Abstract
The experience of foraging under natural conditions increases the volume of mushroom body neuropil in worker honey bees. A comparable increase in neuropil volume results from treatment of worker honey bees with pilocarpine, an agonist for muscarinic-type cholinergic receptors. A component of the neuropil growth induced by foraging experience is growth of dendrites in the collar region of the calyces. We show here, via analysis of Golgi-impregnated collar Kenyon cells with wedge arborizations, that significant increases in standard measures of dendritic complexity were also found in worker honey bees treated with pilocarpine. This result suggests that signaling via muscarinic-type receptors promotes the increase in Kenyon cell dendritic complexity associated with foraging. Treatment of worker honey bees with scopolamine, a muscarinic inhibitor, inhibited some aspects of dendritic growth. Spine density on the Kenyon cell dendrites varied with sampling location, with the distal portion of the dendritic field having greater total spine density than either the proximal or medial section. This observation may be functionally significant because of the stratified organization of projections from visual centers to the dendritic arborizations of the collar Kenyon cells. Pilocarpine treatment had no effect on the distribution of spines on dendrites of the collar Kenyon cells.
Keywords: Apis mellifera, dendritic spine, Golgi technique, mushroom bodies
1. Introduction
Defining the mechanisms by which experience is coupled to lasting changes in the structure of the brain is a fundamental task in the field of neuroscience. Studies in insects (including fruit flies, ants, wasps, and honey bees) have demonstrated that the structure of adult insect brains is altered as a result of changes in the environment or behavior (Groh and Meinertzhagen, 2010). These studies extend work beginning in the 1960s and 1970s, in which numerous manipulations, including enriched environments, free running wheel access, and physical restraint, were shown to alter dendritic structure in adult vertebrates (Bennett et al., 1964; Riege, 1971; Volkmar and Greenough, 1972; Kozorovitskiy et al., 2005; Stranahan et al., 2007; McEwen, 1999). The honey bee as a model organism for studying experience-dependent plasticity strikes a balance between brain and ethological complexities - approximately 1 million neurons in the brain account for a rich behavioral repertoire.
The mushroom bodies of the insect brain are protocerebral structures that display anatomical plasticity both across evolutionary time and during the adult lives of individual insects (Farris and Sinakevitch, 2003; Farris and Roberts, 2005; Fahrbach, 2006; Molina et al., 2009). One of the forms this plasticity takes over both time scales is regulation of the volume of the neuropil associated with protocerebral structures called the mushroom bodies. Changes in mushroom body neuropil volume across the lifespan of the individual are strikingly evident in the brains of relatively long-lived hymenopteran insects at both the light microscopic (e.g. Withers et al., 1993; Gronenberg et al., 1996; O'Donnell et al., 2004; Withers et al., 2008; Stieb et al., 2010) and ultrastructural (e.g. Seid et al., 2005) levels of analysis. The phenomenon has been documented in various species of ants, wasps, and bees, but investigations of the underlying cellular and molecular mechanisms of this plasticity have focused on foraging-associated growth of the mushroom bodies of workers of Apis mellifera, the European honey bee (Ismail et al., 2006; Lutz et al., 2009). In this species, the volume of neuropil associated with the mushroom bodies is significantly larger in experienced foragers than in non-foragers, independent of the age of the foragers (Withers et al., 1993; Durst et al., 1994; Farris et al., 2001; Ismail et al., 2006).
The intrinsic neurons of the mushroom bodies of the honey bee are referred to as Kenyon cells (Kenyon, 1896). The typical Kenyon cell produces a branched axon and a single dendritic arborization. Analyses of mushroom body plasticity have focused almost exclusively on Kenyon cell dendritic arborizations. On each side of the brain, the collected dendritic arborizations of the honey bee Kenyon cells form two cup-shaped neuropils that contain the majority of the somata of the Kenyon cells. These neuropils are postsynaptic to known cholinergic olfactory and presumed cholinergic visual and gustatory afferents originating in the primary sensory neuropils (Mobbs, 1982; Kreissl and Bicker, 1989; Huang and Knowles, 1990; Schröter and Menzel, 2003). Each calyx of the honey bee and other hymenopterans can be subdivided into three regions: lip, basal ring, and collar. Each region contains the dendrites of different subsets of Kenyon cells. The lip processes olfactory information and receives unilateral innervation from the ipsilateral antennal lobe via the antenno-cerebral tract and the basal ring is innervated by both the antennal and optic lobes (Gronenberg, 2001). The largest subdivision of the calyces, the collar, receives bilateral information about the visual world of the compound eyes from the optic lobes via the anterior-superior optic tracts, the anterior-inferior optic tracts, and the lobula tracts (Ehmer and Gronenberg, 2002). Optic lobe afferents to the collar are not uniformly distributed throughout this subdivision. For example, the medulla, which processes color information, projects to the outer two-thirds of the collar neuropil in five alternating dorsal/ventral layers, while the motion-sensitive lobula projects to the inner third of the collar neuropil.
Classic studies of honey bee Kenyon cells have used dendritic field morphology, location of the soma, birth order within the lineage and nature of dendritic specializations (e.g. spines or claws) for classification (Mobbs, 1982; Farris et al., 1999; Strausfeld, 2002). Mobbs (1982) analyzed Golgi-impregnated brains to define Kenyon cells with somata attached directly to the calycal arborization as Ka Kenyon cells and those with somata distinctly more distant from the calycal arborization as Kb Kenyon cells; further classifying the Kenyon cells of the honey bee as dense spiny, sparse spiny, clawed, or bunched. Strausfeld (2002) combined modern cell-labeling techniques with Golgi impregnation to provide additional categorization of Kenyon cells. The collar is formed of the dendritic fields of Ka-type bunched and Kb-type dense spiny neurons that extend arborizations of three morphologies: wedge, rectangular, and bistratified. By contrast, the lip contains only Ka-type dense spiny neurons (which can be subdivided into 4 groups based on the breadth of the dendritic field). The basal ring contains Ka-type bunched, Kb-type bunched, and Kb-type sparse spiny neurons (which can be further divided into six distinct dendritic morphologies). The functional significance of these diverse dendritic morphologies is presently unknown.
In honey bees, the Kenyon cells are produced by the proliferative activity of four large clusters of neuroblasts (one cluster per calyx) which are active during the larval and pupal stages (Farris, 1999). In contrast to some other insects, there is no adult neurogenesis in the mushroom bodies of honey bees; this means that changes in the volume of the mushroom body neuropils must reflect growth or regression of existing elements, rather than the addition of new Kenyon cells (Fahrbach et al., 1995). Therefore, one of the challenges posed by the phenomenon of mushroom body plasticity in adult worker honey bees is the identification of the specific plastic elements. There is strong evidence that mushroom body plasticity is associated with changes in the calycal arborizations of individual mushroom body neurons. Analysis of Golgi-impregnated Kb-type collar Kenyon cells with a wedge morphology revealed that experienced foragers have larger and more complex arborizations than less experienced foragers (Farris et al., 2001). Although no other studies have directly examined the influence of foraging on Kenyon cell cytoarchitecture, this report is consistent with results from studies of the number of microglomeruli within the calyces that demonstrate considerable postembryonic plasticity in these synaptic structures in honey bees (Groh et al., 2004; Groh et al., 2006; Krofczik et al., 2008) and a species of wasp (Jones et al., 2009).
Cholinergic synapses are abundant in the mushroom body calyces of insects; they represent the mechanism for transfer of information from the primary sensory neuropils of the brain to the mushroom bodies (Huang and Knowles, 1990; Bicker, 1999; Yasuyama and Salvaterra, 1999; Yasuyama et al., 2002). Ismail et al. (2006) reported that treatment of caged honey bee foragers with one week of prior foraging experience with the muscarinic cholinergic receptor agonist pilocarpine increased the volume of mushroom body neuropil to the same extent as an additional week of foraging under natural conditions. This result supports the hypothesis that signaling through muscarinic receptors at calycal synapses activated by the enriched sensory world of the forager drives foraging-dependent mushroom body growth. The role of cholinergic signaling in the honey bee mushroom body has been investigated previously from various perspectives including the timing of the initiation of foraging (Shapira et al., 2001) and olfactory association learning (e.g. (Lozano et al., 2001), but never from the perspective of Kenyon cell dendritic complexity. The present study was designed to test the prediction that pharmacological stimulation of muscarinic cholinergic receptors by treatment of worker honey bees with the agonist pilocarpine will increase the complexity of the calycal arborizations of wedge-type collar Kenyon cells. Confirmation of this prediction will be interpreted as supporting the hypothesis that naturally occurring, foraging experience-dependent growth of Kenyon cell arborizations also depends on signaling via cholinergic muscarinic pathways. We also examined the possible role of muscarinic signaling in the regulation of Kenyon cell dendritic arborizations in younger (pre-foraging) workers using treatments with the muscarinic agonist pilocarpine and scopolamine, a muscarinic antagonist.
