and nodose ganglia (lower panel) from rats infused with either vehicle (water, 0.5ml), glucose (60mg) , galactose (60mg) or deoxynojirimycin (DNJ) (0.54mg). Infusion of glucose and DNJ result in a significant increase in immunoreactive pCaMKII in myenteric neurons and vagal afferent neurons compared to infusion of either vehicle or galactose.
Activation of the extrinsic neurons by glucose
There was a significant 3-fold increase of pCaMKII labeled pixels in neurons of the nodose ganglia from rats perfused with glucose compared to vehicle (Fig 3, p<0.05). Only 5.9 ± 1.3% of nodose neurons had significant pCaMKII labeling (≥20%) following vehicle infusion compared to 20.7 ± 5.1 % of neurons with significant pCaMKII labeling (≥20%) in nodose neurons from glucose-treated rats (Table 1; p<0.05).
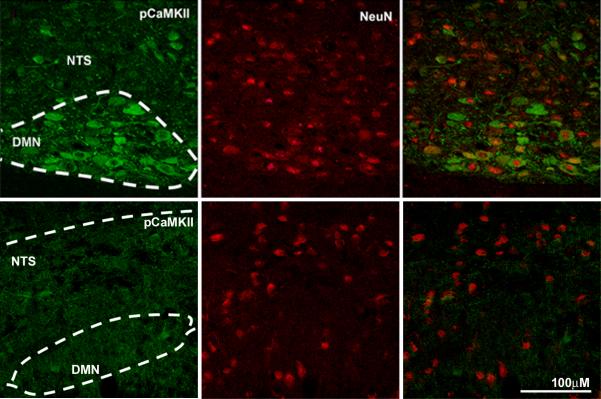
Vagal afferent neurons synapse in the nucleus tractus solitarius (NTS) of the brainstem; there is a topographic pattern of termination of abdominal afferents in the NTS with fibers from the distal portion of the gut terminating in the caudal portion of the NTS (21). Expression of pCaMKII was analyzed in three regions of the NTS, caudal to the area postrema (AP) (approximately 14.6 to 14.3mm caudal of bregma), in the region of the AP (approximately 14.2 to 13.6mm caudal of bregma) and rostral of the AP (approximately 13.5 to 12.8mm caudal of bregma). Rats infused with glucose have increased pCaMKII expression in the NTS, but only in the AP region (p<0.01) (Fig 4, Table 1). Neither the caudal or rostral portions of the NTS showed increased pCaMKII expression in glucose-treated rats (Fig 4).
Figure 4.
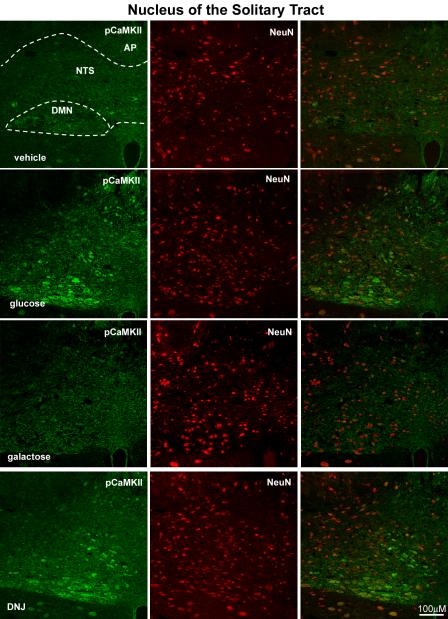
Photomicrographs of the brainstem showing the NTS and DMN of rats following intestinal perfusion with either water, glucose, galactose or DNJ. Immunoreactivity for pCaMKII is shown (right column; green) and for neuron-specific nuclear protein (NeuN) (middle column, red) and the composite image (right panel). Glucose and DNJ increase expression of pCamKII in both NTS and DMN neurons compared to infusion with either vehicle or galactose.
