Abstract
Background
We hypothesize that left sided low-level vagus nerve stimulation (LL-VNS) can suppress sympathetic outflow and reduce atrial tachyarrhythmias in ambulatory dogs.
Methods and Results
We implanted in 12 dogs a neurostimulator to stimulate left cervical vagus nerve and a radiotransmitter for continuous recording of left stellate ganglion nerve activities (SGNA), vagal nerve activities (VNA) and electrocardiograms. Group 1 dogs (N=6) underwent 1 week continuous LL-VNS. Group 2 dogs (N=6) underwent intermittent rapid atrial pacing followed by active or sham LL-VNS on alternate weeks. Integrated SGNA was significantly reduced during LL-VNS (7.8 mV-s; 95% confidence interval [CI] 6.94 to 8.66] vs. 9.4 mV-s [CI, 8.5 to 10.3] at baseline, P=0.033) in Group 1.The reduction was most apparent at 8 AM, along with a significantly reduced heart rate (P=0.008). LL-VNS did not change VNA. The density of tyrosine hydroxylase-positive nerves in the left stellate ganglion one week after cessation of LL-VNS were 99684 µm2/mm2 (CI, 28850 to 170517) in LL-VNS dogs and 186561 µm2/ mm2 (CI, 154956 to 218166; P=0.008) in normal dogs. In Group 2, the frequencies of paroxysmal atrial fibrillation and tachycardia during active LL-VNS were 1.4/day (CI, 0.5/day to 5.1/day) and 8.0/day (CI, 5.3/day to 12.0/day), respectively, significantly lower than during sham stimulation (9.2/day [CI, 5.3/day to 13.1/day], P=0.001 and 22.0/day [CI, 19.1/day to 25.5/day], P<0.001, respectively).
Conclusions
LL-VNS suppresses SGNA and reduces the incidences of paroxysmal atrial tachyarrhythmias in ambulatory dogs. Significant neural remodeling of the left stellate ganglion is evident one week after cessation of chronic LL-VNS.
Keywords: nervous system, autonomic; vagal stimulation; tachyarrhythmias; atrial fibrillation
Introduction
Animal studies suggest that sympathetic stimulation enhances while vagal stimulation reduces ventricular tachyarrhythmias and mortality.1, 2 Increased sympathetic tone and reduced vagal tone, indicated by baroreflex sensitivity analysis, are associated with increased cardiovascular mortality after myocardial infarction (MI)3 and life-threatening ventricular arrhythmias in patients with heart failure (HF).4 Interventions that increase vagal tone are often antiarrhythmic. For example, increased vagal activity elicited by exercise training is associated with strikingly lower cardiac mortality in post-MI patients.5 Chronic vagal stimulation can prevent ventricular fibrillation and sudden cardiac death in conscious dogs with a healed MI.6 Vagal stimulation can also improve cardiac autonomic control and significantly attenuate HF development in both dogs,7 rats8 and humans.9 Spinal cord stimulation, which enhances parasympathetic activity,10 improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model.11 While most of these previous studies used animal models of ventricular arrhythmia and conducted vagal stimulation with stimulus strength sufficient to reduce heart rate, low-level vagus nerve stimulation (LL-VNS) with stimulus strength 1 V below the threshold needed to reduce heart rate is known to be effective in suppressing atrial fibrillation (AF) induction in open-chest anesthetized dogs.12, 13 The mechanisms by which vagal stimulation reduces the incidence of atrial and ventricular arrhythmias remain unclear. Because vagal stimulation opposes sympathetic actions at both pre and post-junctional levels,14, 15 we hypothesize that vagal stimulation may achieve its antiarrhythmic effects by suppressing sympathetic outflow to the heart. To test this hypothesis, we chose to use LL-VNS that did not cause a reduction of heart rate, which may cause a compensatory increase of sympathetic nerve activity and complicate the data interpretation. The purpose of the present study is to perform continuous autonomic nerve recording at baseline and during LL-VNS both in unpaced dogs and in dogs with intermittent rapid atrial pacing to test the hypotheses that LL-VNS suppresses paroxysmal atrial tachyarrhythmias in ambulatory dogs, and that reduced left stellate ganglion nerve activity (SGNA) and sympathetic nerve density underlies the antiarrhythmic mechanisms of LL-VNS.
Methods
The animal protocol was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine and the Methodist Research Institute, Indianapolis, and conforms to the guidelines of the American Heart Association.
Chronic Ambulatory Autonomic Nerve Recordings
Twelve male mongrel dogs (weighing 22.8 to 30.0 kg) were used in this study. Under isoflurane inhalation general anesthesia, a small incision was made on the left anterior side of the neck. The left cervical vagus nerve was identified and isolated from the carotid artery. A bipolar pacing lead was sutured around the nerve and connected to a subcutaneously positioned Medtronic Itrel neurostimulator (Medtronic Inc, Minneapolis, Minnesota). Subsequently, a left thoracotomy was performed through the 4th intercostal space. A Data Sciences International (DSI; St Paul, Minnesota) D70-EEE radiotransmitter with 3 bipolar recording electrodes was implanted to record nerve activity according to methods described in detail elsewhere.16, 17 One pair of bipolar electrodes was sutured onto the caudal half of the left stellate ganglion (LSG) beneath its fascia to record SGNA. Another pair of bipolar electrodes was sutured onto the superior cardiac branch of the left vagal nerve to record vagal nerve activity (VNA). The final pair of electrodes was inserted into the fat pad at the junction of the left superior pulmonary vein and the left atrium18 to record superior left ganglionated plexi nerve activity (SLGPNA). The chest was then closed and the dog was allowed to recover. Following a recovery period of two weeks, the radiotransmitter was turned on and telemetric signals were continuously acquired for 1 week while the dogs were ambulatory.
