Abstract
Enzyme replacement therapy (ERT) with acid α-glucosidase has become available for Pompe disease; however, the response of skeletal muscle, as opposed to the heart, has been attenuated. The poor response of skeletal muscle has been attributed to the low abundance of the cation-independent mannose-6-phosphate receptor (CI-MPR) in skeletal muscle compared to heart. To further understand the role of CI-MPR in Pompe disease, muscle-specific CI-MPR conditional knockout (KO) mice were crossed with GAA-KO (Pompe disease) mice. We evaluated the impact of CI-MPR-mediated uptake of GAA by evaluating ERT in CI-MPR-KO/GAA-KO (double KO) mice. The essential role of CI-MPR was emphasized by the lack of efficacy of ERT as demonstrated by markedly reduced biochemical correction of GAA deficiency and of glycogen accumulations in double KO mice, in comparison with administration of the same therapeutic doses in GAA-KO mice. Clenbuterol, a selective β2-agonist, enhanced CI-MPR expression in skeletal tissue and also increased efficacy from GAA therapy, thereby confirming the key role of CI-MPR with regard to enzyme replacement therapy in Pompe disease. Biochemical correction improved in both muscle and non-muscle tissues, indicating that therapy could be similarly enhanced in other lysosomal storage disorders. In summary, enhanced CI-MPR expression might improve the efficacy of enzyme replacement therapy in Pompe disease through enhancing receptor-mediated uptake of GAA.
Keywords: Mannose-6-phosphate receptor, enzyme replacement therapy, acid alpha-glucosidase, acid maltase, Pompe disease, glycogen storage disease type II
INTRODUCTION
Pompe disease (Glycogen storage disease type II; acid maltase deficiency; MIM 232300) ranges in severity from a severe, infantile-onset hypertrophic cardiomyopathy to a late-onset myopathy, which is caused by a defect in acid α-glucosidase (GAA) varying from complete to partial deficiency of GAA. Infantile-onset Pompe disease affects the heart and skeletal muscle primarily, and causes death early in childhood from cardiorespiratory failure, if initiation of ERT is delayed or the patient fails to respond to ERT due to high, sustained anti-GAA antibodies [1–3]. However, enzyme replacement therapy (ERT) with recombinant human (rh) GAA has been effective for the long-term only in a subset of patients with infantile-onset Pompe disease.
GAA normally functions as an acid hydrolase that metabolizes lysosomal glycogen, and deficient GAA causes lysosomal glycogen accumulation in virtually all tissues [4]. The availability of ERT with rhGAA has prolonged survival and ameliorated the cardiomyopathy in the majority of patients with infantile-onset Pompe disease [2]. In late-onset Pompe disease the clinical response to ERT has been less dramatic than in the infantile-onset presentation, and ERT has largely resulted in stabilization of the disease process from a pulmonary and motor perspective [5]. Many individuals with late-onset Pompe disease have residual gait abnormalities despite adherence to ERT, indicating a relative lack of response of limb-girdle and leg muscles [5]. Muscle weakness was stabilized by ERT in one small series following 5 patients with juvenile-onset Pompe disease, although only one subject approached the normal range for muscle strength as quantified by hand-held dynamometry following 3 years of ERT [6]. A 3 month trial of ERT in 44 subjects with late-onset Pompe disease demonstrated significant improvement in the 6 minute walk test, modified Gowers’ test, and creatine kinase levels, whereas stair climbing and serial arm function tests remained unchanged [5]. Another series of 11 subjects with late-onset Pompe disease was evaluated with dynamometry and quantitative magnetic resonance imaging of leg muscles over the course of 2 years on ERT, and both muscle mass and strength in the anterior thigh improved; however, progression of intramuscular fat accumulation during ERT emphasized the limited efficacy from ERT and the need for early treatment [7]. Taken together, these studies of ERT in juvenile and late-onset Pompe disease emphasized the limited responsiveness of skeletal muscle to the only available therapy.
Documented limitations of ERT in Pompe disease include the requirement for frequent intravenous infusions of high doses of GAA to achieve efficacy, degree of pre-ERT muscle damage, and the possibility of humoral immunity [2; 3; 8]. The rhGAA doses are markedly higher than those required for ERT in other lysosomal storage disorders, possibly reflecting the higher threshold for correction of GAA deficiency in the skeletal muscle of Pompe disease patients [9]. The paucity of cation-independent mannose-6-phosphate receptor (CI-MPR) in adult mammals’ muscle has underscored the concept that CI-MPR is limiting for ERT in Pompe disease. Previously, low levels of CI-MPR were demonstrated in skeletal muscle of GAA-KO mice, specifically in muscles comprised primarily of type II myofibers [10; 11]. Further evidence for the importance of CI-MPR expression to ERT in Pompe disease was demonstrated by the increased efficacy of rhGAA modified to increase mannose-6-phosphate content [12–14]. Furthermore, Pompe disease patient fibroblasts were found to be deficient in CI-MPR recycling and uptake of rhGAA was impaired [15]. However, until now the effect of CI-MPR manipulation in vivo has never been analyzed in Pompe disease.