2. Materials and Methods
2.1 Honey bee collection and experimental design
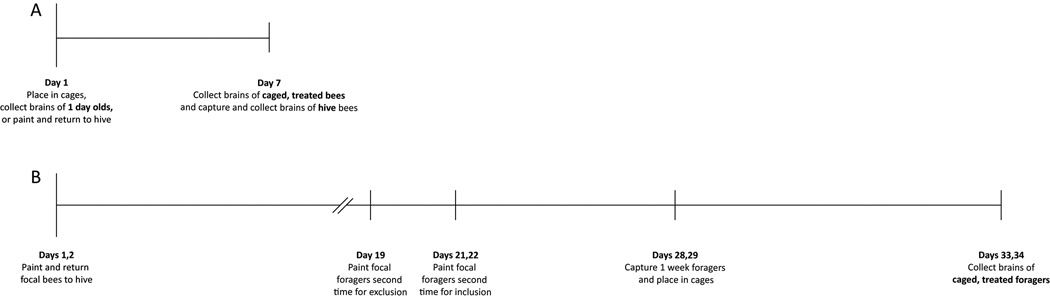
Honey bees (Apis mellifera) were obtained from research apiaries maintained at Wake Forest University (Forsyth County, NC, USA) according to standard commercial techniques. To obtain newly emerged bees, brood comb containing pharate adult workers was removed from field colonies and placed in an incubator (Percival Scientific, Inc., Perry, IA, USA) maintained at 33°C, 35 – 45 % relative humidity. For studies of pre-foragers, honey bees were collected within 6 h of emergence from their natal cell and immediately placed in small cages. To obtain hive-reared bees, 1,000 honey bees (< 12 h post-emergence) were each marked on the dorsal thorax with a single dot of enamel paint (Testors PLA, Rockford, IL, USA) and then returned to a typical field colony containing a naturally-mated queen. The hive was opened one week later to collect the marked bees (hereafter designated as hive 7-day-olds). To obtain known-age, known-experience foragers, 6,000 – 8,000 honey bees (< 12 h post-emergence; “focal bees”) were each marked on the dorsal thorax with a single dot of enamel paint and then returned to a typical field colony containing a naturally mated queen. This experiment was repeated three times. For each experiment, honey bees were marked and returned to a field colony over the course of two days, as slightly staggering the age of the bees allowed brain dissections to be completed across multiple days while still permitting a comparison of bees of the same age and closely-matched experiences. The hive entrance was observed for 4 – 6 h 19 days later. Any focal bee seen foraging was marked with a second color of paint on the thorax. Because it is unknown when these bees initiated foraging, they were excluded from the experiment. Beginning two days later, focal foragers (those returning to the hive entrance bearing a single paint mark) were painted with a new color on the thorax each day for two consecutive days. These bees were allowed to forage under natural conditions in the field for an additional week before being collected and brought into the laboratory for drug treatments. All of the studies reported here used worker bees collected during the summers of 2008 and 2009. A timeline of the studies is provided (Fig. 1).
Fig. 1.
Experimental timeline. A, To study the effects of manipulating cholinergic signaling in pre-foragers, bees were collected within 6 h of emergence. They were either caged in groups of ten, paint marked and returned to the source colony, or immediately processed for Golgi impregnation (1-day-olds). 7 days later, the brains of paint marked bees collected from the hive and caged bees fed pilocarpine or scopolamine dissolved in sucrose, or sucrose alone collected and processed for Golgi impregnation. B, To obtain age and foraging experience matched honey bees, newly emerged bees were painted over 2 days and returned to the source colony (focal bees). Any focal bee seen foraging was painted a second time on the abdomen and excluded from the study. Beginning 2 days later, all focal bees with a single paint mark seen foraging were marked for 2 days with a new color each day. These bees were collected one week later, caged in groups of ten in the laboratory, and fed pilocarpine or scopolamine dissolved in sucrose, or sucrose alone. An additional week later, the brains of caged, treated one-week foragers were collected and processed for Golgi impregnation. Names used to designate the different groups of brains analyzed are given in bold
Foragers collected in the field were housed in the laboratory in Plexiglas cages (10 cm × 10 cm × 7 cm, 10 bees per cage). For the studies of pre-foragers caged immediately upon their emergence from the brood comb, bees were kept in the Percival incubator (28°C, 35 – 45 % relative humidity) in darkness for one week. Caged bees were fed a 1:1 solution of sucrose (Sigma, St. Louis, MO, USA) dissolved in deionized water (control; hereafter designated as sucrose 7-day-olds) or a sucrose solution of the same concentration with either pilocarpine (Sigma; 10−4 M; muscarinic agonist; designated pilocarpine 7-day-olds) or scopolamine (Sigma; 10−2 M; muscarinic antagonist; designated scopolamine 7-day-olds). For studies using foragers, caged honey bees were kept on the benchtop in a dark room (26 – 28°C, 25 – 30 % relative humidity) and fed 1:1 sucrose (sucrose foragers) or pilocarpine (10−6 M; pilocarpine foragers) or scopolamine (10−4 M; scopolamine foragers) dissolved in the same concentration of sucrose for five days. These doses were chosen to match earlier studies demonstrating neuroanatomical and/or behavioral effects of these drugs (Ismail et al., 2006; Ismail et al., 2008). Feeding tubes were changed daily to prevent fermentation of the sucrose solution; dead bees were removed via illumination with a red light invisible to bees (Peitsch et al., 1992). Bees that do not ingest carbohydrates typically do not survive more than 24 h; therefore bees that survived until the end of the treatment period are guaranteed to have been exposed to the drug for several days (Lorenz et al., 2001). Great care was taken to ensure that the effects reported, which are quantitative rather than qualitative in nature, reflect muscarinic activation or antagonism, rather than differences in age or foraging experience. For example, social aspects of the environment, such as rearing caged bees under solitary conditions or allowing dead bees to remain in the cage for the duration of the study, have also been shown to influence the volume of the mushroom bodies in honey bees (Maleszka et al., 2009). In the present study, all caged bees were initially housed in groups of ten. Dead bees were removed daily and data were not collected from bees reared in cages of fewer than 6. We therefore attribute the measured changes in dendritic arborizations of the collar Kenyon cells to the specific treatments, rather to age or early experience.
2.2 Golgi impregnation
A combined Colonnier-rapid Golgi impregnation protocol was used to label individual Kenyon cells (Li and Strausfeld, 1997). Honey bees were cooled at 4°C until immobilized. Brains were dissected from the head capsule in iced 2.5 % potassium dichromate [Electron Microscopy Sciences (EMS), Hatfield, PA, USA] solution containing 1.3 % sucrose (Sigma) and 4 % glutaraldehyde (EMS) in a downdraft fume extractor (Hacker Instruments, Winnsboro, SC, USA). Brains were then fixed in a fresh glass vial containing the dissection solution for five days in the dark at 4°C. This dissection solution was replaced with fresh solution on the fifth day, followed by 4 washes for 30 min each in fresh 2.5 % potassium dichromate, the brains were moved to a fresh glass vial containing 2.5 % potassium dichromate and 0.1 % osmium tetroxide (EMS) for 5 days in the dark at 4°C. The brains were then individually moved with a plastic pipette to a fresh bath of 0.75 % silver nitrate solution (EMS) twice before being incubated in a new glass vial with 0.75 % silver nitrate for 2 days at 4°C. They were then dehydrated through increasing ethanol concentrations and cleared in propylene oxide (Sigma) before embedding in Durcupan (EMS, Hatfield, PA, USA). After being cured at 60°C for at least 36 h, the embedded brains were sectioned at 50 µm thickness using a tungsten carbide C microtome blade. The sections were mounted in a drop of deionized water on Superfrost Plus slides (Fisher Scientific, St. Louis, MO) and coverslipped using Permount (Fisher Scientific).
2.3 Analysis of Golgi impregnated dendritic arbors
Only wedge Kenyon cells of the Kb type (hereafter simply wedge Kenyon cells) in the collar subdivision of the mushroom body were selected for analysis. The collar represents the largest subdivision of the honey bee calyx and in previous studies has been shown to display significant modulation of volume in association with foraging experience (Durst et al., 1994; Farris et al., 2001). The wedge collar Kenyon cells are unequivocally identifiable and are characterized by a somewhat flattened cytoarchitecture favorable for reconstruction and quantitative analysis. To be included in this study, the entire arbor of the wedge collar Kenyon cell had to reside within a single 50 µm-thick section with relatively few overlapping branches from other Kenyon cells. A single Golgi-impregnated bee brain typically contained 0 to 2 collar Kenyon cells that met these stringent criteria. Slides selected for analysis were encoded by a third party to ensure that subsequent measures were performed blind with respect to group identity. A camera lucida attached to an Olympus BH2 upright microscope (Olympus, Center Valley, PA, USA) with a 100× Olympus lens (D Plan, 1.30 NA), a 1.25× ring adapter (Olympus), and Olympus 10× eye pieces were used to trace the dendritic arbor at 1250× total magnification. The drawings were then enlarged (3.5×) before completing branch order and Sholl ring analysis. For spine density analysis, coded slides were imaged on a Zeiss AxioObserver inverted microscope (Thornwood, NY, USA) with a 40× Zeiss lens (Plan Neofluar, 0.85NA,Thornwood, NY, USA), 1.6× optivar, and a 10× eyepiece for a total magnification of 640×. A series of consecutive optical sections were acquired using a Hamamatsu (Bridgewater, NJ, USA) ORCA ERA camera with a Z-step of 0.2 µm and were imported into the program Volocity (Improvision, Coventry, England). The analysis program was calibrated so that the pixel to distance conversion allowed for accurate measurements.