Chronic LL-VNS in Normal Dogs (Group 1)
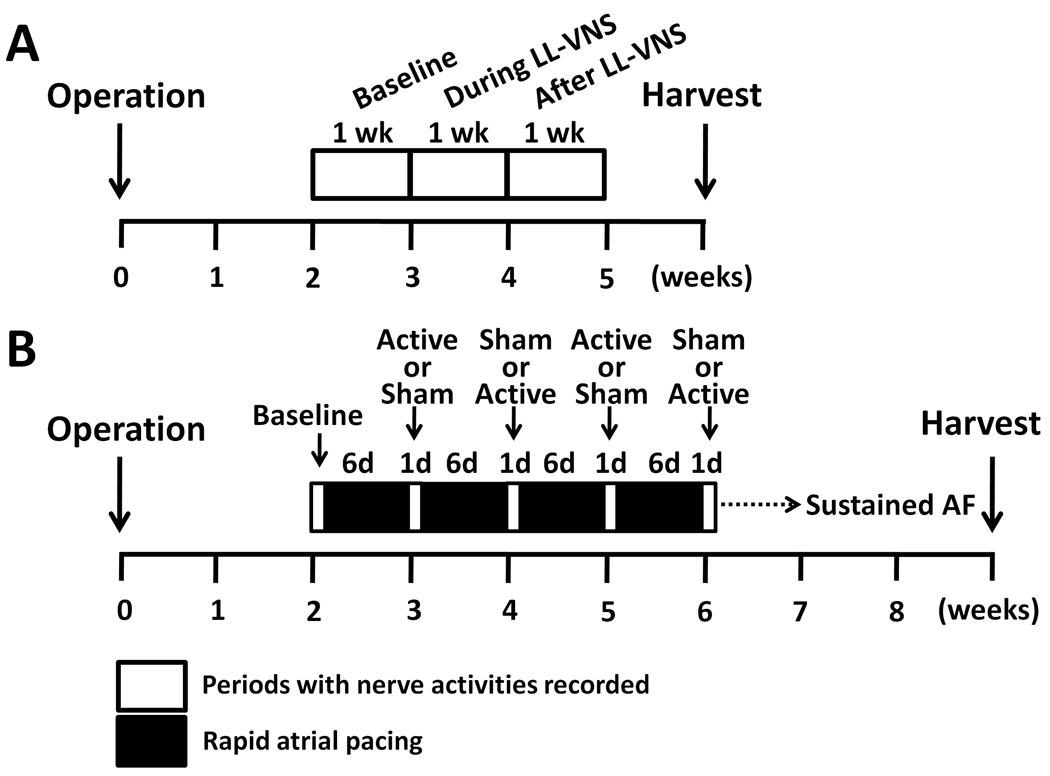
In the first 6 dogs (Group 1), LL-VNS was administered following the protocol illustrated in Figure 1A. After one week of baseline recording, LL-VNS was then commenced while the dog was ambulatory. We first defined the stimulation threshold for each dog by stimulating the left cervical vagus nerve at 13 Hz (450-µs pulse duration). The stimulus amplitude (V) that elicited an abrupt decrease of heart rate by >20% from baseline or caused AV conduction block was defined as the stimulation threshold. We then programmed the pacemaker output to 1 V below the stimulation threshold12 and confirmed that this stimulus voltage (4±2 V, range 1–6 V) did not cause any heart rate changes. The stimulation parameters chosen resulted in no serious adverse reactions. However, transient cough and drooling were observed in 4 dogs and nausea occurred in 2 dogs. The LL-VNS was kept for one week during which continuous nerve signals were acquired. After the LL-VNS was terminated, the dogs were monitored for another week before being euthanized. The stellate ganglia were harvested for histological analysis.
Figure 1.
Study protocols. A, For dogs without rapid atrial pacing (Group 1, N=6), one week of baseline recording was performed two weeks after surgery. Chronic low-level vagus nerve stimulation (LL-VNS) was then performed for one week. After LL-VNS terminated, nerve activities were recorded for another week before euthanasia. B, For dogs undergoing intermittent rapid atrial pacing (Group 2, N=6), after 2 weeks of postoperative recovery, nerve activities and heart rhythm were recorded for 1 day before the onset of pacing. High-rate (640 bpm) atrial pacing was then given for 6 days, followed by 1 day of pacing-free recording. During this recording period only, active or sham stimulation sessions were given on alternate weeks. This protocol was repeated until sustained AF was documented. LL-VNS, low-level vagus nerve stimulation; AF, atrial fibrillation.
LL-VNS in Dogs With Intermittent Rapid Atrial Pacing (Group 2)
To determine if LL-VNS has atrial antiarrhythmic effects, we performed LL-VNS in a high-yield canine model of atrial tachyarrhythmias16, 17 in the remaining six dogs (Group 2). In addition to the radiotransmitter and Itrel neurostimulator, a Medtronic EnPulse pacemaker (Medtronic Inc, Minneapolis, Minnesota) was implanted with a pacing lead sutured onto the left atrial appendage for intermittent high-rate atrial pacing according to a protocol that is described in detail elsewhere.16, 17 After 2 weeks of postoperative recovery, the DSI radiotransmitter was turned on to record baseline rhythm for 1 day. Baseline is defined as the observational period before the onset of pacing. High-rate (640 bpm, twice the diastolic threshold) atrial pacing was then given for 6 days, followed by 1 day of monitoring during which the atrial pacemaker was turned off. During this recording period, active or sham LL-VNS was performed on alternate weeks as shown in Figure 1B. The rhythm was monitored to determine the presence of paroxysmal atrial tachyarrhythmias. The atrial pacing protocol was repeated until sustained (>48 hrs) AF was documented.