In order to gain understanding of the influence of CI-MPR expression upon therapy in Pompe disease, we have characterized muscle-specific CI-MPR-KO/GAA-KO mice, evaluating ERT in these double (D) KO mice and demonstrating impaired responsiveness of skeletal muscle in DKO mice. In order to confirm the relevance of CI-MPR to ERT in Pompe disease, we sought to up-regulate expression of CI-MPR in skeletal muscle. The only drug known to have this effect was β2-agonist therapy with clenbuterol, which increased insulin-like growth factor 2 (Igf-2) receptor (also known as CI-MPR) expression in the masseter muscle of mice, along with Igf-1 and Igf-2 [16]. Therefore, we chose to evaluate the effect of clenbuterol treatment upon receptor-mediated uptake and biochemical correction of skeletal muscle during ERT in GAA-KO mice. The effectiveness of clenbuterol in increasing the response to ERT suggests that this might be valuable as an adjunctive therapy for Pompe disease.
RESULTS
Evaluation of GAA uptake and glycogen clearance in absence of CI-MPR expression
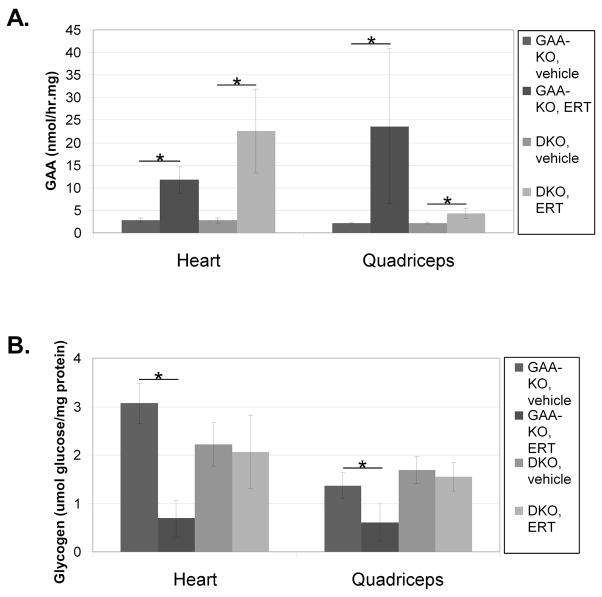
To understand the role of CI-MPR in recombinant human GAA (rhGAA) uptake and glycogen clearance specifically in Pompe disease, muscle-specific CI-MPR-KO mice were crossed with GAA-KO (Pompe disease) mice [17]. Evaluation of GAA activity demonstrated no significant differences in Pompe disease characteristics between the GAA-KO and the DKO mouse strains (Fig. 1A). GAA-KO and DKO mice were administered four weekly doses of rhGAA (20 mg/kg body weight), and euthanized three days after the last injection to evaluate GAA enzyme activity and glycogen content in striated muscle. GAA activity analysis demonstrated significantly decreased enzyme levels in the quadriceps of DKO mice following ERT, in comparison with GAA-KO mice (p=0.003; Fig. 1A). However, in the heart increased GAA activity was observed in DKO mice following ERT, in comparison with GAA-KO mice (p=0.03; Fig. 1A). The latter observation suggested an alternative mechanism for uptake of rhGAA in the heart that did not utilize CI-MPR.
Fig. 1. Impaired rhGAA uptake in DKO mice.
The homozygous DKO mice (n=4) and GAA-KO mice (n=4) were administered four weekly doses of rhGAA and sacrificed three days after the last injection. (A) GAA enzyme levels and (B) glycogen content were evaluated in the target tissues. Mean +/− standard deviation are shown.
The reversal of glycogen storage, or clearance, marks the biochemical correction in Pompe disease. ERT achieved significant glycogen clearance in the heart and quadriceps only in GAA-KO mice, not in DKO mice (Fig. 1B). Furthermore, glycogen content was increased in the heart of DKO mice following ERT, in comparison with GAA-KO mice (2.1 +/− 0.8 versus 0.7 +/− 0.4 umol glucose/mg protein, respectively; p=0.003). Similarly, resistance to the clearance of glycogen was observed in the quadriceps of DKO mice following ERT, in comparison with GAA-KO mice (p=0.0002; Fig. 1B).