2.3.1 Branch order analysis
Volkmar and Greenough (1972) used branch order analysis to examine the complexity of the dendritic arborizations of visual cortex neurons in rats; Farris et al. (2001) adapted this method for use with honey bee collar Kenyon cells as follows. Insect neurons differ in cytoarchitecture from those of vertebrates in that the typical insect neuron is unipolar and soma of the insect neuron is contained in a separate compartment than the neuropil (Burrows, 1996). Collar Kenyon cells extend a single (primary) neurite from the soma before it branches to form a distinct dendritic arbor and an axonal projection to the lobes. The point at which the dendritic arborization branches from the primary neurite was used as the point of origin for the analysis. All dendritic segments arising from this point on the main neurite were designated 1, as were any dendritic segments arising proximal to this branch point. If the dendrite branches again, those segments were assigned a 2 and so on until the end of the arbor was reached. The total number of dendritic branches and total number of segments of a given order were compared across experimental groups. This provides a comparison of the total extent of branching in each dendritic arbor.
2.3.2 Sholl ring analysis
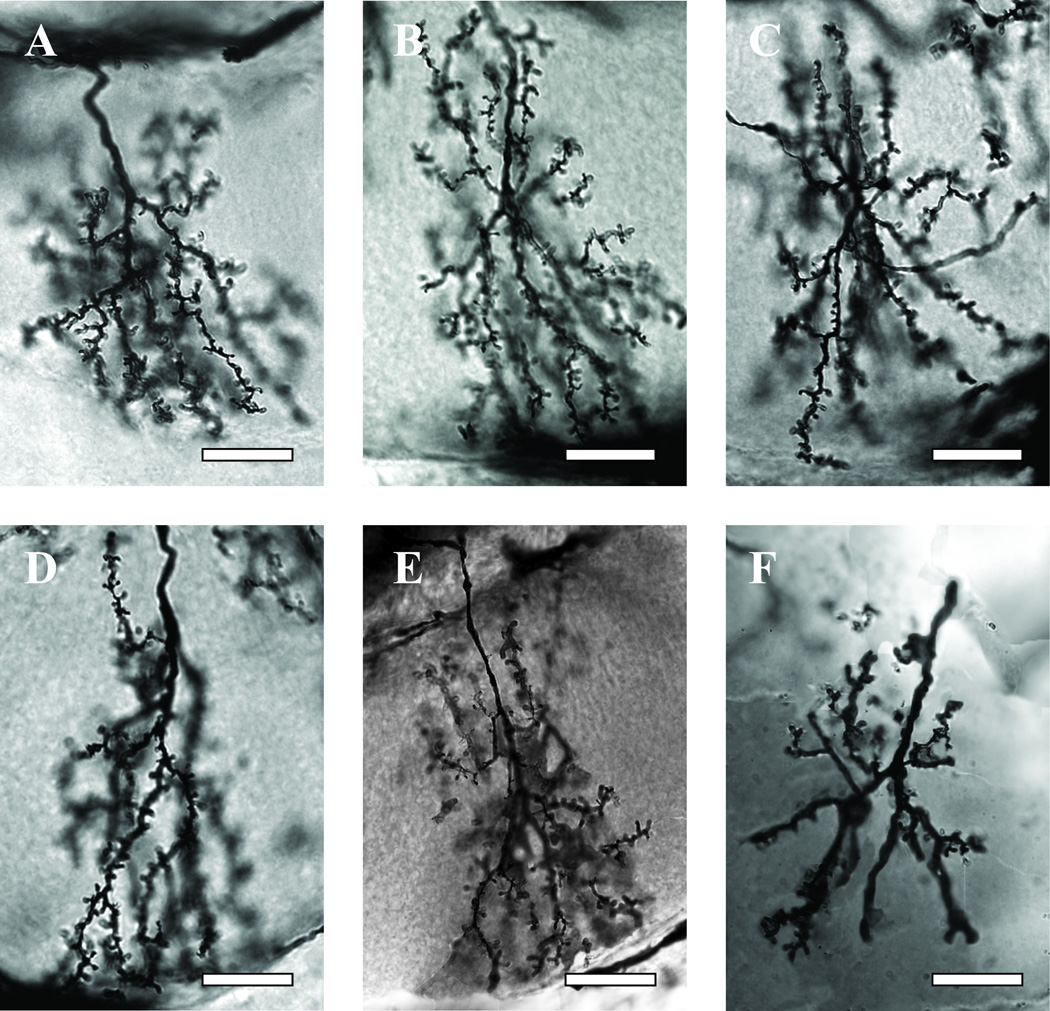
Dendritic length was quantified using a slight modification of the traditional Sholl ring analysis (Sholl, 1953). A Sholl ring analysis centers a series of concentric circles on a vertebrate neuron’s soma. For the same reason noted in the preceding section, a series of concentric circles spaced 10 mm apart (corresponding in our analysis to 5 µm of tissue) printed on clear acetate was instead centered at the base of the readily identifiable main branch point of drawings of the dendritic arbor (Fig. 2). The number of segments that cross at each of the consecutive rings were counted as an estimate of the number of segments of a given length that are present in each arbor. The total number of Sholl ring intersections, summed across all rings, provides a global estimate of dendritic complexity.
Fig 2.
Golgi-stained dendritic arborizations of wedge collar Kenyon cells in bees of different ages and treatments. A, Kenyon cell dendrites in a 1-day-old bee. B,C,D, Kenyon cell dendrites in seven-day-old bees treated chronically from the first day of adult life with pilocarpine (B), sucrose (control) (C), and scopolamine (D). E, F, Kenyon cell dendrites in foragers with one week of foraging experience followed by caging, fed either pilocarpine (E) and scopolamine (F) for five days. Scale bars = 10 µm.
2.3.3 Spine density analysis
Two centrally located, clearly visible long branches were selected for analysis from each wedge collar Kenyon cell included in this study. Each branch selected was divided into three non-overlapping 10 µm segments designated proximal, medial, or distal. The proximal segment was defined as the first 10 µm stretch of branch starting at the first spine. The medial segment was from 20 µm to 30 µm from the main branch point. The distal segment was defined as the final 10 µm section of the branch. The density of the four different spine morphologies were calculated within each segment. A spine was defined as a protuberance from the dendrite. Four spine morphologies were included in our analysis: mushroom, filopodia-like, branched, and tooth. Spines were classified by first determining whether the spine was branched. If unbranched, the head-to-neck ratio was measured. If the head was greater than twice the size of the neck, the spine was classified as a mushroom and as filopodia-like or tooth if the spine was less than twice the size of the neck. The latter category was subdivided according to the following criterion: if the spine was less than 1 µm in length, it was classified as a tooth, otherwise it was labeled filopodia-like.
2.4 Statistical analysis and preparation of images for publication
Data were decoded and grouped appropriately before analysis in Prism 5 (GraphPad Software, La Jolla, CA, USA) utilizing one-way ANOVAs with Newman-Keuls multiple comparisons post-test to assess differences in total branches, total Sholl ring intersections, and total spine density among the treatment groups. Where appropriate, t-tests were also used for pairwise comparisons. Adobe Photoshop CS4 Extended version 11.0.1 (Adobe Systems Incorporated, San Jose, CA) was used to increase the contrast in photomicrographs of Golgi-impregnated Kenyon cells for presentation as figures.