Data Analyses
We analyzed recordings from all channels using custom-written software. Nerve activities were considered present if there was a 3-fold increase in the amplitude over baseline noise. We analyzed both the frequency of sympathetic discharge episodes and the corresponding heart rate increments after each discharge from 7 AM to 9 AM at baseline and during LL-VNS. In addition, we also determined the occurrence of paroxysmal atrial tachyarrhythmia, which was defined as a tachycardia with a rate of >160 bpm that lasted for >10 sec.19 To reduce the probability of including sinus tachycardia in this analysis, we required the tachycardia to have both an abrupt onset (>50 bpm increment) and a reduction in bipolar electrogram amplitude. The ones with irregular atrial activations are paroxysmal atrial fibrillations (PAFs), whereas the ones with regular activations are paroxysmal atrial tachycardias (PATs). Quantitative analyses were also performed with the aid of custom-design software to automatically import, filter and analyze the recordings. To optimize nerve signals, data from SGNA and VNA were high-pass filtered at 100 Hz.16, 19 Spike-triggered averaging was performed to allow removal of the atrial electrograms from SLGPNA recordings by subtracting an atrial electrogram template obtained from averaged atrial electrograms in the observation window.20 The filtered or transformed signals were then rectified, integrated with a 100-ms time constant, and summed to represent integrated nerve activity of 6-sec segments over 24 hrs of baseline recordings. We did not directly record a surface ECG. Instead, we applied bandpass filtering (5 to 100 Hz) on the VNA recording to obtain an ECG for analysis.16, 17
Immunohistochemistry Studies
LSG samples of all Group 1 dogs were obtained from the recording sites and fixed in 4% formalin for 45–60 min, followed by storage in 70% alcohol.21 The tissues were processed routinely, paraffin embedded and cut into 5-µm thick sections. Immunohistochemical staining was performed with antibodies against tyrosine hydroxylase (TH) using mouse monoclonal anti-TH (Accurate Chemical, Westbury, NY). We included LSG samples from 5 normal dogs as control. The density of TH-positive nerves was expressed as µm2/mm2. A second method of analysis was to determine the number of TH-negative ganglion cells among all ganglion cells in a stained slide. A blinded observer was asked to randomly select 10 high power (20X) fields with highest ganglion cell density in the LSG from each dog and obtained a digital picture. A second blinded investigator used these digital pictures to manually determine the percentage of TH-negative ganglion cells among all ganglion cells in the stained slides. The mean of those 10 selected fields was used as the value for that LSG.
Statistical Analyses
The data are presented as mean and 95% confidence interval (CI). Repeated measures analysis of variance (ANOVA) followed by Fisher’s protected least significant difference post hoc test was performed to compare the mean values and daily changes of nerve activity at baseline, during LL-VNS, and after LL-VNS. A paired t-test was used to compare the immunostaining of the LSG from Group 1 dogs and normal control dogs, and to compare episodes of atrial tachyarrhythmias during active LL-VNS and during sham stimulation. A Poisson regression model was used to fit the data of frequency of paroxysmal atrial tachyarrhythmias for comparisons in Group 2 dogs. The regression parameters were estimated through generalized estimating equation (GEE) with exchangeable correlation structure to account for correlations of measures from the same dog. Over-dispersion parameters were estimated by Pearson residuals. All tests were performed at a two-tailed significance level of P=0.05 and a 95% confidence interval (CI) was calculated for each mean values. Online Supplemental Figure 1 shows that the major variables are normally distributed. The statistics were computed using the PASW Statistics (version 18; SPSS Inc, Chicago, IL) and SAS 9.2 (SAS Inc, Cary, NC).