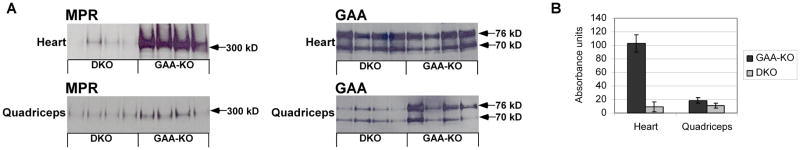
The basis for resistance to ERT in DKO mice was further analyzed by Western blot analysis of heart, which revealed more than 90% reduction of CI-MPR in DKO mouse in comparison with GAA-KO mice. In skeletal muscle (quadriceps), DKO mice had 40% decreased CI-MPR expression in comparison with GAA-KO mice (Fig. 2). These data revealed that CI-MPR expression was significantly reduced, establishing a correlation between reduced CI-MPR expression and resistance to glycogen clearance from ERT in striated muscle of DKO mice.
Fig. 2. Western blot analysis of CI-MPR expression and GAA content of heart and skeletal muscle.
(A) Western blot detection of CI-MPR and human GAA in the tissues of DKO and GAA-KO mice is shown, with molecular weights indicated. Each lane represents an individual mouse. Equivalent quantities of tissue homogenate were loaded for each mouse. (B) Signal for CI-MPR as quantified by densitometry of Western blots. Mean +/− standard deviation are shown.
Enhanced efficacy from simultaneous ERT and clenbuterol administration: motor function, GAA uptake and glycogen clearance
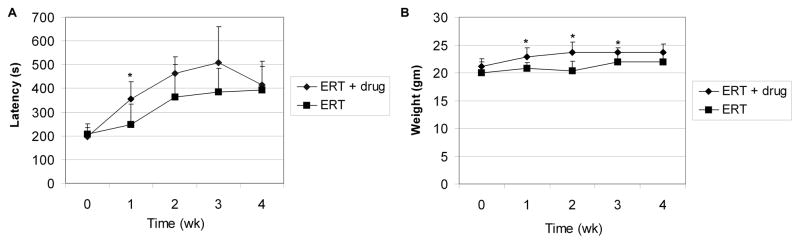
The implication that CI-MPR expression was crucial to both enzyme uptake and glycogen clearance, and intracellular processing of GAA in Pompe disease led us to attempt to manipulate CI-MPR levels in GAA-KO mice. This strategy differs from previous attempts to address the limiting role of CI-MPR expression during ERT in Pompe disease, which relied upon increasing the mannose-6-phosphate content of rhGAA [10; 13]. We attempted to increase CI-MPR in skeletal muscle to demonstrate the dependence of biochemical correction upon receptor-mediated endocytosis and uptake of GAA. Therefore, ERT was enhanced by the addition of a drug, clenbuterol, which was previously demonstrated to increase the expression of the Igf 2 receptor; identical to CI-MPR, in the masseter muscle of mice [16]. Groups of 3 month-old GAA-KO mice were treated with four weekly doses of rhGAA (20 mg/kg body weight), with or without concurrent clenbuterol treatment. The efficacy of clenbuterol treatment was demonstrated by an early increase in Rotarod latency in groups of male GAA-KO mice (p=0.04 at week 1), in comparison with GAA-KO mice treated with ERT alone (Fig. 3A). Increased weight gain in GAA-KO mice treated with clenbuterol suggested that muscle hypertrophy was stimulated as described [16], in comparison with GAA-KO mice treated with ERT alone (Fig. 3B; p<0.05 at weeks 1–3).
Fig. 3. Enhanced Rotarod performance and weight gain following ERT plus clenbuterol treatment.
GAA-KO mice were administered four weekly doses of rhGAA (20 mg/kg), and treated with clenbuterol (n=7) or untreated (n=5). (A) Rotarod latency and (B) weight at indicated time points. Mean +/− standard deviation are shown. Statistically significant alterations associated with clenbuterol treatment indicated (*).