3. Results
3.1 Muscarinic signaling influences growth of the dendritic arbor in pre-foragers
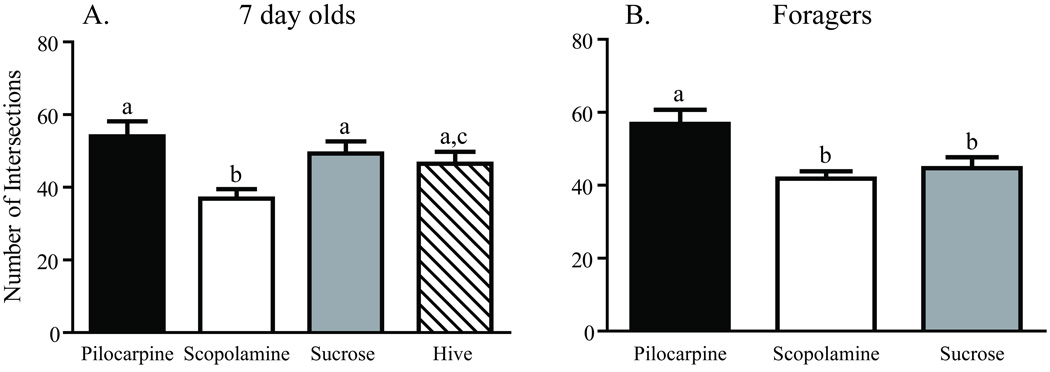
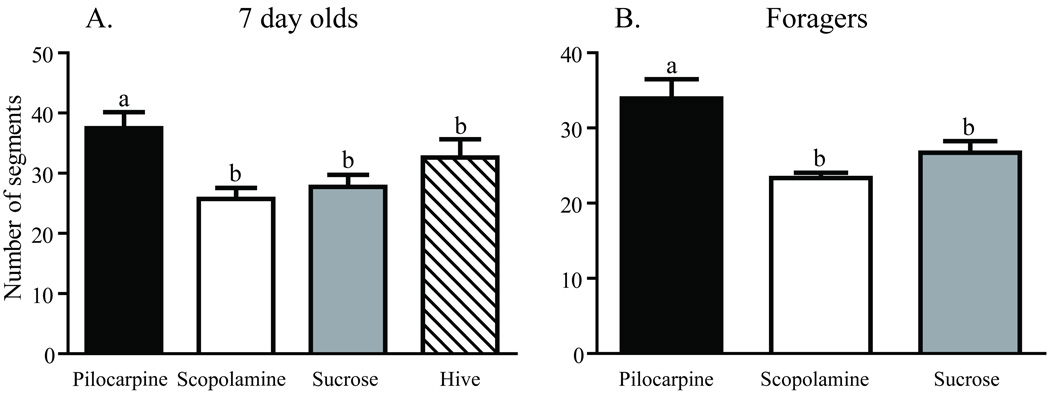
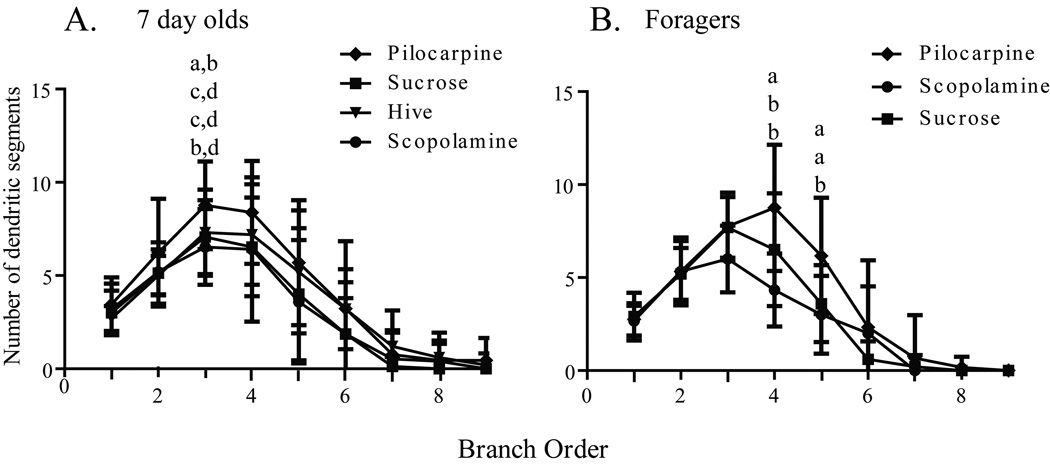
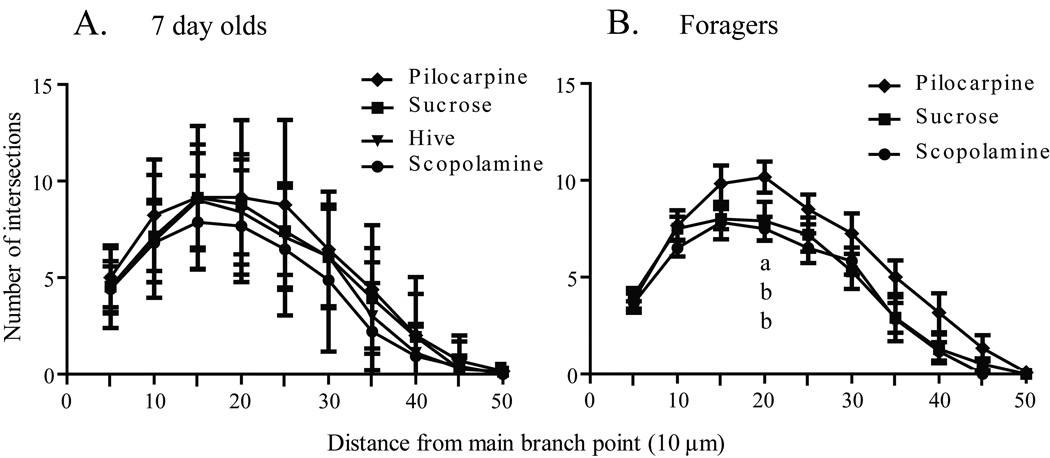
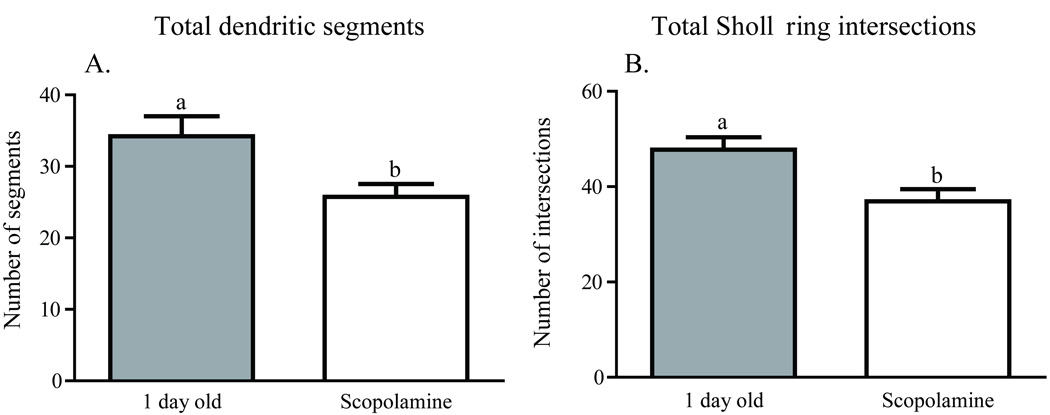
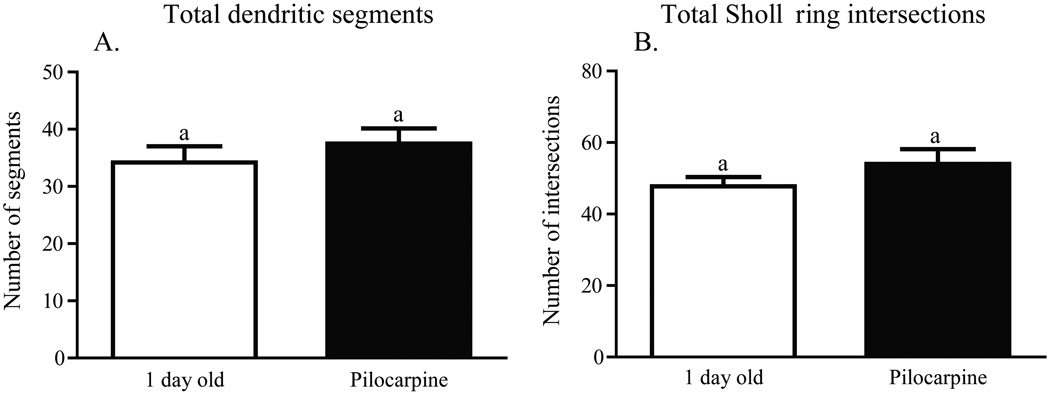
Worker honey bees were collected as they emerged from their cells in a brood comb. Brains were dissected immediately from a subset of these bees (one-day-olds), while others were transferred to cages held in a laboratory incubator and treated for one week. One group of caged bees was fed sucrose syrup (sucrose 7-day-olds); a second group was fed sucrose syrup containing pilocarpine (pilocarpine 7-day-olds), a muscarinic cholinergic receptor agonist; a third group was fed sucrose syrup containing scopolamine (scopolamine 7-day-olds), a muscarinic cholinergic receptor antagonist. A fifth group of age-matched bees (hive 7-day-olds) was marked at emergence and returned to a typical field colony for collection one week later. Examples of the resulting Golgi-impregnated Kenyon cells are shown in Fig. 2. Treatments resulted in significantly different counts of total Sholl ring intersections [one-way ANOVA (df = 2, 37), F = 6.152; p = 0.004] and total branches [one-way ANOVA (df = 2, 40), F = 8.138; p = 0.001]. One week of pilocarpine treatment of newly emerged bees resulted in significantly more total Sholl ring intersections (Newman-Keuls, p < 0.01) and total dendritic segments (Newman-Keuls, p < 0.01) than age-matched bees treated with scopolamine (Figs. 3A and 4A). Sucrose-only 7-day-olds (controls) had fewer total branches (Newman-Keuls, p < 0.01) but equivalent total Sholl ring intersections compared with pilocarpine-fed 7-day-olds. An equal number of total branches but more total Sholl ring intersections (Newman-Keuls, p < 0.05) differentiated sucrose-only controls and scopolamine-fed 7 day old. Hive 7 day olds had fewer total branches than pilocarpine-treated 7-day-olds and were not different from scopolamine and sucrose-fed 7-day-olds (Newman-Keuls, p < 0.05). Total Sholl ring intersections of hive 7-day-olds were greater than scopolamine treated but equal to sucrose and pilocarpine-fed 7-day-olds (Newman-Keuls, p < 0.05). Only at branch order 3 was a difference found between the treatment groups (Fig. 5A), but there was no difference in individual Sholl ring intersections (Fig. 6A). One day old bees had more total Sholl ring intersections (unpaired, two-way t-test, p = 0.023) and total branches (unpaired, two-way t-test, p = 0.028) than scopolamine-fed 7-day-olds (Fig. 7), but were equivalent to pilocarpine-treated 7-day-olds (Sholl ring: unpaired, two-way t-test, p = 0.391; Branch order: unpaired, two-way t-test, p = 0.495; Fig. 8).