Results
Group 1
Immediate Effects of LL-VNS
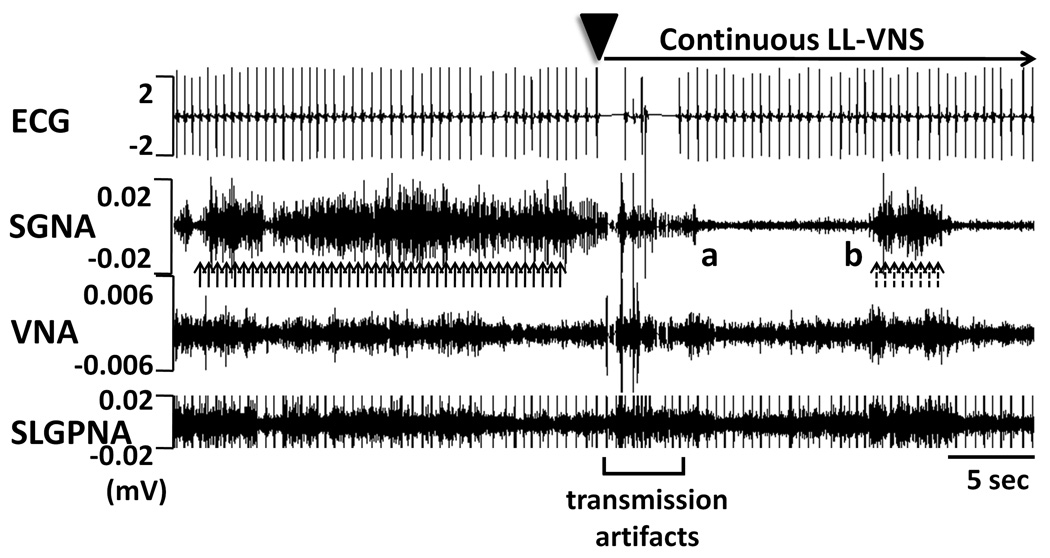
After the optimal stimulus strength was chosen, we turned on the neurostimulator for chronic LL-VNS. Figure 2 illustrates an example of the simultaneous nerve activity and ECG recordings before and immediately after the commencement of LL-VNS (arrowhead). The applied voltage was 1 V less than the threshold that immediately reduced the heart rate, and therefore no obvious heart rate deceleration was observed. There were abundant sympathetic discharges (solid arrows) prior to the LL-VNS. However, immediately following LL-VNS, there was suppression of SGNA for approximately 10 sec (from point a to b) before sporadic SGNA reappeared (dashed arrows). There were no apparent changes in either VNA or SLGPNA in this example. Except for the transmission artifacts produced by pacemaker programmer in the initial 5 sec of LL-VNS, chronic LL-VNS did not produce any stimulus artifacts.
Figure 2.
Immediate effects of LL-VNS. The administration of LL-VNS (without affecting sinus rate) immediately suppressed SGNA for approximately 10 sec (from point a to b) before sporadic SGNA reappeared. Upward dashed arrows point to SGNA. Except for the first 5 sec during programmer transmission, chronic LL-VNS did not produce any stimulus artifacts. ECG, electrocardiogram; SGNA, stellate ganglion nerve activity; VNA, vagal nerve activity; SLGPNA, superior left ganglionated plexi nerve activity.
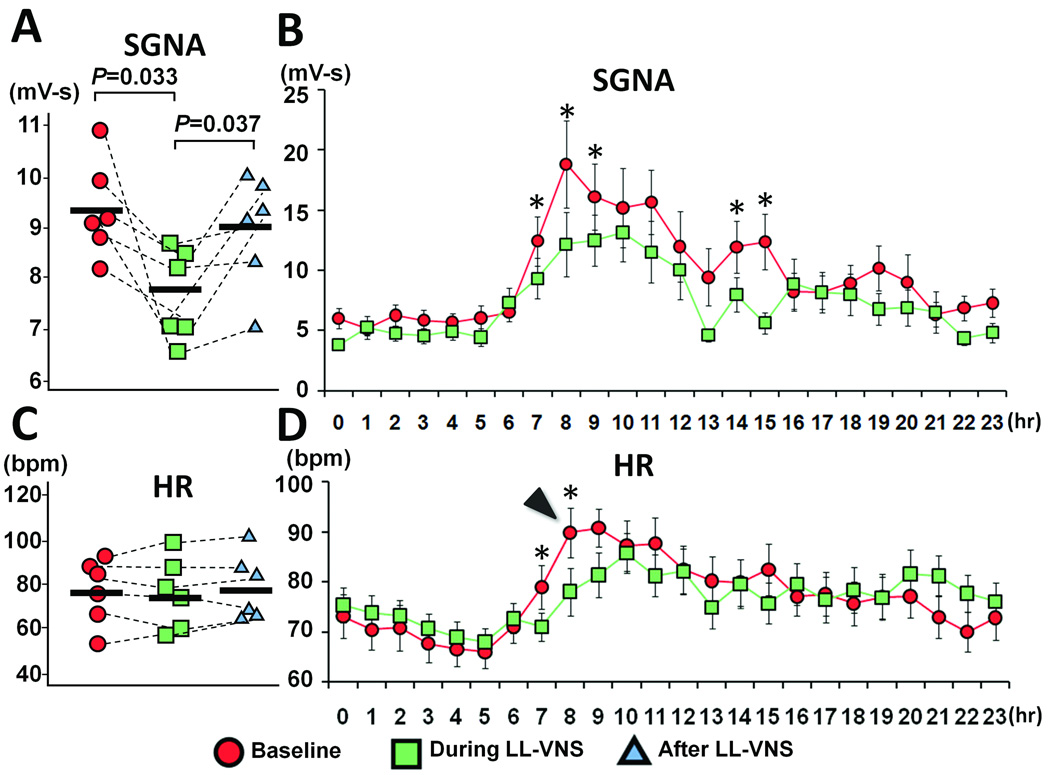
Effects of LL-VNS on Integrated Nerve Activity and Heart Rate
We evaluated the chronic effects of LL-VNS by calculating integrated nerve activity and average heart rate. We analyzed five days at baseline and during LL-VNS for each Group 1 dog. During LL-VNS, the integrated SGNA (7.8 mV-s [CI, 6.94 to 8.66]) was significantly reduced compared to baseline (9.4 mV-s [CI, 8.5 to 10.3], P=0.033) with an overall reduction of 17% over 24 hrs (Figure 3A). The nerve activity was first integrated over 6 sec and then averaged over 5 days in each of the 6 dogs. The mean value for each dog was then averaged to get the integrated nerve activity. We also used the same method to obtain an hourly average of integrated nerve activity over 5 days of all 6 dogs. The reduction in integrated SGNA was particularly striking at 8 AM (35% reduction; 12.2 mV-s [CI, 5.0 to 17.4] versus 18.8 mV-s [CI, 11.7 to 25.8] at baseline, P=0.002). Figure 3B shows the hourly average of SGNA over a 24-hour period. The reduction in average heart rate during this period (14% of reduction; 78.1 bpm [CI, 68.7 to 87.5] versus 90.6 bpm [CI, 80.8 to 100.4] at baseline, P=0.008) paralleled the reduction in SGNA. Figure 3B also reveals that SGNA was suppressed to a greater degree during the daytime (from 7 AM to 5 PM) than during the nighttime. The morning surge of heart rate that is normally observed at baseline in ambulatory dogs (arrowhead in Figure 3D) was considerably attenuated during LL-VNS as well. The 24-hr average heart rate, however, was not significantly reduced (76.7 bpm [CI, 59.9 to 93.5] versus 77.5 bpm [CI, 61.9 to 93.1] at baseline, P=0.726, Figure 3C). Of note, LL-VNS did not change either the 24-hr integrated VNA (4.2 mV-s [CI, 1.3 to 7.1] versus 4.0 mV-s [CI, 1.4 to 6.6] at baseline, P=0.164) or 24-hr SLGPNA (6.6 mV-s [CI, 2.1 to 11.1] versus 5.7 mV-s [CI, 3.0 to 8.4] at baseline, P=0.241). Hourly averaged VNA and SLGPNA did not show a significant difference in any hour of the 24-hr period (Online Supplemental Figure 2).