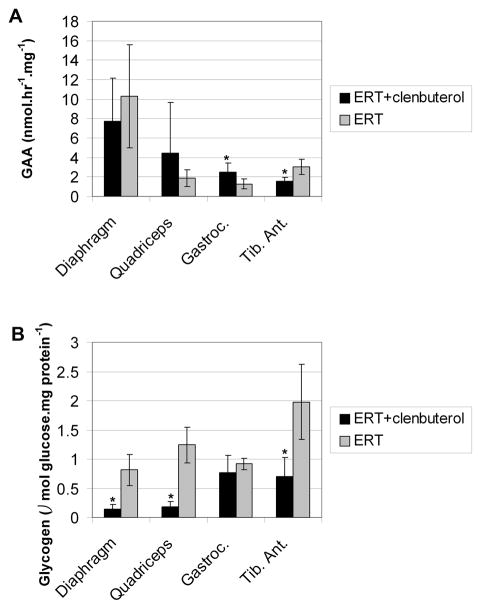
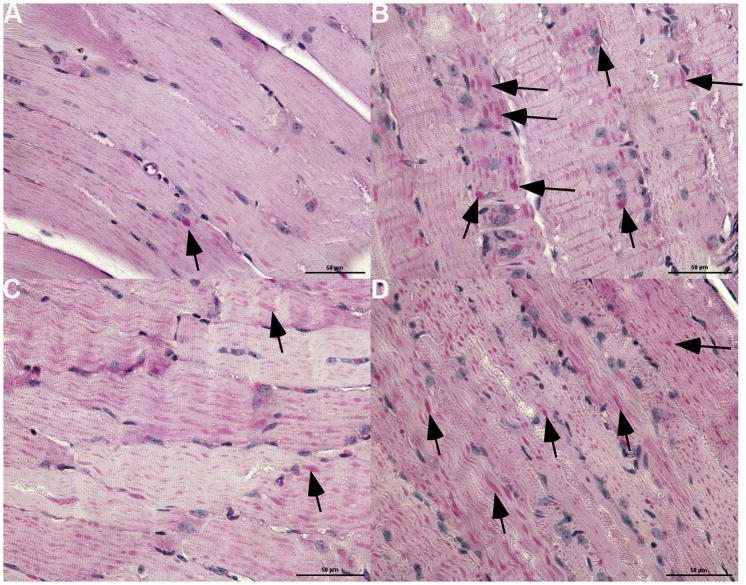
The enhanced efficacy of ERT in conjunction with clenbuterol treatment was further demonstrated by improved biochemical correction of GAA deficiency in both viscera and skeletal muscle. The GAA activity of gastrocnemius (p=0.04) was significantly increased for the clenbuterol-treated group, suggesting that CI-MPR-mediated uptake was enhanced in skeletal muscle (Fig. 4A). Furthermore, glycogen content was significantly reduced for diaphragm (p=0.0002), quadriceps (p=0.00002), and tibialis anterior (p=0.002) following clenbuterol treatment (Fig. 4B). The absence of increased glycogen clearance in the gastrocnemius from clenbuterol treatment, despite significantly increased GAA activity, suggested that additional factors might influence the reversal of glycogen storage in that muscle. Microscopic examination of the diaphragm and quadriceps revealed reductions in the size of glycogen vacuoles following ERT and clenbuterol treatment (Fig. 5A and 5C), in comparison with ERT alone (Fig. 5B and 5D). Thus, the addition of clenbuterol enhanced the biochemical correction of muscles that are severely affected in Pompe disease.
Fig. 4. Enhanced efficacy from ERT plus clenbuterol treatment.
Male GAA-KO mice were administered four weekly doses of 20 mg/kg body weight of rhGAA and sacrificed one week following the last injection. Groups of mice were treated with clenbuterol (n=6) or untreated (n=5) during ERT. Mice were euthanized for tissue analysis 4 weeks after vector injection. (A) GAA enzyme levels and (B) glycogen content were evaluated in the target tissues, including the tibialis anterior (Tib. Ant.). Statistically significant alterations associated with clenbuterol treatment indicated (*).
Fig. 5. Decreased glycogen accumulation in skeletal muscle following clenbuterol administration.
Periodic-acid Schiff staining for glycogen in paraffin-embedded sections. Original magnification 400X. (A) Diaphragm following clenbuterol treatment and ERT. (B) Diaphragm following ERT alone. (C) Quadriceps following clenbuterol treatment and ERT. (D) Quadriceps following ERT alone.
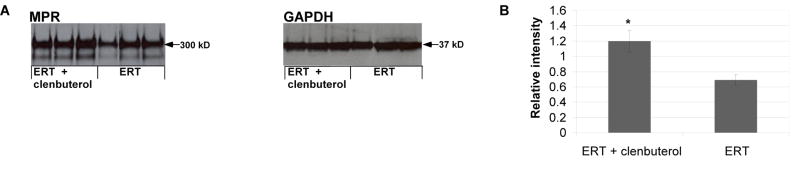
The basis for enhanced biochemical correction following addition of clenbuterol therapy was further analyzed by Western blot analysis of skeletal muscle. CI-MPR was increased in the tibialis anterior following clenbuterol treatment (Fig. 6A). CI-MPR expression was significantly increased following clenbuterol (p=0.003), when normalized to the signal for glycerol-3-aldehyde phosphate dehydrogenase (Fig. 6B).
Fig. 6. Western blot analysis of CI-MPR expression and GAA content of skeletal muscle following clenbuterol treatment.
(A) Western blot detection of CI-MPR and human GAA in the tibialis anterior of GAA-KO mice is shown, with molecular weights indicated. Each lane represents an individual mouse. Equivalent quantities of tissue homogenate were loaded for each mouse. (B) Signal for CI-MPR as quantified by densitometry of Western blot images. Mean +/− standard deviation are shown. Statistically significant alterations associated with CI-MPR absence indicated (*).