Fig. 3.
Total number of Sholl ring intersections of collar Kenyon cells from age- and experience-matched honey bees. A, Comparison of seven-day-old honey bees after one week of chronic treatment. B, Comparison of foragers with one week of foraging experience after caging for 5 days of chronic treatment with pilocarpine, scopolamine, or sucrose. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Groups assigned the same letter are not different (Newman-Keuls post hoc tests).
Fig. 4.
Total number of dendritic segments of collar Kenyon cells from age- and experience-matched honey bees. A, Comparison of seven-day-old honey bees after one week of chronic treatment. B, Comparison of one-week foragers after 5 days of chronic treatment. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Groups assigned the same letter are not different (Newman-Keuls post hoc tests).
Fig. 5.
Distribution of dendritic branch order for age- and experience-matched honey bees. A, Comparison of seven-day-old honey bees after one week of chronic treatment. B, Comparison of one-week foragers after 5 days of chronic treatment. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Letters indicate significant differences in segment number for each branch order as determined by post hoc pairwise comparisons (unpaired, two-tailed t-test). Groups assigned the same letter are not different.
Fig. 6.
Treatment related changes in the distribution of Sholl ring intersections of collar Kenyon cells. A, Comparison of seven-day-old honey bees after one week of chronic treatment. B, Comparison of one-week foragers after 5 days of chronic treatment. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Letters indicate significant differences in for each 5 µm interval as determined by post hoc pairwise comparisons (unpaired, two-tailed t-test). Groups assigned the same letter are statistically similar to each other.
Fig. 7.
The dendritic arbors of one-day-old honey bee collar Kenyon cells are more complex that of one-week old, scopolamine fed bees. A, A greater number of total dendritic segments were observed in one-day-old bees than seven-day-old scopolamine treated bees. B, A greater number of total Sholl ring intersections were observed in one-day-old bees than seven-day-old scopolamine treated bees. Letters indicate significant differences as determined by post hoc pairwise comparisons (unpaired, two-tailed t-test).
Fig. 8.
The dendritic arbors of one-day-old honey bee collar Kenyon cells are not different from that of one-week old, pilocarpine fed bees. A, No difference in total dendritic segments was observed in one-day-old bees than seven-day-old pilocarpine-treated bees. B, No difference in the number of total Sholl ring intersections was observed in one-day-old bees than seven-day-old pilocarpine-treated bees. Groups assigned the same letters are significantly similar to each other as determined by post hoc pairwise comparisons (unpaired, two-tailed t-test).
3.2 Pilocarpine increases dendritic complexity in foragers
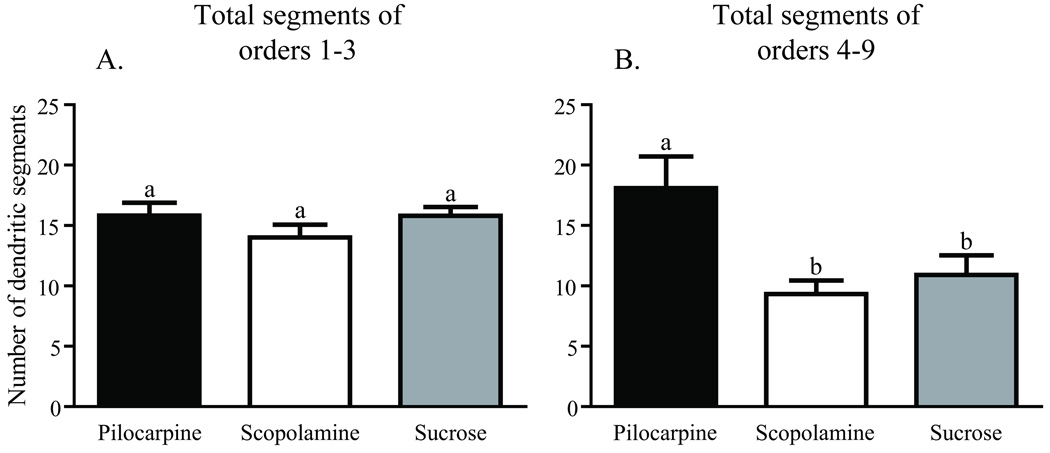
Dendritic complexity was assessed in three age-matched, foraging experience-matched groups of forager honey bees using branch order and Sholl ring analyses. All bees initiated foraging and accumulated a week of foraging experience in a typical field colony prior to caging in the laboratory fed either pilocarpine (pilocarpine foragers) or scopolamine (scopolamine foragers) dissolved in sucrose syrup, or sucrose syrup alone (sucrose foragers). These three groups of caged one-week foragers had significantly different total Sholl ring intersections [one-way ANOVA (df = 5, 49), F = 3.260; p = 0.012] and total branches [one-way ANOVA (df = 2, 25), F = 6.007; p = 0.007]. Foragers treated with pilocarpine had significantly more total Sholl ring intersections (Newman-Keuls, p < 0.05) and total branches (Newman-Keuls, p < 0.05) than age- and foraging experience-matched foragers fed either scopolamine or sucrose (Figs. 3B and 4B). Sucrose-only bees (controls) did not differ from scopolamine-treated bees on either measure (Newman-Keuls, p < 0.05). Pilocarpine treatment exerted its greatest effect on the higher branch orders. There was no difference across treatments in the total number of branches of orders 1–3 [one-way ANOVA (df = 2, 25), F = 0.8575; p = 0.436], but pilocarpine-treated foragers had significantly more total branches of orders 4–9 [Fig. 9; (one-way ANOVA (df = 2, 25), F = 4.457; p = 0.022); Newman-Keuls, p < 0.05]. Pilocarpine-treated foragers had higher counts of branch orders 4 (unpaired, two-way t-test, p = 0.041) and 5 (unpaired, two-way t-test, p = 0.009), significantly more than those of scopolamine-fed foragers. Sucrose controls differed from pilocarpine-fed foragers on branch order 5 (unpaired, two-way t-test, p = 0.038; Fig, 6B). Correspondingly, a higher number of intersections at the Sholl ring corresponding to 20 µm were found in the pilocarpine-treated foragers compared with sucrose or scopolamine-treated foragers (unpaired, two-way t-test, p = 0.044; Fig. 6B).
Fig. 9.
Pilocarpine treatment increases the number of higher order branches preferentially in one-week foragers. A, Comparison of the total number of segments of orders 1 – 3. B, Comparison of the total number of segments of orders 4 – 9. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Groups assigned the same letter are not different (Newman-Keuls post hoc tests).