Figure 3.
Effects of LL-VNS on SGNA and heart rate (HR). A, Chronic LL-VNS significantly reduced SGNA over 24 hrs. The SGNA normalized to baseline level after cessation of LL-VNS. B, Hourly averages of SGNA show that the reduction in integrated SGNA was particularly striking at 8 AM. All values are averaged over 5 days and 6 dogs. C, The administration and cessation of chronic LL-VNS did not change the overall heart rate. D, Hourly averages of heart rate reveal that the morning surge of heart rate (arrowhead) was markedly attenuated during LL-VNS. HR, heart rate. * P<0.05.
Effects of LL-VNS on Sympathetic Discharge Episodes
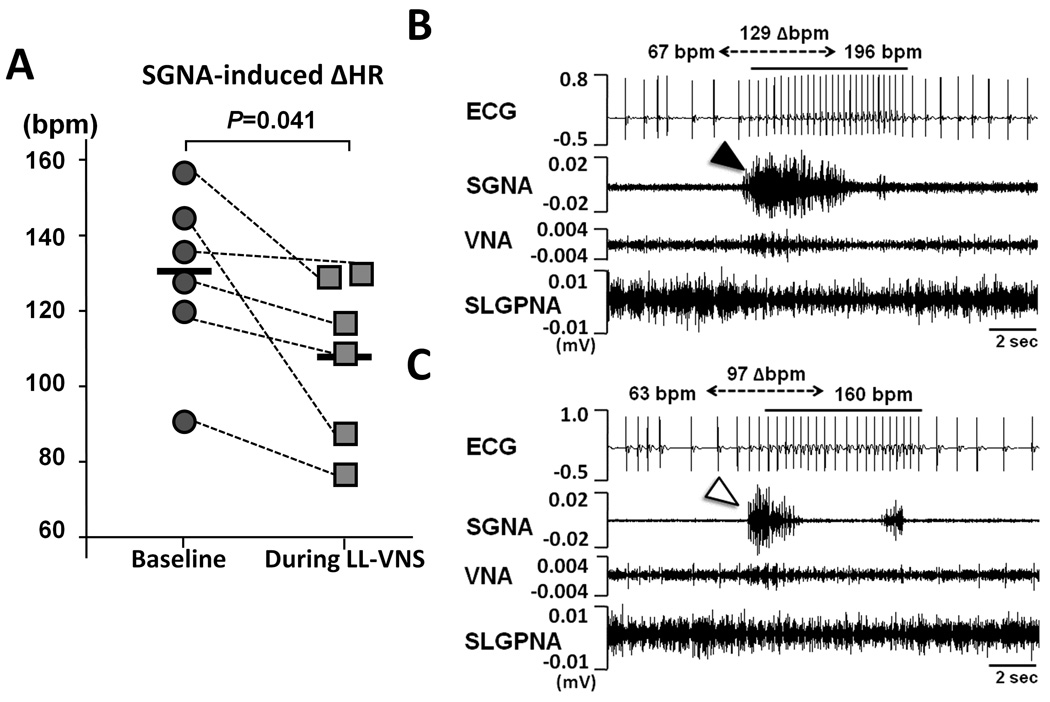
The presence or absence of SGNA within each 60-sec window from 7 to 9 AM each day was used to represent the frequency of sympathetic discharge episodes.19 During LL-VNS, the sympathetic discharge episodes were present 31% (CI, 18 to 44%) of the time, significantly less frequent than at baseline (44% [CI, 29 to 59%] of the time, P=0.021). The average duration of each sympathetic discharge episode (1.9 sec [CI, 1.4 to 2.3]) was also significantly shortened compared to baseline (3.0 sec [CI, 2.1 to 3.9], P=0.029). We further analyzed the heart rate responses to each episode by comparing the average heart rate at 5 sec before the onset of the sympathetic discharge episodes (averaged over 3 sec; from 8 to 5 sec before the onset of SGNA, see Online Supplemental Figure 3) with that during the episodes (from the onset to 3 sec after the onset). At baseline, the SGNA-induced heart rate acceleration was 129.2 bpm [CI, 105.2 to 153.1] (increased from 57.9 to 187.1 bpm, Figure 4A). However, during LL-VNS, the SGNA-induced heart rate acceleration was 107.9 bpm [CI, 84.8 to 131.1] (increased from 57.8 to 166.2 bpm), which was significantly attenuated (P=0.041). Figure 4B is an example at baseline, in which an episode of sympathetic discharge (black arrowhead) led to heart rate increase from 67 to 196 bpm (129 bpm increment). This contrasts with Figure 4C, which shows an episode of sympathetic discharge (white arrowhead) led to a heart rate increase from 63 to 160 bpm (97 bpm increment).