DISCUSSION
The modulating effect of CI-MPR has now been confirmed by analyzing the efficacy from GAA replacement therapy in mice with Pompe disease, either when expression was depleted or enhanced. DKO mice featured defective rhGAA uptake in skeletal muscle during ERT. Decreased rhGAA uptake in DKO mice resulted in residual glycogen storage, in comparison with GAA-KO mice treated simultaneously with ERT. The up-regulation of CI-MPR by treatment with clenbuterol enhanced the response to ERT in GAA-KO mice. CI-MPR was increased in the tibialis anterior, a skeletal muscle comprised primarily of type II myofibers, following clenbuterol treatment. The increased efficacy of ERT following clenbuterol treatment can be attributed at least in part to the up-regulation of CI-MPR; however, other effects such as enhanced perfusion of skeletal muscle and increased glycogenolysis cannot be excluded from the current study. The enhanced efficacy from clenbuterol treatment promises to improve the response to ERT in Pompe disease, by either reducing dosage requirements or increasing the efficacy of this standard of care therapy.
The DKO mouse model was designed to eliminate CI-MPR expression only in cells expressing MCK-cre recombinase; therefore, it is very likely that the lack of CI-MPR expression in the muscle fibers significantly impairs rhGAA uptake and trafficking to the glycogen laden lysosomes in this critical cell type. Of note, the presence of non-muscle cell types (e.g. vascular endothelium, adipose tissue and/or fibroblasts) in the quadriceps tissue samples may explain the significant residual levels of CI-MPR expression in this tissue. In these cell types, muscle creatine kinase (MCK)-cre recombinase is not expressed and therefore the CI-MPR gene is not subject to cre recombination and elimination of expression [18].
Clenbuterol has long been recognized for its hypertrophic effect upon skeletal muscle in rodent models, working through stimulation of β2-adrenergic receptors, acting through the local expression of Igf-1 and Igf-2 [19; 20]. Hypertrophy following clenbuterol administration was associated with increased muscle weight in the limb muscles, including gastrocnemius, soleus, and extensor digitorum longus (EDL), as well as increased body weight [21–23]. Fiber type switching was demonstrated following clenbuterol treatment, with a conversion from slow-twitch, oxidative type I to type IIA, and from slow-twitch, oxidative type I to fast twitch, glycolytic type IIb fibers in the soleus [19; 23; 24]. The masseter muscle responded to clenbuterol treatment with increased weight and elevated Igf-1 and Igf-2 expression; moreover, the expression of Igf-2 receptor, identical to CI-MPR, was demonstrated in the hypertrophied masseter muscle [16]. Taken together, these data suggest that the mechanism for enhanced efficacy from replacement therapy by the addition of clenbuterol is the expression of CI-MPR by type II myofibers that were previously unresponsive to ERT [10; 11]. Consistent with this hypothesis, we demonstrated the increased clearance of accumulated glycogen from the tibialis anterior, which contains primarily type II myofibers.
Clenbuterol is a β2 agonist drug that is used widely for the treatment of asthma due to bronchodilator effects, and it has been used illicitly by performance athletes and in agricultural animal production for its hypertrophic effects upon muscle [19]. Side effects including muscle tremor, tremor and insomnia have been reported [19]. Caution has been urged due to the relatively high dosages used to achieve muscle hypertrophy in rodent models, and this study used approximately 210 μg/kg/day, given an estimated water consumption for mice of 7 ml/day. However, infusion of a low amount of clenbuterol (only10 μg/kg/day) induced hypertrophy of type II fast, oxidative glycolytic myofibers in rats [25]. Dosing as low as 5 μg/kg/day increased muscle force generation in horses, indicating that much lower doses might be effective [26]. Indeed, clenbuterol was well-tolerated in a long-term study of four Duchenne muscular dystrophy patients receiving 30 to 40μg/kg/day, and the power and volume of well preserved muscles was increased [27]. In the current study efficacy was demonstrated for the lowest dose of clenbuterol, approximately 11 5 μg/kg/day, which supports the potential translation to adjunctive therapy with ERT in the clinic. Other β2 agonist drugs might be considered due to similar hypertrophic effects in rodent models, including formoterol or salmeterol at 25 μg/kg/day [28] or fenoterol at 1.4 mg/kd/day [29], when injected intraperitoneally. Finally, low dose oral albuterol was previously administered to 5 patients with late-onset Pompe disease, resulting in improvement of a functional score in absence of side effects [30; 31]. One of these subjects developed pulmonary edema following an intial treatment with injected albuterol. The same subject tolerated subsequent treatment with oral albuterol [31]. Clearly this use of albuterol requires evaluation of safety and efficacy, but encouraging efficacy has currently been demonstrated for the equivalent dose of albuterol in combination with ERT in mice with Pompe disease.