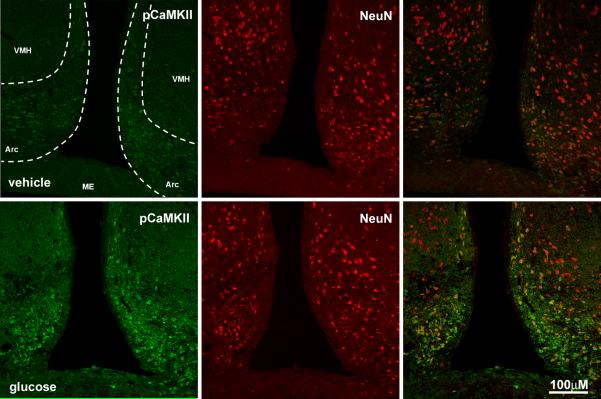
The dorsal motor nucleus of the vagus (DMN) lies ventral to the NTS and was analyzed in two regions; caudal of the AP and at the AP. In the DMN at the level of the AP, there was a 3-fold increase (p<0.05) of pCaMKII in response to infused glucose (Table 1, Fig 3B). In the DMN at the level caudal of the AP, there was no significant difference between vehicle and glucose-treated rats. We also determined whether duodenal glucose perfusion influenced neuronal activity in the arcuate nucleus of the hypothalamus. There was an almost 3-fold increase (p<0.05) of pCaMKII immunoreactivity in the arcuate nucleus of rats infused with glucose compared to those infused with vehicle (Fig 5).
Figure 5.
Photomicrographs of immunoreactivity for pCaMKII (right column; green) in the arcuate nucleus of the hypothalamus of rats infused with water or glucose. There is an increase in immunoreactivity for pCaMKII in tissue from taken from rats infused with glucose compared to vehicle. Most pCaMKII expression is associated neuron-specific nuclear protein (NeuN) labeled neurons (middle panel; red).
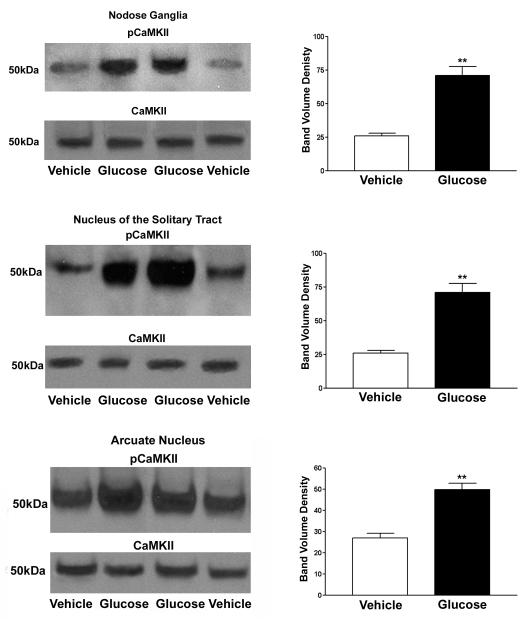
Western blots for pCaMKII in the nodose ganglia, NTS, and arcuate nucleus showed similar results to those found with immunohistochemistry. pCaMKII expression in the nodose ganglia showed a mean band volume density 3.8 times greater (p<0.05) than water perfused rats. Average Western band density in the NTS and arcuate were 2.7 times greater (p<0.01) and 1.8 (p<0.01) in rats perfused with glucose than water (Fig 6).
Figure 6.
Western blots show increased pCaMKII expression levels in the nodose ganglia and arcuate nucleus of rats infused with glucose relative to vehicle infused rats. After stripping the Western blot membranes, levels of CaMKII (unphosphorylated) are also shown. N=8 rats in each group; p<0.01 vehicle vs glucose treatments.