Figure 4.
Effects of LL-VNS on SGNA-induced heart rate changes. A, Graph of statistical dot plot shows that the SGNA-induced heart rate acceleration was markedly attenuated during LL-VNS compared to baseline. B, An example at baseline shows a burst of SGNA (black arrowhead) led to heart rate increase from 67 to 196 bpm (129 bpm increment). C, An example during LL-VNS shows a burst of SGNA (white arrowhead) led to heart rate increase from 63 to 160 bpm (97 bpm increment).
Normalization of SGNA After the Cessation of LL-VNS
The Group 1 dogs were further monitored for 1 week after cessation of LL-VNS. During this week, SGNA and the frequency of sympathetic discharges normalized to near baseline level. The integrated SGNA rose to 9.0 mV-s (CI, 8.2 to 9.9, P=0.037 compared with the values during LL-VNS, Figure 3A). The frequency of sympathetic discharges from 7 to 9 AM increased to near baseline values (44% [CI, 31 to 56%] of the time during which SGNA was present, P=0.041 compared with the values during LL-VNS). The heart rate acceleration value induced by SGNA also rose to 130.6 bpm [CI, 105.2 to 153.1] (increased from 57.4 to 188.0 bpm), which was almost identical to baseline values (P =0.883). Despite a trend towards increased SGNA, the 24-hr average heart rate did not significantly differ from the values during LL-VNS (79.2 bpm [CI, 63.6 to 94.7] versus 76.7 bpm [CI, 59.9 to 93.5], P=0.281, Figure 3C). The cessation of LL-VNS did not alter either VNA (4.5 mV-s [CI, 2.1 to 7.0] versus 4.2 mV-s [CI, 1.3 to 7.1] during LL-VNS, P=0.522) or SLGPNA (6.4 mV-s [CI, 1.6 to 11.3] versus 6.6 mV-s [CI, 2.1 to 11.1] during LL-VNS, P=0.379). An overview of daily changes of SGNA of all Group 1 dogs is provided in Figure 5. The SGNA was significantly decreased on the first day (D1) of LL-VNS (8.0 mV-s [CI, 7.1 to 8.9] versus 9.6 mV-s [CI, 8.2 to 10.9] on the D5 of baseline, P=0.017). On the D4 during LL-VNS, the SGNA was further decreased (5.6 mV-s [CI, 4.8 to 6.4] versus 7.7 mV-s [CI, 6.5 to 8.9], P<0.001). The SGNA remained significantly suppressed 1 day after the cessation of LL-VNS (7.4 mV-s [CI, 5.9 to 8.9] versus 9.6 mV-s [CI, 8.2 to 10.9] on the D5 of baseline, P=0.001). Afterwards, the SGNA gradually normalized to the near-baseline level. However, there was no rebound increase of SGNA as compared with baseline.
Figure 5.
Daily changes of SGNA in response to the LL-VNS. The SGNA was significantly decreased on the D1 of LL-VNS and was further decreased on the D4 and D5 of LL-VNS. After the cessation of LL-VNS, the SGNA remained suppressed for more than 24 hrs before gradually normalizing to baseline level. * P<0.05 compared to the D5 of baseline. † P<0.05 compared to the D3 during LL-VNS.