The current experiments demonstrated that the efficacy of GAA replacement therapy was enhanced by β2 agonist administration. This preclinical data promises that the response to ERT in Pompe disease might be improved by treatment with clenbuterol or a similarly active β2 agonist drug.
MATERIALS AND METHODS
Generation of muscle-specific CI-MPR-KO and DKO mouse models
CI-MPR-KO mice were generated using a muscle-specific promoter (muscle creatine kinase; CK) and the cre/loxP conditional knock out system as described previously [32]. The muscle-specific CI-MPR-KO mice were crossed with GAA-KO mice to generate muscle specific DKO mice. This mouse colony was subsequently screened to be GAA−/−, M6PR flox/flox and MCK-Cre positive. These DKO mice and age matched Pompe mice were administered four weekly doses of 20 or 100 mg/kg rhGAA and sacrificed 3 days after the last injection. Selected tissues were collected for GAA enzyme activity levels, glycogen content depletion and Western blot analysis. Pompe and DKO mice injected with vehicle were used as controls. At the indicated time points post-injection, tissue samples were obtained and processed as described below. All animal procedures were done in accordance with Duke University Institutional Animal Care and Use Committee-approved guidelines. GAA activity and glycogen content were analyzed as described [33].
GAA-KO genotyping was done with a PCR-based assay to determine the deletion of exon 6 in GAA-KO mice. Genomic mouse DNA (100 ng) was used as template in a PCR reaction (35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute) using the primers: exon5 F(5′-CCTTTCTACCTGGCACTGGAGGAC-3′), exon7 R (GGACAATGGCGGTCGAGGAGTA-3′) and neomycin F (5′-CCTCGTGCTTTACGGTATCGC-3′). This genotyping was carried out till GAA knock out mice colony was successfully established.
Mouse CI-MPR Genotyping was done with a PCR-based assay to determine the presence of the loxP site in M6P/IGF2R intron 9. 100 ng of mouse genomic DNA was used as template in a PCR reaction (35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute ) using the primers INT9F2 (5′-CCTTCCCTCCAGGCCGTTAC-3′) and INT9R1 (5′-AGGTCTCCATCTGAGTACC-3′). Routine genotyping of this colony was needed until the muscle-CI-MPR-KO mouse colony was successfully established.
Mouse Cre genotyping was done with a PCR-based assay was used to determine presence of Cre recombinase expressed in specific tissue. Every DKO colony litter was genotyped using 100 ng of mouse genomic DNA as template in a PCR reaction (35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute) using the primers: Cre F ( 5′-ATGTCCAATTTACTGACCG-3′), Cre R(5′-CGCCGCATA ACCAGTGAAA-3′). Mouse testing positive for MCK-Cre were used for experimentation.
Rotarod testing was performed as described [33]. Western blotting of hGAA was performed as described using the hGAA monoclonal antibody (courtesy of Genzyme Corp., Framingham, MA) and the CI-MPR antibody (catalog number GTX28093; Gene Tex, Irvine, CA) [34].
Statistical analyses
Comparison of two groups was assessed by a homoscedastic Student t-test. A p-value of <0.05 was considered to be statistically significant.
Acknowledgments
This work was supported by NIH Grant R01 HL081122 from the National Heart, Lung, and Blood Institute. Partial research grant support from Genzyme Corporation to YT Chen, DB and AMW do this work, under an SRA is highly appreciated. BS was supported by a Development Grant from the Muscular Dystrophy Association. GAA-KO mice were provided courtesy of Dr. Nina Raben at the National Institutes of Health (Bethesda, MD). Muscle specific CI-MPR-KO mice were provided courtesy of Dr. Randy Jirtle, Duke University (Durham, NC). rhGAA was provided under agreement with Genzyme Corporation.