Role of member of SGLT protein family in glucose sensing
Galactose had no significant effect on levels of immunoreactive pCaMKII in 5-HT expressing cells or any of the neuronal populations studied (Figs 1, 3). Duodenal infusion of DNJ (0.54mg: 0.5ml) produced an increase of pCaMKII in 5HT cells similar to that of glucose (Table 1). There was an approximate seven-fold increase (p<0.05) of immunoreactivity for pCaMKII in 5HT-immunopositive cells from DNJ- compared to vehicle-treated rats (Fig 1). The action of DNJ on epithelial cells in the duodenum was specific to 5HT-immunopositive cells; there was no significant pCaMKII immunoreactivity in duodenal CCK cells in rats treated with DNJ. Similar to glucose, DNJ produced a significant increase of pCaMKII expression in the myenteric plexus (p<0.01), nodose ganglia (p<0.05), NTS (p<0.01), DMN (p=0.05), and arcuate nucleus (p=0.01) relative to vehicle-treated rats (Figs 3,4, 5; Table 1).
Role of 5-hydroxytryptamine in glucose-induced activation
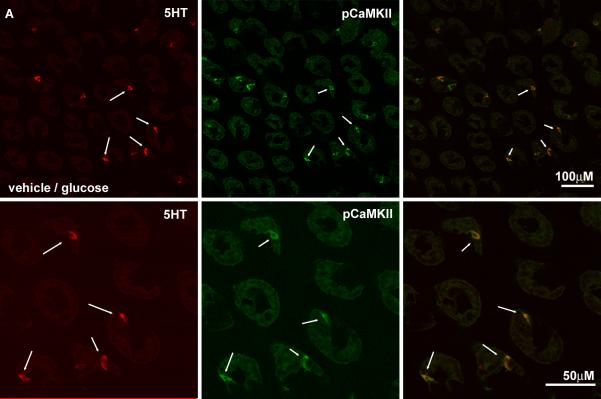
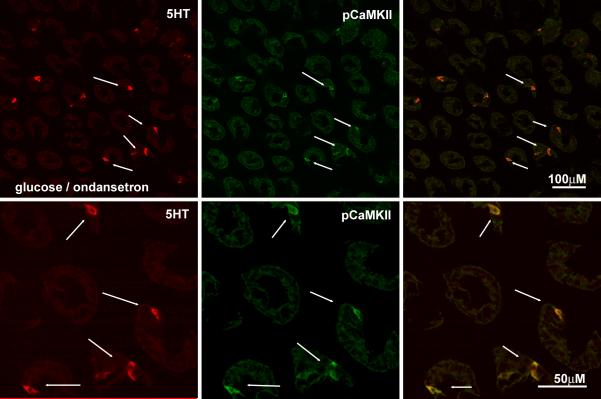
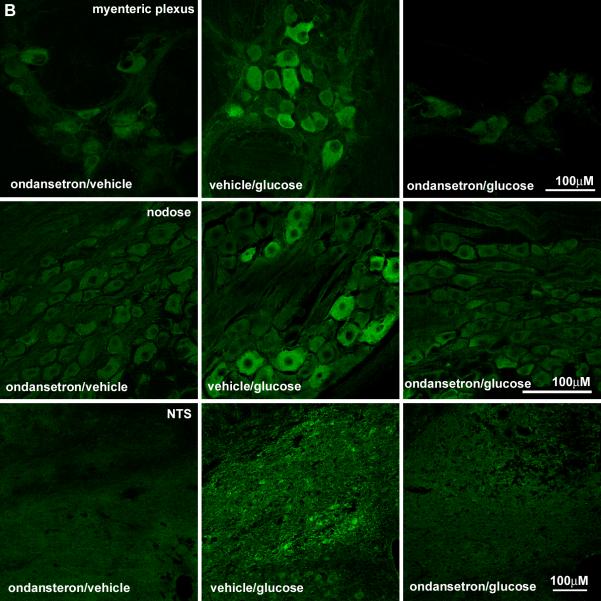
Pretreatment with ondansetron had no significant effect on the increase in immunoreactive pCaMKII in ECs in response to duodenal glucose perfusion compared to pretreatment with vehicle (NS). (Fig 7). However, there was a significant attenuation of the glucose-induced increase in pCaMKII expression levels in myenteric and vagal afferent neurons in rats pretreated with ondansetron compared to vehicle pretreatment (p<0.01) (Table 2, Fig 8) and in both the AP/rostral regions of the NTS and caudal/AP portions of the dorsal motor nucleus of the vagus in the ondansetron/glucose treated rats relative to vehicle/glucose rats (Fig 8, Table 2). The expression of pCaMKII in the arcuate nucleus was also diminished in the ondansetron/glucose treated rats relative to vehicle/glucose rats (Table 2).
