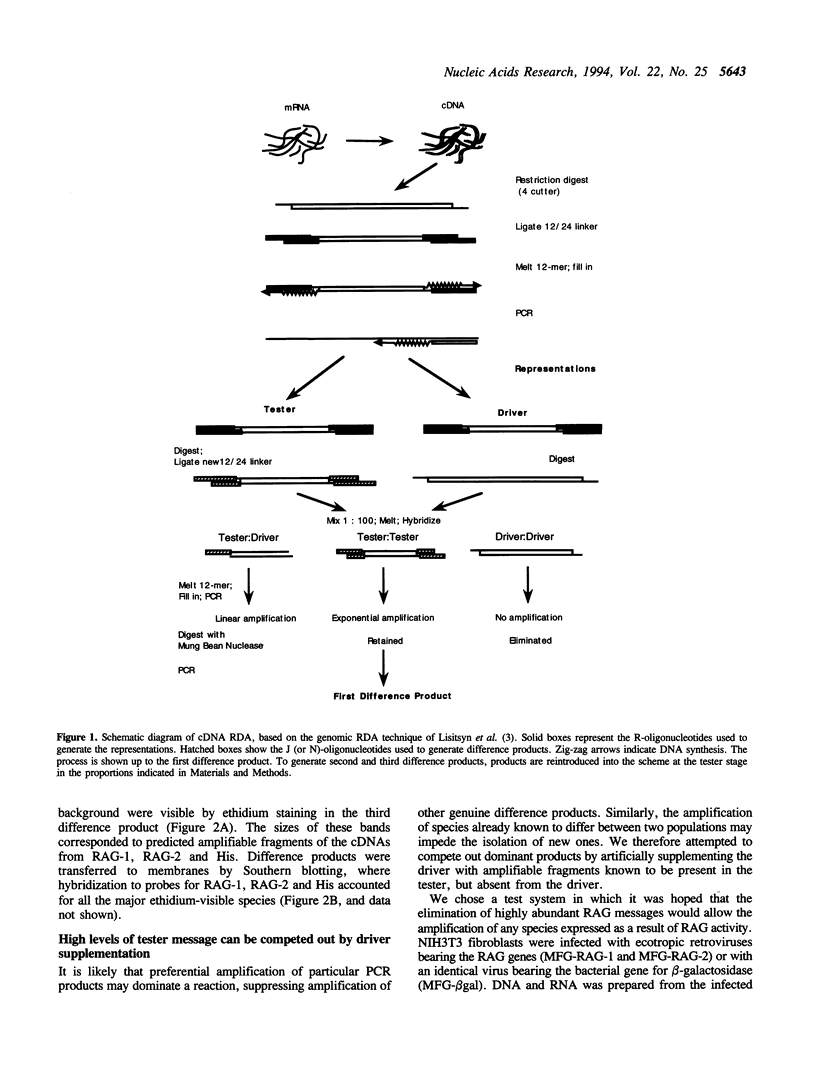
Abstract
Detection of differentially regulated genes has been severely hampered by technical limitations. In an effort to overcome these problems, the PCR-coupled subtractive process of representational difference analysis (RDA) [Lisitsyn, N. et al. (1993) Science 259, 946-951] has been adapted for use with cDNA. In a model system, RAG-1 and RAG-2, the genes responsible for activating V(D)J recombination, were identified in a genomic transfectant by cDNA RDA in a small fraction of the time taken by conventional means. The system was also modified to eliminate expected difference products to facilitate the identification of novel genes. Additional alterations to the conditions allowed isolation of differentially expressed fragments. Several caffeine up-regulated clones were obtained from the pre-B cell line 1-8, including IGF-1B, and a predicted homologue of the natural killer cell antigen, NKR-P1. The approach was found to be fast, extremely sensitive, reproducible, and predominantly lacked false positives. cDNA RDA has the capacity and adaptability to be applied to a wide range of biological problems, including the study of single gene disorders, characterization of mutant and complemented cell types, developmental or post-event expression time courses, and examination of pathogen-host interactions.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baier T. G., Jenne E. W., Blum W., Schönberg D., Hartmann K. K. Influence of antibodies against IGF-I, insulin or their receptors on proliferation of human acute lymphoblastic leukemia cell lines. Leuk Res. 1992 Aug;16(8):807–814. doi: 10.1016/0145-2126(92)90160-9. [DOI] [PubMed] [Google Scholar]
- Bauer D., Müller H., Reich J., Riedel H., Ahrenkiel V., Warthoe P., Strauss M. Identification of differentially expressed mRNA species by an improved display technique (DDRT-PCR). Nucleic Acids Res. 1993 Sep 11;21(18):4272–4280. doi: 10.1093/nar/21.18.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Stempien M. M., Fong N. M., Rall L. B. Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res. 1986 Oct 24;14(20):7873–7882. doi: 10.1093/nar/14.20.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. T., Williamson J. A., Skolnick M. H. A model for restriction fragment length distributions. Am J Hum Genet. 1983 Sep;35(5):795–815. [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Donohue P. J., Alberts G. F., Hampton B. S., Winkles J. A. A delayed-early gene activated by fibroblast growth factor-1 encodes a protein related to aldose reductase. J Biol Chem. 1994 Mar 18;269(11):8604–8609. [PubMed] [Google Scholar]
- Jung M., Kondratyev A. D., Dritschilo A. Elongation factor 1 delta is enhanced following exposure to ionizing radiation. Cancer Res. 1994 May 15;54(10):2541–2543. [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A., Segre J. A., Kusumi K., Lisitsyn N. M., Nadeau J. H., Frankel W. N., Wigler M. H., Lander E. S. Direct isolation of polymorphic markers linked to a trait by genetically directed representational difference analysis. Nat Genet. 1994 Jan;6(1):57–63. doi: 10.1038/ng0194-57. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Cyclic AMP induces insulin-like growth factor I synthesis in osteoblast-enriched cultures. J Biol Chem. 1990 Sep 15;265(26):15353–15356. [PubMed] [Google Scholar]
- McKearn J. P., Rosenberg N. Mapping cell surface antigens on mouse pre-B cell lines. Eur J Immunol. 1985 Mar;15(3):295–298. doi: 10.1002/eji.1830150316. [DOI] [PubMed] [Google Scholar]
- Menetski J. P., Gellert M. V(D)J recombination activity in lymphoid cell lines is increased by agents that elevate cAMP. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9324–9328. doi: 10.1073/pnas.87.23.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio Y., Aiello L. P., King G. L. Glucose induced genes in bovine aortic smooth muscle cells identified by mRNA differential display. FASEB J. 1994 Jan;8(1):103–106. doi: 10.1096/fasebj.8.1.8299882. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Neveu M., Liang P., Sotiropoulou G. Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993 Jul;7(10):964–970. doi: 10.1096/fasebj.7.10.8344495. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988 Apr 8;53(1):107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schuler W., Bosma M. J. Nature of the scid defect: a defective VDJ recombinase system. Curr Top Microbiol Immunol. 1989;152:55–62. doi: 10.1007/978-3-642-74974-2_8. [DOI] [PubMed] [Google Scholar]
- Waring M., Britten R. J. Nucleotide sequence repetition: a rapidly reassociating fraction of mouse DNA. Science. 1966 Nov 11;154(3750):791–794. doi: 10.1126/science.154.3750.791. [DOI] [PubMed] [Google Scholar]
- Wieland I., Bolger G., Asouline G., Wigler M. A method for difference cloning: gene amplification following subtractive hybridization. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2720–2724. doi: 10.1073/pnas.87.7.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]