Immunohistochemical Studies
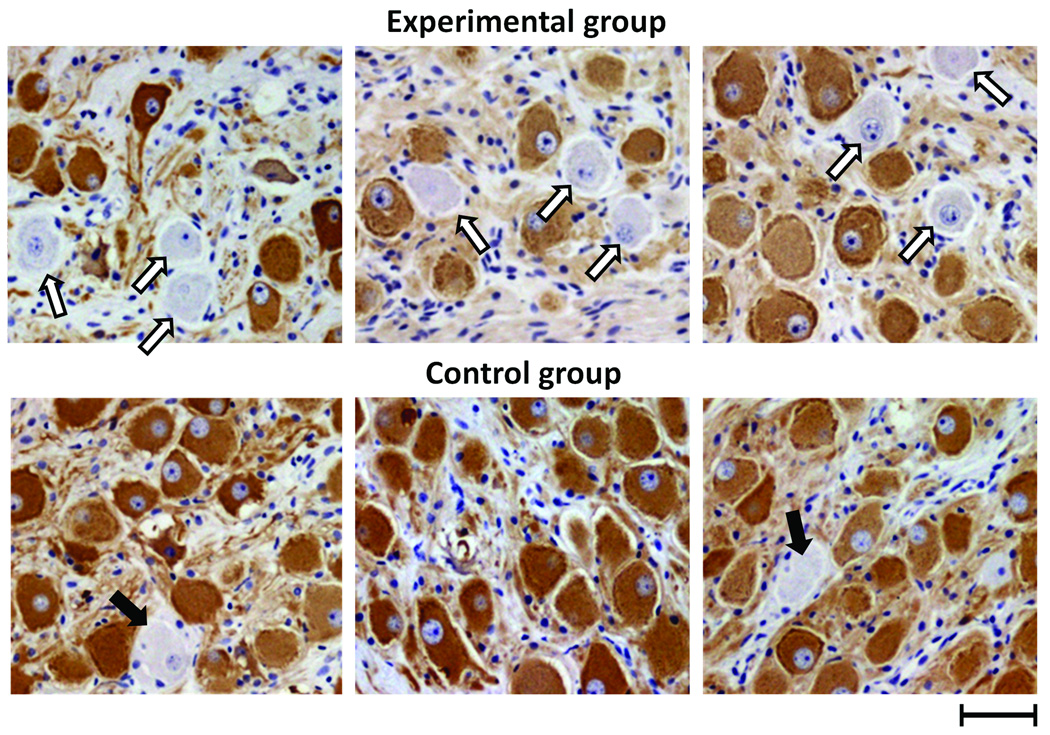
We compared the results of immunostaining of the LSG between Group 1 dogs that underwent LL-VNS (N=6; upper panel of Figure 6) and normal control dogs (N=5; lower panel of Figure 6). For dogs with LL-VNS, there was a significantly decreased density of TH-positive nerves in the LSG (99684 µm2/mm2 [CI, 28850 to 170517]) compared to normal dogs (N=5; 186561 µm2/mm2 [CI, 154956 to 218166], P=0.008). There were significantly more ganglion cells without immunoreactivity to TH (as pointed by unfilled and solid arrows in Figure 6) in dogs with LL-VNS (5.5% [CI, 4.3 to 6.7%] in 60 randomly selected windows, 10 windows for each dog) than in control (1.4% [CI, 0.2 to 2.6%] in 50 randomly selected windows, 10 windows for each dog, P<0.001 after Bonferroni correction).
Figure 6.
Tyrosine hydroxylase (TH) immunostaining of left stellate ganglion. Upper panel shows the left stellate ganglion (LSG) of three representative dogs with vagus nerve stimulation (all belong to Group 1). Lower panel shows the LSG of three representative normal control dogs. In experimental dogs, there was a significantly decreased density of TH-positive nerve structures in the LSG and significantly more ganglion cells lacking immunoreactivity to TH (unfilled arrows), which were less common in the control dogs (solid arrows). Scale bar=50 µm.
Effects of LL-VNS on PAT
PAT episodes are observed in both groups of dogs. In Group 1 dogs, most episodes of PAT (104 of total 130 episodes observed; 80%) occurred from 4 AM to 12 PM. During LL-VNS, the frequency of PAT was significantly reduced (1.2/day [CI, 0.5/day to 1.9/day] versus 4.3/day [CI, 1.1/day to 7.6/day], P=0.048). The reduction was particularly apparent from 4 AM to 12 PM, when most episodes of PAT occurred (0.6/day [CI, 0.1/day to 1.2/day] versus 3.9/day [CI, 0.5/day to 7.1/day], P=0.026). No PAF was noted in Group 1.
Group 2
Effects of LL-VNS on Paroxysmal Atrial Tachyarrhythmias
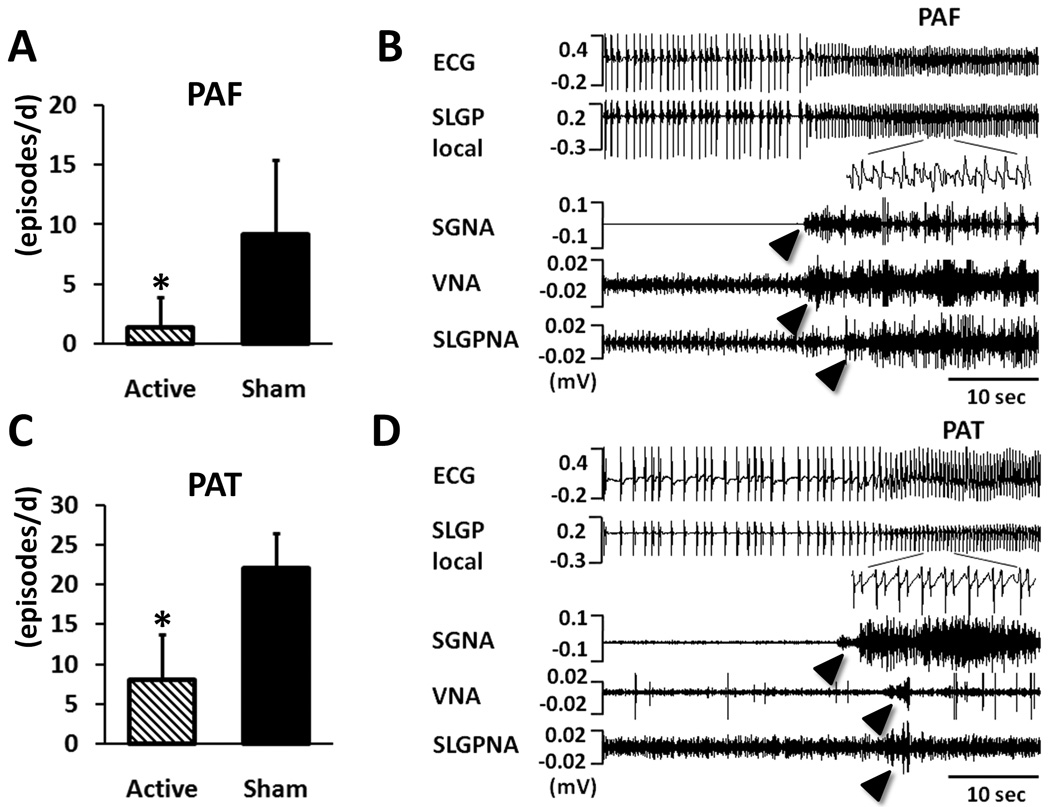
For Group 2 dogs that underwent rapid atrial pacing, the number of PAT significantly increased, along with the occurrence of PAF. We compared the periods with active LL-VNS (N=12) and those with sham stimulation (N=14). The frequency of PAF during the periods with active LL-VNS (1.4/day [CI, 0.5/day to 5.1/day]) was significantly lower than during the periods with sham stimulation (9.2/day [CI, 5.3/day to 13.1/day], P=0.001) (Figure 7A). This reduction was even more obvious for PAT (8.0/day [CI, 5.3/day to 12.0/day] versus 22.0/day [CI, 19.1/day to 25.5/day], P<0.001) (Figure 7C). Along with the decrease of paroxysmal atrial tachyarrhythmias, SGNA was significantly suppressed during active LL-VNS (11.0 mV-s [CI, 7.8 to 12.7]) compared to during sham stimulation (14.3 mV-s [CI, 11.3 to 17.5], P=0.030). There were no significant differences in VNA (6.7 mV-s [CI, 5.3 to 8.0] under active LL-VNS versus 6.8 mV-s [CI, 5.2 to 8.3] under sham stimulation, P=0.897), SLGPNA (6.9 mV-s [CI, 4.5 to 9.2] under active LL-VNS versus 8.6 mV-s [CI, 4.8 to 12.3] under sham stimulation, P=0.370) or heart rate (92.6 bpm [CI, 84.6 to 103.6] under active LL-VNS versus 88.3 bpm [CI, 76.0 to 99.4] under sham stimulation, P=0.285). Figure 7B is an example of PAF. The ECG showed fast and irregular ventricular response and the LA local electrograms recorded from the SLGP revealed fractionated electrograms (inset). Figure 7D is an episode of PAT, in which the atrial rate abruptly accelerated to 228 bpm and lasted for more than 20 sec.