Footnotes
Conflict of interest: YT, DB and DDK have received research/grant support from Genzyme Corporation in the past. rhGAA, in the form of Genzyme’s product, Myozyme™ and Lumizyme™ is now approved by the US FDA and the European Union as therapy for Pompe disease. Duke University and inventors for the method of treatment and predecessors of the cell lines used to generate the enzyme (rhGAA) used in various clinical trials will receive royalty payments pursuant to the University’s Policy on Inventions, Patents and Technology. AMW is currently employed by Genzyme Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II: Acid α-Glucosidase (Acid Maltase) Deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis for Inherited Disease. McGraw-Hill; New York: 2001. [Google Scholar]
- 2.Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, Leslie N, Levine J, Spencer C, Mcdonald M, Li J, Dumontier J, Halberthal M, Chien YH, Hopkin R, Vijayaraghavan S, Gruskin D, Bartholomew D, Van Der PA, Clancy JP, Parini R, Morin G, Beck M, De La Gastine GS, Jokic M, Thurberg B, Richards S, Bali D, Davison M, Worden MA, Chen YT, Wraith JE. Recombinant human acid {alpha}-glucosidase: Major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- 3.Kishnani PS, Goldenberg PC, Dearmey SL, Heller J, Benjamin D, Young S, Bali D, Smith SA, Li JS, Mandel H, Koeberl D, Rosenberg A, Chen YT. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab. 2010;99:26–33. doi: 10.1016/j.ymgme.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponce E, Witte DP, Hirschhorn R, Huie ML, Grabowski GA. Murine Acid α-Glucosidase: Cell-Specific mRNA Differential Expression during Development and Maturation. Am J Pathol. 1999;154:1089–1096. doi: 10.1016/s0002-9440(10)65361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strothotte S, Strigl-Pill N, Grunert B, Kornblum C, Eger K, Wessig C, Deschauer M, Breunig F, Glocker FX, Vielhaber S, Brejova A, Hilz M, Reiners K, Muller-Felber W, Mengel E, Spranger M, Schoser B. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J Neurol. 2010;257:91–97. doi: 10.1007/s00415-009-5275-3. [DOI] [PubMed] [Google Scholar]
- 6.Van Capelle CI, Van Der Beek NA, Hagemans ML, Arts WF, Hop WC, Lee P, Jaeken J, Frohn-Mulder IM, Merkus PJ, Corzo D, Puga AC, Reuser AJ, Van Der Ploeg AT. Effect of enzyme therapy in juvenile patients with Pompe disease: a three-year open-label study. Neuromuscul Disord. 2010;20:775–782. doi: 10.1016/j.nmd.2010.07.277. [DOI] [PubMed] [Google Scholar]
- 7.Ravaglia S, Danesino C, Moglia A, Costa A, Cena H, Maccarini L, Carlucci A, Pichiecchio A, Bini P, De Filippi P, Rossi M. Changes in nutritional status and body composition during enzyme replacement therapy in adult-onset type II glycogenosis. Eur J Neurol. 2010;17:957–962. doi: 10.1111/j.1468-1331.2010.02959.x. [DOI] [PubMed] [Google Scholar]
- 8.Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, Mackey J, Kishnani P, Smith W, Mcvie-Wylie A, Sullivan JA, Hoganson GE, Phillips JA, Iii, Schaefer GB, Charrow J, Ware RE, Bossen EH, Chen YT. Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease type II: Results of a phase I/II clinical trial. Genet Med. 2001;3:132–138. [PubMed] [Google Scholar]
- 9.Desnick RJ. Enzyme replacement and enhancement therapies for lysosomal diseases. J Inher Metab Dis. 2004;27:385–410. doi: 10.1023/B:BOLI.0000031101.12838.c6. [DOI] [PubMed] [Google Scholar]
- 10.Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, Mattaliano RJ, Nagaraju K, Plotz PH. Enzyme replacement therapy in the mouse model of Pompe disease. Molecr Genet Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Raben N, Fukuda T, Gilbert AL, De Jong D, Thurberg BL, Mattaliano RJ, Meikle P, Hopwood JJ, Nagashima K, Nagaraju K, Plotz PH. Replacing acid alpha-glucosidase in Pompe disease: Recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Li X, Mcvie-Wylie A, Jiang C, Thurberg BL, Raben N, Mattaliano RJ, Cheng SH. Carbohydrate-remodeled acid alpha-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Neuromuscr Dis. 2005;15:712–713. doi: 10.1042/BJ20050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mcvie-Wylie AJ, Lee KL, Qiu H, Jin X, Do H, Gotschall R, Thurberg BL, Rogers C, Raben N, O’callaghan M, Canfield W, Andrews L, Mcpherson JM, Mattaliano RJ. Biochemical and pharmacological characterization of different recombinant acid alpha-glucosidase preparations evaluated for the treatment of Pompe disease. Mol Genet Metab. 2008;94:448–455. doi: 10.1016/j.ymgme.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Jiang JL, Gumlaw NK, Zhang J, Bercury SD, Ziegler RJ, Lee K, Kudo M, Canfield WM, Edmunds T, Jiang C, Mattaliano RJ, Cheng SH. Glycoengineered Acid alpha-Glucosidase With Improved Efficacy at Correcting the Metabolic Aberrations and Motor Function Deficits in a Mouse Model of Pompe Disease. Mol Ther. 2009 doi: 10.1038/mt.