Figure 7.
Photomicrographs of the duodenal mucosa from rats pretreated with ondansetron or vehicle followed by perfusion of the intestine with glucose. The intensity of pCaMKII immunolabeling (middle column; green) is shown in epithelial cells in sections colocalizing 5-HT immunoreactivity (right column, red) and the overlay of the images shown in the right column. for rats pre-treated with the vehicle (A) or 5HT3R antagonist, ondansetron (B). Pre-treatment with ondansetron does not significantly diminish the ability of glucose to increase pCaMKII expression in ECs.
Table 2.
Effect of pretreatment with ondansetron on levels of immunoreactivity for pCaMKII in response to intestinal perfusion with glucose in various cell types. Data expressed as percentage labeled pixels and given as mean ± SEM, n=4-6 rats in each group; data taken from 20-30 images..
| Treatment | Vehicle/glucose | Ondansetron/glucose |
|---|---|---|
| ECs | 3.9 ± 0.4 | 4.0 ± 0.4 |
| Myenteric neurons | 5.3 ± 0.6 | 2.6 ± 0.4* |
| Nodose | 9.4 ± 0.9 | 2.8 ± 0.1** |
| NTS | 2.8 ± 0.4 | 0.1 ± 0.4* |
| DMN | 4.7 ± 0.5 | 0.7 ± 0.1* |
P<0.05 vehicle vs glucose or DNJ;
P<0.01 vehicle vs glucose or DNJ.
Figure 8.
Photomicrographs of the duodenal mucosa from rats pretreated with ondansetron or vehicle followed by duodenal infusion with glucose. (A) The intensity of immunolabeling for pCaMKII is shown in the myenteric plexus (A), nodose ganglion (B) and brainstem (C) of rats pre-treated with vehicle or the 5HT3R antagonist, ondansetron. Scale bar = 100μM. Neurons in all three regions show a decrease in pCaMKII in response to glucose when pretreated with ondansetron.
Discussion
Intestinal glucose activates a number of responses, both within the gut and in other organs, such as the pancreas and central nervous system. The present results show that glucose is detected directly in the lumen of the gut; its presence is then rapidly signaled to the enteric nervous system and via the vagus nerve to the central nervous system. Luminal glucose detection by ECs to release 5-HT likely involves SGLT-3, a membrane protein that may function as a glucose sensor. Transmission from gut ECs to the enteric and central nervous systems appears to be partially dependent on the release of 5HT from ECs in the gut mucosa and activation of 5HT3Rs. This data is consistent with previous observations from functional experiments showing release of 5-HT in response to lumenal glucose and vagally-dependent changes in gastrointestinal and pancreatic function in mediated by 5HT3Rs (1, 3, 6-7, 12).
ECs and EECs were activated following acute glucose perfusion into the lumen of the duodenum. We also show that glucose activates cytokeratin 18-immunopositive brush cells; these cells share distinct features with the taste receptor cells of the tongue and are thus considered to be putative chemo-receptive cells (20, 22). We found that GIP and GLP-1 containing cells were activated by glucose consistent with their role as incretin hormones and previous data showing release of these hormones in response to luminal glucose (see 23). There was no activation of CCK-containing ECs cells, consistent with the lack of release of CCK in response to glucose. The data suggest a specific activation of a subpopulation of specialized epithelial cells by luminal glucose in vivo which can be visualized by positive immunostaining of pCaMKII. Vagal afferent nerves signal the presence of luminal glucose; somas of these afferent neurons, which reside in the nodose ganglia, show increased levels of CaMKII phosphorylation in response to luminal glucose. Moreover, neurons of the nucleus tractus solitarius (NTS), which are primary synaptic sites for vagal afferents, have increased levels of pCaMKII. Luminal glucose results in increased pCaMKII in dorsal motor nucleus neurons. This suggests that luminal glucose is rapidly detected in the gut and this information conveyed to the central nervous system affecting autonomic efferent outflow to the viscera. The arcuate nucleus of the hypothalamus also shows increased levels of pCaMKII. Thus, information about luminal glucose is rapidly conveyed via the vagus nerve and integrated in central nervous system in regions that process visceral information. This likely represents the neural substrates mediating vago-vagal regulation of gastric emptying and possibly also pancreatic secretion (6). Initiation of glucose-mediated neuronal signaling appears to be dependent on release of 5-HT as ondansetron, a specific 5HT3 receptor antagonist, decreased glucose-mediated pCaMKII signaling in intrinsic and extrinsic neurons whereas pCaMKII expression in 5HT cells remains elevated. This shows that activation of both the enteric nervous system and the vagally-mediated pathway into the central nervous system is dependent on 5HT release and 5HT3R activation.