3.3 Spine density analysis
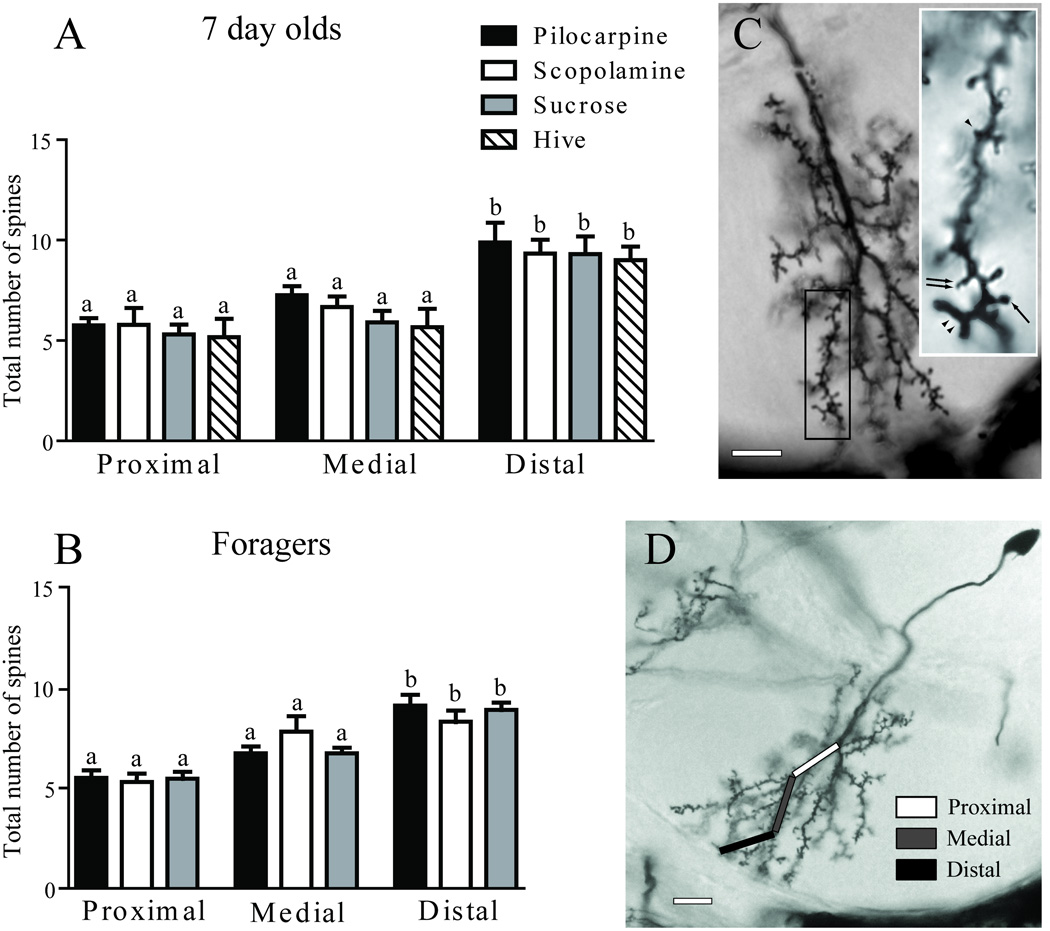
In each region (proximal, medial, or distal relative to the main branch point), the number of mushroom, tooth-like, filopodia-like, and branched spines were counted in both 7-day-old and forager groups (Fig. 10C). No difference was found among the individual spine morphologies (7-day-old: mushroom, one-way ANOVA (df = 14, 90), F = 1.371; p = 0.183; tooth-like, one-way ANOVA (df = 5, 36), F = 0.968; p = 0.450; filopodia-like, one-way ANOVA (df = 14, 90), F = 1.973; p = 0.058; branched, one-way ANOVA (df = 14, 90), F = 0.605; p = 0.854; Forager: mushroom, one-way ANOVA (df = 5, 87), F = 1.679; p = 0.148; tooth-like, one-way ANOVA (df = 6, 135), F = 1.992; p = 0.314; filopodia-like, one-way ANOVA (df = 6, 135), F = 0.992; p = 0.433; branched, one-way ANOVA (df = 6, 135), F = 0.849; p = 0.534). Hence, the data were pooled to evaluate total spine density in subsequent comparisons. Treatment with pilocarpine or scopolamine had no effect on total spine density in the proximal, medial, or distal regions of either seven day old (proximal: one-way ANOVA (df = 4, 30), F = 0.409; p = 0.810; medial: one-way ANOVA (df = 4, 30), F = 1.175; p = 0.342; distal: one-way ANOVA (df = 4, 30), F = 0.258; p = 0.902; Fig. 10A) or forager (proximal: one-way ANOVA (df = 2, 46), F = 0.059; p = 0.942; medial: one-way ANOVA (df = 2, 41), F = 1.659; p = 0.203; distal: one-way ANOVA (df = 2, 46), F = 0.538; p = 0.586; Fig. 10B) Kenyon cells. Regardless of treatment, the most distal region of wedge collar Kenyon cell dendrite had a greater total spine density than either proximal or medial regions (Seven day old: one-way ANOVA (df = 11, 87), F = 6.26, p < 0.0001; Foragers: one-way ANOVA (df = 11, 272), F = 17.69, p < 0.0001).
Fig 10.
Dendritic spine density varies by location, but not treatment, in collar Kenyon cells. A, Comparison of total dendritic spines in each dendritic region of seven-day-old honey bees after one week of chronic treatment. B, Comparison of total dendritic spines in each dendritic region of one-week foragers after 5 days of chronic treatment. C, The dendritic arbor of a collar Kenyon cell from a typical forager is shown. The boxed region is enlarged in the inset showing spines of difference morphologies. Four spine morphologies are found on collar Kenyon cells, mushroom (arrow), tooth-like (arrowhead), filopodia-like (double-arrow), and branched (double-arrowhead). D, A single segment which extends from the main branch point was divided into 3 regions indicated by the boxes for spine density analysis. Sample sizes for each group are listed in Table 1. Statistical analysis of these data used a one-way ANOVA model. Groups assigned the same letter are statistically similar to each other. Scale bars = 10 µm.
4. Discussion
The principal significance of these results is that they provide evidence that changes in cholinergic receptor activation can affect the morphology of mushroom body neurons. Activation of muscarinic cholinergic receptors via pilocarpine treatment induced an increase in several measures of dendritic complexity of wedge collar Kenyon cells in both young pre-forager and forager honey bees. Spine density varied with sampling location: the distal portion of the dendritic field had greater total spine density than either the proximal or medial section. Neither pilocarpine nor scopolamine treatment altered the distribution of spines.
Previous studies have repeatedly demonstrated that the experience of foraging under natural conditions increases the volume of the mushroom body neuropil, at least in part through stimulation of Kenyon cell dendritic outgrowth (Withers et al., 1993; Ismail et al., 2006; Farris et al., 2001). Caged one-week foragers fed the muscarinic agonist pilocarpine for a week were found to have an increase in volume of mushroom body neuropil of approximately the same extent as age matched foragers allowed to experience a second week of foraging (Ismail et al., 2006). Changes in volume may reflect many aspects of brain structure, including increased inputs from projection neurons, a growth of inhibitory feedback neurons, changes in Kenyon cell dendritic arbor complexity, and/or possibly technical artifacts. We show here that a comparable pilocarpine treatment resulted in increased dendritic complexity of wedge collar Kenyon cells.
Using a different sampling scheme (comparison of two groups of precocious foragers with different amounts of foraging experience), Farris et al. (2001) found that longer foraging experience was correlated with increased numbers of dendritic segments with the highest branch orders, as well as a greater number of intersections in the Sholl rings corresponding to 20 µm, 30 µm, and 40 µm. Although the differences in experimental design make a direct comparison impossible, it appears that muscarinic receptor-regulated dendritic growth differs slightly from growth induced by natural foraging experience; the number of branches of fourth and fifth order and counts of Sholl ring intersections corresponding to 20 µm of tissue were significantly higher in pilocarpine-treated foragers than scopolamine-treated foragers. This suggests that muscarinic activation in and of itself, although growth promoting, is not a complete replacement for foraging experience. Other factors associated with foraging may modulate the effects of muscarinic signaling via alterations in gene expression or co-release of other neurotransmitters. The present results, however, support the hypothesis that one of the important differences between hive bees and experienced foragers is the amount of cholinergic neurotransmission in the mushroom body calyces. The results also suggest that this mechanism can be engaged at any time in the life of a worker honey bee, independent of age or prior foraging experience. This is in accord with previous demonstrations of foraging-induced growth of the mushroom body neuropil in bees induced to forage precociously (Withers et al., 1993; Durst et al., 1994; Farris et al., 2001). Kenyon cell development is also potentially impacted by levels of circulating hormones, such as ecdysteroids (Velarde et al., 2009). We hypothesize that a baseline level of growth resulting from nuclear receptor activation may be locally modulated through cholinergic synaptic transmission.
Only wedge-shaped collar Kenyon cells were included in our analysis, and at present no information is available that permits these results to be generalized to other categories of Kenyon cells or other compartments of the calycal neuropil. Wedge-shaped collar Kenyon cells are a homogenous population that allow “like-versus-like” comparisons of dendritic morphology. By focusing a single subtype of Kenyon cell, we were able to provide quantitative measures for an exemplar of experience-dependent structural plasticity. Previous reports found that foraging experience is positively correlated with their complexity (Farris et al., 2001). Other mushroom body neurons likely undergo dendritic reorganization as well. In the paper wasp, foraging increases dendritic complexity of both lip and collar Kenyon cells (Jones et al., 2009). Changes in honey bee mushroom body volume in response to experience occur in the lip, collar, basal ring, and the peduncle (Kenyon cell axons) (Withers et al., 1993; Durst et al., 1994; Withers et al., 1995).
In addition to linking the pilocarpine-induced growth of mushroom body neuropil reported in experienced foragers by Ismail et al. (2006) to changes in dendritic complexity, we also probed the capacity of wedge collar Kenyon cells in young pre-foragers to respond to the muscarinic agonist. Farris et al. (2001) found the wedge collar Kenyon cells of one-day-old bees had more total dendritic branches than the 9–10 day old nurse bees also included in that study. This suggested that the dendritic arbors of the wedge collar Kenyon cells undergo regression (retraction or pruning) during the first week of adult life. A similar finding was also reported in the ant mushroom body by Seid, Harris, and Traniello (2005). Young worker ants have a greater number of axonal boutons (presynaptic specializations) than older workers. Pruning continues throughout the ant’s behavioral development as experienced foragers have reduced numbers of boutons in the lip and collar compared with younger callows (Seid and Wehner, 2009). The data presented here confirm previous suggestions of mushroom body pruning by Farris et al. (2001) and Seid et al. (2005) and Seid and Wehner (2009). Regression of Kenyon cell arbors, and possibly presynaptic boutons, may be regulated during normal development of young adult workers via cholinergic signaling because chronic stimulation of muscarinic receptors by feeding pilocarpine resulted in dendritic arbors similar in complexity to those observed in one-day-olds, whereas blocking muscarinic activation by feeding scopolamine resulted in neurons with simpler arborizations than one-day-olds. Furthermore, 7-day-old pilocarpine-treated bees had more total Sholl ring intersections than scopolamine-treated 7-day-olds, and more total branches than either scopolamine-treated, hive, or sucrose 7-day-olds. This suggests that the extent of cholinergic neurotransmitter release by mushroom body afferents (reflecting changes in sensory experience) regulates the shape of Kenyon cell dendritic arbor during the first week of adult life.