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardone M, Porto C, Tarallo A, Vicinanza M, Rossi B, Polishchuk E, Donaudy F, Andria G, De Matteis MA, Parenti G. Abnormal mannose-6-phosphate receptor trafficking impairs recombinant alpha-glucosidase uptake in Pompe disease fibroblasts. Pathogenetics. 2008;1:6. doi: 10.1186/1755-8417-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto T, Akutsu S, Wakana N, Morito M, Shimada A, Yamane A. The expressions of insulin-like growth factors, their receptors, and binding proteins are related to the mechanism regulating masseter muscle mass in the rat. Arch Oral Biol. 2006;51:603–611. doi: 10.1016/j.archoralbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Raben N, Kanneboyina N, Lee, Kessler EP, Byrne B, Lee L, Lamarca M, King C, Ward J, Sauer B, Plotz P. Targeted Disruption of the Acid α-Glucosidase Gene in Mice Causes an Illness with Critical Features of Both Infantile and Adult Human Glycogen Storage Disease Type II. J Biol Chem. 1998;273:19086–19092. doi: 10.1074/jbc.273.30.19086. [DOI] [PubMed] [Google Scholar]
- 18.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 19.Lynch GS, Ryall JG. Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 20.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 21.Mounier R, Cavalie H, Lac G, Clottes E. Molecular impact of clenbuterol and isometric strength training on rat EDL muscles. Pflugers Arch. 2007;453:497–507. doi: 10.1007/s00424-006-0122-1. [DOI] [PubMed] [Google Scholar]
- 22.Maclennan PA, Edwards RH. Effects of clenbuterol and propranolol on muscle mass. Evidence that clenbuterol stimulates muscle beta-adrenoceptors to induce hypertrophy. Biochem J. 1989;264:573–579. doi: 10.1042/bj2640573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awede BL, Thissen JP, Lebacq J. Role of Igf-1 and IGFBPs in the changes of mass and phenotype induced in rat soleus muscle by clenbuterol. Am J Physiol Endocrinol Metab. 2002;282:E31–37. doi: 10.1152/ajpendo.2002.282.1.E31. [DOI] [PubMed] [Google Scholar]
- 24.Shi H, Zeng C, Ricome A, Hannon KM, Grant AL, Gerrard DE. Extracellular signal-regulated kinase pathway is differentially involved in beta-agonist-induced hypertrophy in slow and fast muscles. Am J Physiol Cell Physiol. 2007;292:C1681–1689. doi: 10.1152/ajpcell.00466.2006. [DOI] [PubMed] [Google Scholar]
- 25.Burniston JG, Mclean L, Beynon RJ, Goldspink DF. Anabolic effects of a non-myotoxic dose of the beta2-adrenergic receptor agonist clenbuterol on rat plantaris muscle. Muscle Nerve. 2007;35:217–223. doi: 10.1002/mus.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant DR, Kearns CF, Mckeever KH, Lynch GS. Therapeutic clenbuterol treatment does not alter Ca2+ sensitivity of permeabilized fast muscle fibres from exercise trained or untrained horses. J Muscle Res Cell Motil. 2003;24:471–476. doi: 10.1023/a:1027377731137. [DOI] [PubMed] [Google Scholar]
- 27.Oya Y, Morita H, Ogawa M, Nonaka I, Tsujino S, Kawai M. Adult form of acid maltase deficiency presenting with pattern of muscle weakness resembling facioscapulohumeral dystrophy. Rinsho Shinkeigaku. 2001;41:390–396. [PubMed] [Google Scholar]
- 28.Ryall JG, Sillence MN, Lynch GS. Systemic administration of beta2-adrenoceptor agonists, formoterol and salmeterol, elicit skeletal muscle hypertrophy in rats at micromolar doses. Br J Pharmacol. 2006;147:587–595. doi: 10.1038/sj.bjp.0706669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol. 2004;555:175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelini C, Menegazzo E, Marcon M, Marsala SZ, Roni V. An open trial of albuterol and branched chain aminoacids in adult acid maltase deficiency. Neurology. 1998;50:A369–A369. [Google Scholar]
- 31.Angelini EPC, Marsala SZ, Vergani L, Nascimbeni AC, Fulizio L, Fanin M. Adult acid maltase deficiency: an open trial with albuterol and branched-chain aminoacids. Basic Appl Myol. 2004;14:71–78. [Google Scholar]
- 32.Wylie AA, Pulford DJ, Mcvie-Wylie AJ, Waterland RA, Evans HK, Chen YT, Nolan CM, Orton TC, Jirtle RL. Tissue-specific inactivation of murine M6P/IGF2R. Am J Pathol. 2003;162:321–328. doi: 10.1016/S0002-9440(10)63823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun BD, Zhang HY, Franco LM, Young SP, Schneider A, Bird A, Amalfitano A, Chen YT, Koeberl DD. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Amalfitano A, Mcvie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, Chen YT. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Nat’l Acad Sci (USA) 1999;96:8861–8866. doi: 10.1073/pnas.96.16.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]