Several mechanisms have been identified by which cells detect and respond to extracellular glucose. Pancreatic β-cells and glucose excitatory neurons of the central nervous system utilize the ATP-sensitive K+ channel to sense glucose (24-26). Metabolism of glucose elevates ATP/ADP ratios, which increases ATP binding at the ATP-sensitive K+ potassium channel, resulting in cellular depolarization and an influx of calcium via voltage-gated channels. There is evidence that a similar mechanism is involved in release of incretin hormones from EC cells in the gut (3,14). Recent evidence has suggested another mechanism for glucose-sensing in the gut wall. Taste receptors in the oral cavity detect glucose and artificial sweeteners via the type 1 taste receptor (T1R), a heterodimer of T1R2 and T1R3 subunits; there is evidence to suggest that the T1Rs may have a role in signaling gut luminal glucose (27-28). Dietary sugars and sweeteners upregulate SGLT-1 expression in wild-type mice but do not in Ggust or T1R3 knockout mice (29). Both T1R receptors and Ggust are expressed in enteroendocrine cells and GLUTag cells, which also secrete hormones involved in SGLT-1 upregulation in response to sweeteners (23, 27). However, there is currently little or no evidence to suggest that these mechanisms are involved in release of 5-HT. In the current study, we investigated the role of SGLT-3 as a glucose sensor in the intestine. The sodium/glucose cotransporter family (SLCA5 gene family) consists of many related proteins in bacterial and animal cells in tissues from epithelia to the nervous system (30-31). SGLT-1 transports the dietary sugars, D-glucose and D-galactose, from the lumen of the intestine into enterocytes. A variant of the SGLT-1 transporter, SGLT-3, is found in human skeletal muscle and small intestine (11). When human SGLT-3 is expressed in the Xenopus laevis oocyte, electrophysiological studies show that glucose is not transported, but results in a specific, phloridzin-sensitive, sodium-dependent membrane depolarization. Galactose does not produce a sodium-dependent depolarization in SGLT-3 transfected oocytes. Thus it appears that SGLT-3 responds specifically to glucose resulting in membrane depolarization, but transports little glucose into the cell and consequently SGLT-3 protein has been proposed as a glucose sensor. We have previously shown that gastric emptying, measured in awake rats, is significantly inhibited by perfusion of the intestine with glucose (60 and 90 mg) (10). This effect was mimicked by α-methyl glucose (nonmetabolizable substrate of SGLT-1 and-3) but not by 2-deoxy-D-glucose or isoosmotic mannitol (substrates for GLUT-2). Glucose-mediated inhibition of gastric motility in awake rats is attenuated by phloridzin, which again indicates an SGLT mediated mechanism (13). Duodenal glucose, but not galactose, stimulates the release of 5HT into mesenteric lymph and stimulates the discharge of duodenal vagal afferent fibers (10). The SGLT-3 ligand, DNJ, is a potent agonist of SGLT-3, with very low binding affinity for SGLT-1. Here we show that DNJ activates gut epithelial cells that are known to be activated by glucose, including EC cells, brush cell and also EECs expressing GLP-1. These results indicate, but do not confirm, that 5HT cells can be directly activated by luminal gut glucose and serve as glucose sensor cells, possibly using SGLT3. Taken together with the results from the present study, these data suggest that binding of glucose to SGLT-3 is important in the ability of the gut to detect the presence of luminal glucose and for subsequent 5HT-mediated activation of the vagal afferents. SGLT-3 expression has been demonstrated via in situ hybridization and immunohistochemistry on human tissue to occur in cholinergic neurons of submucosal and myenteric plexus but not in gut enterocytes. Recently, our laboratory has shown via real time PCR that SGLT-3 mRNA occurs in the mucosa of the rat intestine (10). Thus SGLT-3 may occur in epithelial cells other than enterocytes.