The dynamics of the structural plasticity reported here cannot be determined using end-point measurements such as Golgi-based reconstructions. In addition, the particular synapses directly affected by the muscarinic agents cannot be specified. The honey bees in these studies orally ingested either a muscarinic agonist or antagonist dissolved in sucrose. After ingestion, the drugs presumably diffuse from the midgut into the hemolymph and bathe the entire brain. It is enticing to assume the primary effect of these drugs was directly on the Kenyon cell dendritic arborizations, but the possibility cannot be excluded that the inputs into these cells, either afferents from the optic lobe or mushroom body feedback neurons, are the main site of action and that changes in their activity drive the changes measured here. Repeated in vivo imaging of an individual Kenyon cell treated with muscarinic active compounds would be required to address issues of structural dynamics; microinjections of agonists and antagonists directly into various brain regions could determine if Kenyon cell synapses or their inputs are the primary driver of structural plasticity.
Three previous studies have directly examined spine density on dendrites of honey bee Kenyon cells (Coss et al., 1980; Brandon and Coss, 1982; Farris et al., 2001). Our study differs from these earlier studies in that we examined the density of spines in three distinct regions (proximal, medial, and distal with respect to the primary neurite) of the dendritic field rather than pooling all spines visible in a single plane of focus (Coss et al., 1980; Brandon and Coss, 1982) or measuring a single region (which corresponds to our defined medial region; Farris et al., 2001). We found that, while manipulation of muscarinic signaling did not affect total spine density, the distal region always had nearly twice as many total spines as either the proximal or medial regions. Because collar Kenyon cells receive stratified input from the optic lobe, this distribution of dendritic spines may have functional significance. Afferents from the lobula, shown to be motion sensitive, project into the proximal region of the collar, whereas those from the color sensitive medulla project more distally (Ehmer and Gronenberg, 2002). Although Golgi impregnation cannot indicate whether a spine forms a functional synapse, the higher number of spines in the distal region may represent unequal representation of different attributes of the visual world in the collar, with heavier weighting given to color in more experienced foragers. Honey bees have trichromatic color vision and foraging bees attend to color cues associated with flowers, feeders, and hive entrances (e.g. Menzel and Blakers, 1976; Winston, 1987; reviewed in Mujagic et al., 2010).
Studies of the calyces of the mushroom bodies based on different cell labeling techniques have yet to be integrated into a single compelling scenario that incorporates structural plasticity. On one hand, we have the Golgi-based catalog of the different categories of Kenyon cells and several studies documenting quantitative changes in Kenyon cell dendritic arborizations associated with foraging and, now, treatments that affect cholinergic neurotransmission. On the other hand, we have the results of studies combining labeling of f-actin and postsynaptic specializations demonstrating that projection neurons from primary sensory areas form microglomerular synaptic complexes involving Kenyon cell spines (Frambach et al., 2004; Groh et al., 2004; Groh et al., 2006). Changes in the density of microglomeruli, but not changes in neuropil volume, have been reported to occur in the lip region of the calyx after olfactory conditioning of the proboscis extension response led to the formation of a memory that persisted for 72 h (Hourcade et al., 2010). Our data suggest that new dendritic branches serve as the basis of new microglomeruli rather than being added to existing microglomerular structures. The lack of a link between increased density of microglomeruli and neuropil volume in the olfactory conditioning paradigm likely reflects the delicate and selective nature of the synaptic plasticity associated with learning a single odor/reward pairing. By contrast, the transition to foraging (or pharmacological treatment with a muscarinic agonist) would be expected to impact the majority of synapses in the mushroom body calyces, resulting in changes that can be observed both at the level of individual Kenyon cells and in measures of neuropil volume.
The behavioral implications of Kenyon cell plasticity have yet to be determined. Neuronal morphology influences the number and type of synaptic contacts; neurons with longer dendrites and/or broader dendritic fields have a greater opportunity to interact with more synaptic partners. Changing dendritic morphology, either through normal development, accumulating foraging experience, or pharmacological treatment, therefore leads to functional changes of the affected neurons. A feedback cycle can be envisioned whereby experience influences neuronal structure, changes in structural influence sensory processing, which ultimately leads to an adapted behavior. It is unknown how foraging-related mushroom body changes influence behavior, but foraging experience has been linked to improved foraging efficiency (Dukas and Visscher, 1994; Schippers et al., 2006).
In summary, we have demonstrated that pilocarpine (muscarinic agonist) increased the dendritic complexity of wedge collar Kenyon cells in young and foraging-aged bees and that scopolamine (muscarinic antagonist) enhanced normal developmental pruning. These results suggest a mechanism by which foraging experience is coupled to structural plasticity in the adult honey bee brain.
Table 1.
Number of samples analyzed and age of worker honey bees used in Golgi analyses.
| Treatment group | Age (d) | No. of bees used | No. of neurons analyzed for dendritic complexity analysis |
No. of branches used in spine analysis |
|---|---|---|---|---|
|
Pre-foragers | ||||
| 1-day-old | 1 | 6 | 6 | N/A |
| Pilocarpine | 7 | 12 | 13 | 8 |
| Scopolamine | 7 | 13 | 15 | 9 |
| Sucrose-only | 7 | 15 | 15 | 10 |
| Hive | 7 | 10 | 10 | 6 |
| Forager (1 week) | ||||
| Pilocarpine | 30 – 31 | 9 | 12 | 20 |
| Scopolamine | 30 – 31 | 5 | 6 | 10 |
| Sucrose-only | 30 – 31 | 9 | 10 | 18 |
Acknowledgements
Thanks to Rodrigo A. Velarde for assistance with experimental design, Jeffrey T. Jackson for assistance with beekeeping and Claudia C. Lutz and Erika Vardeman for reviewing the manuscript. S.E.D. was supported by NIH Award GM073644 to G.E.R. and S.E.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett EL, Diamond MC, Krech P, Rosenzweig MR. Chemical and anatomical plasticity of the brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Bicker G. Histochemistry of classical neurotransmitters in antennal lobes and mushroom bodies of the honeybee. Microscopy Research and Technique. 1999;45:174–183. doi: 10.1002/(SICI)1097-0029(19990501)45:3<174::AID-JEMT5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Brandon J, Coss R. Rapid dendritic spine shortening during one-trial learning: the honeybee's first orientation flight. Brain Research. 1982;252:51–61. doi: 10.1016/0006-8993(82)90977-5. [DOI] [PubMed] [Google Scholar]
- Burrows M. The neurobiology of an insect brain. Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- Coss RG, Brandon JG, Globus A. Changes in morphology of dendritic spines on honeybee calycal interneurons associated with cumulative nursing and foraging experience. Brain Research. 1980;192:49–59. doi: 10.1016/0006-8993(80)91007-0. [DOI] [PubMed] [Google Scholar]
- Dukas R, Visscher P. Lifetime learning by foraging honey bees. Animal Behaviour. 1994;48:1007–1012. [Google Scholar]
- Durst C, Eichmuller S, Menzel R. Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behavioral Neural Biology. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- Ehmer B, Gronenberg W. Segregation of visual input to the mushroom bodies in the honeybee (Apis mellifera) Journal of Comparative Neurology. 2002;451:362–373. doi: 10.1002/cne.10355. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annual Review of Entomology. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Strande JL, Robinson GE. Neurogenesis is absent in the brains of adult honey bees and does not explain behavioral neuroplasticity. Neuroscience Letters. 1995;197:145–148. doi: 10.1016/0304-3940(95)11913-h. [DOI] [PubMed] [Google Scholar]
- Farris SM, Roberts NS. Coevolution of generalist feeding ecologies and gyrencephalic mushroom bodies in insects. Proceedings of the National Academy of Sciences USA. 2005;102:17394–17399. doi: 10.1073/pnas.0508430102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Davis RL, Fahrbach SE. Larval and pupal development of the mushroom bodies in the honey bee, Apis mellifera. Journal of Comparative Neurology. 1999;414:97–113. doi: 10.1002/(sici)1096-9861(19991108)414:1<97::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Farris SM, Robinson GE, Fahrbach SE. Experience- and age-related outgrowth of intrinsic neurons in the mushroom bodies of the worker honeybee. Journal of Neuroscience. 2001;21:6395–6404. doi: 10.1523/JNEUROSCI.21-16-06395.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SM, Sinakevitch I. Development and evolution of the insect mushroom bodies: towards the understanding of conserved developmental mechanisms in a higher brain center. Arthropod Structure and Development. 2003;32:79–101. doi: 10.1016/S1467-8039(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Frambach I, Rossler W, Winkler M, Schürmann FW. F-actin at identified synapses in the mushroom body neuropil of the insect brain. Journal of Comparative Neurology. 2004;475:303–314. doi: 10.1002/cne.20165. [DOI] [PubMed] [Google Scholar]