In conclusion, the data from the present study demonstrates the feasibility of a method to study the response of individual gut endocrine and enterochromaffin cells to luminal stimuli in vivo. The data also suggest that native EC cells respond to glucose, most likely via an SGLT-3-dependent mechanism, to activate intrinsic and extrinsic neurons and thereby regulate GI function.
Supplementary Material
Supplementary Information
Supplemental Figure 1. Photomicrographs to show immunoreactivity for pCaMKII (panels ii & iv, green) in sections of rat duodenum from rats infused in which the duodenum was infused with either 0.5ml of vehicle (water) or glucose (60mg, 0.5ml) co-stained for glucose-dependent insulinotropic peptide (GIP) labeled cells (panels i & iii, red). Scale bar = 10μM.
Supplemental Figure 2. Photomicrographs for pCaMKII immunoreactivity (panels ii & iv, red) in shown in cytokeratin-18 immunopositive brush cells (panels i & iii, green) for rats in which the duodenum was infused either 0.5ml of vehicle or glucose (60mg:0.5ml) (A). The brush cells of rats infused with glucose have significantly (p=0.003) greater levels of pCaMKII expression than rats infused with vehicle (B). Scale bar = 10uM. Number of rats examined 8. Number of cells analyzed, 169.
Acknowledgements
Work supported by NIH DK58588 (HER).
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Raybould HE. Sensing of glucose in the gastrointestinal tract. Auton Neurosci. 2007;133(1):86–90. doi: 10.1016/j.autneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Dyer J, Daly K, Salmon KS, Arora DK, Kokrashvili Z, Margolskee RF, et al. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans. 2007;35(Pt 5):1191–4. doi: 10.1042/BST0351191. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Racke K, Reimann A, Schworer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res. 1996;73(1-2):83–7. doi: 10.1016/0166-4328(96)00075-7. [DOI] [PubMed] [Google Scholar]
- 5.Gershon MD. Review article: serotonin receptors and transporters -- roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther. 2004;20(Suppl 7):3–14. doi: 10.1111/j.1365-2036.2004.02180.x. [DOI] [PubMed] [Google Scholar]
- 6.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, et al. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G367–72. doi: 10.1152/ajpgi.00292.2001. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Wu XY, Zhu JX, Owyang C. Intestinal serotonin acts as paracrine substance to mediate pancreatic secretion stimulated by luminal factors. Am J Physiol Gastrointest Liver Physiol. 2001;281(4):G916–23. doi: 10.1152/ajpgi.2001.281.4.G916. [DOI] [PubMed] [Google Scholar]
- 8.Day J, King B, Haque SM, Kellum JM. A nonneuronal 5-hydroxytryptamine receptor 3 induces chloride secretion in the rat distal colonic mucosa. Am J Surg. 2005;190(5):736–8. doi: 10.1016/j.amjsurg.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Cooke HJ, Javed NH, Carey HV, Christofi F, Raybould HE. D-glucose releases 5-hydroxytryptamine from human BON cells as a model of enterochromaffin cells. Gastroenterology. 2001;121(6):1400–6. doi: 10.1053/gast.2001.29567. [DOI] [PubMed] [Google Scholar]
- 10.Freeman SL, Bohan D, Darcel N, Raybould HE. Luminal glucose sensing in the rat intestine has characteristics of a sodium-glucose cotransporter. Am J Physiol Gastrointest Liver Physiol. 2006;291(3):G439–45. doi: 10.1152/ajpgi.00079.2006. [DOI] [PubMed] [Google Scholar]
- 11.Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, et al. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A. 2003;100(20):11753–8. doi: 10.1073/pnas.1733027100. PMCID: 208830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu JX, Zhu XY, Owyang C, Li Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol. 2001;530(Pt 3):431–42. doi: 10.1111/j.1469-7793.2001.0431k.x. PMCID: 2278417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Raybould HE, Zittel TT. Inhibition of gastric motility induced by intestinal glucose in awake rats: role of Na(+)-glucose co-transporter. Neurogastroenterol Motil. 1995;7(1):9–14. doi: 10.1111/j.1365-2982.1995.tb00203.x. [DOI] [PubMed] [Google Scholar]