- Groh C, Tautz J, Rossler W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proceedings of the National Academy USA. 2004;101:4268–4273. doi: 10.1073/pnas.0400773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh C, Ahrens D, Rossler W. Environment- and age-dependent plasticity of synaptic complexes in the mushroom bodies of honeybee queens. Brain Behavior Evolution. 2006;68:1–14. doi: 10.1159/000092309. [DOI] [PubMed] [Google Scholar]
- Groh C, Meinertzhagen IA. Brain plasticity in Diptera and Hymenoptera. Frontiers in Bioscience. 2010;2:268–288. doi: 10.2741/s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronenberg W. Subdivisions of hymenopteran mushroom body calyces by their afferent supply. Journal of Comparative Neurology. 2001;435:474–489. doi: 10.1002/cne.1045. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Heeren S, Holldöbler B. Age-dependent and task-related morphological changes in the brain and mushroom bodies of the ant Camponotus floridanus. Journal of Experimental Biology. 1996;199:2011–2019. doi: 10.1242/jeb.199.9.2011. [DOI] [PubMed] [Google Scholar]
- Hourcade B, Muenz TS, Sandoz J, Rössler W, Devaud J. Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? Journal of Neuroscience. 2010;30:6461–6465. doi: 10.1523/JNEUROSCI.0841-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Knowles C. Nicotinic and muscarinic cholinergic receptors in honey bee (Apis mellifera) brain. Comparative Biochemistry and Physiology. 1990;97C:275–281. [Google Scholar]
- Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proceedings of the National Academy USA. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Christine S, Robinson GE, Fahrbach SE. Pilocarpine improves recognition of nestmates in young honey bees. Neuroscience Letters. 2008;439:178–181. doi: 10.1016/j.neulet.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Donlan NA, O'Donnell S. Growth and pruning of mushroom body Kenyon cell dendrites during worker behavioral development in the paper wasp, Polybia aequatorialis (Hymenoptera: Vespidae) Neurobiology of Learning and Memory. 2009;92:485–495. doi: 10.1016/j.nlm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Kenyon FC. The brain of the bee. A preliminary contribution to the morphology of the nervous system of the arthropoda. Journal of Comparative Neurology. 1896;6:133–210. [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proceedings of the National Academy USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreissl S, Bicker G. Histochemistry of acetylcholinesterase and immunocytochemistry of an acetylcholine receptor-like antigen in the brain of the honeybee. Journal of Comparative Neurology. 1989;286:71–84. doi: 10.1002/cne.902860105. [DOI] [PubMed] [Google Scholar]
- Krofczik S, Khojasteh U, de Ibarra NH, Menzel R. Adaptation of microglomerular complexes in the honeybee mushroom body lip to manipulations of behavioral maturation and sensory experience. Developmental Neurobiology. 2008;68:1007–1017. doi: 10.1002/dneu.20640. [DOI] [PubMed] [Google Scholar]
- Li Y, Strausfeld N. Morphology and sensory modality of mushroom body extrinsic neurons in the brain of the cockroach, Periplaneta americana. Journal of Comparative Neurology. 1997;387:631–650. [PubMed] [Google Scholar]
- Lorenz MW, Kellner R, Völkl W, Hoffmann KH, Woodring J. A comparative study on hypertrehalosaemic hormones in the Hymenoptera: sequence determination, physiological actions and biological significance. Journal of Insect Physiology. 2001;47:563–571. doi: 10.1016/s0022-1910(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Lozano VC, Armengaud C, Gauthier M. Memory impairment induced by cholinergic antagonists injected into the mushroom bodies of the honeybee. Journal of Comparative Physiology A. 2001;187:249–254. doi: 10.1007/s003590100196. [DOI] [PubMed] [Google Scholar]
- Lutz CC, Rodriguez-Zas SL, Fahrbach SE, Robinson GE. Effects of foraging experience on gene expression in the honey bee brain; Society for Neuroscience Annual Meeting; San Diego: 2009. [Google Scholar]
- Maleszka J, Barron AB, Helliwell PG, Maleszka R. Effect of age, behaviour and social environment on honey bee brain plasticity. Journal of Comparative Physiology A. 2009;195:733–740. doi: 10.1007/s00359-009-0449-0. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Menzel R, Blakers M. Colour receptors in the bee eye – morphology and spectral sensitivity. Journal of Comparative Physiology A. 1976;108:11–33. [Google Scholar]
- Mobbs PG. The brain of the honeybee Apis mellifera L. The connections and spatial organization of the mushroom bodies. Philosophical Transactions Royal Society of London B. 1982;298:309–354. [Google Scholar]
- Molina Y, Harris RM, O'Donnell S. Brain organization mirrors caste differences, colony founding and nest architecture in paper wasps (Hymenoptera: Vespidae) Proceedings of the Royal Biological Society B. 2009;276:3345–3351. doi: 10.1098/rspb.2009.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujagic S, Sarkander J, Erber B, Erber J. Sucrose acceptance and different forms of associative learning of the honey bee (apis mellifera L.) in the field and laboratory. Frontiers in Behavioral Neuroscience. 2010;4:46. doi: 10.3389/fnbeh.2010.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S, Donlan NA, Jones TA. Mushroom body structural change is associated with division of labor in eusocial wasp workers (Polybia aequatorialis, Hymenoptera: Vespidae) Neuroscience Letters. 2004;356:159–162. doi: 10.1016/j.neulet.2003.11.053. [DOI] [PubMed] [Google Scholar]
- Peitsch D, Fietz A, Hertel H, deSouza J, Ventura DF, Menzel R. The spectral input systems of hymenopteran insects and their receptor-based colour vision. Journal of Comparative Physiology A. 1992;170:23–40. doi: 10.1007/BF00190398. [DOI] [PubMed] [Google Scholar]
- Riege WH. Environmental influences on brain and behavior of year-old rats. Developmental Psychobiology. 1971;4:157–167. doi: 10.1002/dev.420040207. [DOI] [PubMed] [Google Scholar]
- Schippers M, Dukas R, Smith R, Wang J, Smolen K, McClelland G. Lifetime performance in foraging honeybees: behaviour and physiology. Journal of Experimental Biology. 2006;209:3828–3836. doi: 10.1242/jeb.02450. [DOI] [PubMed] [Google Scholar]
- Schröter U, Menzel R. A new ascending sensory tract to the calyces of the honeybee mushroom body, the subesophageal-calycal tract. Journal of Comparative Neurology. 2003;465:168–178. doi: 10.1002/cne.10843. [DOI] [PubMed] [Google Scholar]
- Seid M, Harris K, Traniello J. Age-related changes in the number and structure of synapses in the lip region of the mushroom bodies in the ant Pheidole dentata. Journal of Comparative Neurology. 2005;488:269–277. doi: 10.1002/cne.20545. [DOI] [PubMed] [Google Scholar]
- Seid MA, Wehner R. Delayed axonal pruning in the ant brain: a study of developmental trajectories. Developmental Neurobiology. 2009;69:350–364. doi: 10.1002/dneu.20709. [DOI] [PubMed] [Google Scholar]
- Shapira M, Thompson CK, Soreq H, Robinson G. Changes in neuronal acetylcholinesterase gene expression and division of labor in honey bee colonies. Journal of Molecular Neuroscience. 2001;17:1–12. doi: 10.1385/JMN:17:1:1. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of Anatomy. 1953;87:387–407. [PMC free article] [PubMed] [Google Scholar]
- Stieb SM, Muenz TS, Wehner R, Rössler W. Visual experience and age affect synaptic organization in the mushroom bodies of the desert ant Cataglyphis fortis. Developmental Neurobiology. 2010;70:408–423. doi: 10.1002/dneu.20785. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. Journal of Comparative Neurology. 2002;450:4–33. doi: 10.1002/cne.10285. [DOI] [PubMed] [Google Scholar]
- Velarde RA, Robinson GE, Fahrbach SE. Coordinated responses to developmental hormones in the Kenyon cells of the adult worker honey brain (Apis mellifera L.) Journal of Insect Physiology. 2009;55:59–69. doi: 10.1016/j.jinsphys.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176:1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- Withers GS, Day NF, Talbot EF, Dobson HE, Wallace CS. Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachilidae) Developmental Neurobiology. 2008;68:73–82. doi: 10.1002/dneu.20574. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Effects of experience and juvenile hormone on the organization of the mushroom bodies of honey bees. Journal of Neurobiology. 1995;26:130–144. doi: 10.1002/neu.480260111. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen I, Schürmann F. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. Journal of Comparative Neurology. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Salvaterra PM. Localization of choline acetyltransferase-expressing neurons in Drosophila nervous system. Microscopy Research Techniques. 1999;45:65–79. doi: 10.1002/(SICI)1097-0029(19990415)45:2<65::AID-JEMT2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]