- 14.Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587(Pt 1):27–32. doi: 10.1113/jphysiol.2008.164012. PMCID: 2670019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276(6):3719–22. doi: 10.1074/jbc.R000013200. [DOI] [PubMed] [Google Scholar]
- 16.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi L, Diez-Sampedro A. A single amino acid change converts the sugar sensor SGLT3 into a sugar transporter. PLoS One. 2010;5(4):e10241. doi: 10.1371/journal.pone.0010241. PMCID: 2857651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss AA, Diez-Sampedro A, Hirayama BA, Loo DD, Wright EM. Imino sugars are potent agonists of the human glucose sensor SGLT3. Mol Pharmacol. 2007;71(2):628–34. doi: 10.1124/mol.106.030288. [DOI] [PubMed] [Google Scholar]
- 19.Freeman SL, Glatzle J, Robin CS, Valdellon M, Sternini C, Sharp JW, et al. Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterology. 2006;131(1):97–107. doi: 10.1053/j.gastro.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Sbarbati A, Osculati F. The taste cell-related diffuse chemosensory system. Prog Neurobiol. 2005;75(4):295–307. doi: 10.1016/j.pneurobio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283(2):248–68. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 22.Sbarbati A, Osculati F. A new fate for old cells: brush cells and related elements. J Anat. 2005;206(4):349–58. doi: 10.1111/j.1469-7580.2005.00403.x. PMCID: 1571484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8(6):532–9. doi: 10.1016/j.cmet.2008.11.002. PMCID: 2697331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53(10):2521–8. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 25.Holsbeeks I, Lagatie O, Van Nuland A, Van de Velde S, Thevelein JM. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem Sci. 2004;29(10):556–64. doi: 10.1016/j.tibs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci. 2005;360(1464):2211–25. doi: 10.1098/rstb.2005.1762. PMCID: 1569593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr Opin Endocrinol Diabetes Obes. 2008;15(1):73–8. doi: 10.1097/MED.0b013e3282f43a73. PMCID: 2943060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33(Pt 1):302–5. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 29.Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104(38):15075–80. doi: 10.1073/pnas.0706678104. PMCID: 1986615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology (Bethesda) 2004;19:370–6. doi: 10.1152/physiol.00026.2004. [DOI] [PubMed] [Google Scholar]
- 31.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447(5):510–8. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplemental Figure 1. Photomicrographs to show immunoreactivity for pCaMKII (panels ii & iv, green) in sections of rat duodenum from rats infused in which the duodenum was infused with either 0.5ml of vehicle (water) or glucose (60mg, 0.5ml) co-stained for glucose-dependent insulinotropic peptide (GIP) labeled cells (panels i & iii, red). Scale bar = 10μM.
Supplemental Figure 2. Photomicrographs for pCaMKII immunoreactivity (panels ii & iv, red) in shown in cytokeratin-18 immunopositive brush cells (panels i & iii, green) for rats in which the duodenum was infused either 0.5ml of vehicle or glucose (60mg:0.5ml) (A). The brush cells of rats infused with glucose have significantly (p=0.003) greater levels of pCaMKII expression than rats infused with vehicle (B). Scale bar = 10uM. Number of rats examined 8. Number of cells analyzed, 169.