Figure 7.
Effects of LL-VNS on paroxysmal atrial tachyarrhythmias. A, Chronic LL-VNS significantly prevented the occurrence of paroxysmal atrial fibrillation (PAF). B, An example of PAF that shows fast and irregular ventricular responses and fractionated atrial electrograms (inset). The PAF episodes were following burst firings of SGNA, VNA and SLGPNA (arrowheads). C, LL-VNS also significantly prevented the occurrence of paroxysmal atrial tachycardia (PAT). D, An example of PAT shows that following burst firings of SGNA, VNA and SLGPNA (arrowheads), the atrial rate abruptly accelerated to 228 bpm and lasted for more than 20 sec. As contrasted to PAF, the atrial local electrograms in PAT were regular (inset). * P<0.05 comparing active LL-VNS with sham. PAF, paroxysmal atrial fibrillation; PAT, paroxysmal atrial tachycardia.
Discussion
New Observations
We showed in ambulatory dogs that chronic left LL-VNS (1) effectively suppresses left SGNA, particularly in the morning; (2) reduces the density of TH-positive ganglion cells and increases the density of TH-negative ganglion cells in the LSG one week after cessation of chronic LL-VNS; and (3) significantly reduces the frequency of paroxysmal atrial tachyarrhythmias.
Chronic LL-VNS Suppresses SGNA
It has been proposed that the primary electrophysiological effect of vagal activity is the direct consequence of antagonizing the effects of sympathetic activity.6 However, this relationship has not been proven through direct recording of nerve activity in conscious, ambulatory animals. In this study, continuous LL-VNS was administered to ambulatory dogs and its ability to suppress sympathetic nerve activity was assessed by directly examining SGNA. Without significantly reducing the heart rate or increasing VNA, LL-VNS was able to suppress the overall SGNA throughout the day, especially in the morning (8AM). These findings are particularly important because of the circadian variation of sudden cardiac death, with increased incidence in the morning,22, 23 and SGNA’s role as a direct trigger of sudden cardiac death.24 Furthermore, the heart rate responses to SGNA were significantly blunted during LL-VNS as compared with baseline. This may have been due to reduced SGNA duration through prejunctional inhibition of sympathetic nerve terminals14, 15 or due to post-junctional actions of VNA. Myocardium is known to produce antiadrenergic neurotransmitters such as chromogranin A25 and vasostatin-126, 27 (one of chromogranin A’s derivatives). It is possible that the production of these neuromodulators is increased by LL-VNS and exerts direct antiadrenergic effects on the myocardium. Alternatively, these substances can be transmitted to the LSG through the retrograde axonal transport to cause neural remodeling in that structure, similar to the mechanisms of LSG remodeling induced by myocardial infarction.28 Since no change in heart rate is observed, the LL-VNS may activate the afferent nerves that result in neural remodeling in the central nervous system. The latter remodeling processes may then cause secondary neural remodeling in the LSG. Finally, after the cessation of LL-VNS, there was a gradual normalization of SGNA back to approximately baseline level. This “washout” effect further validates the causal relationship between LL-VNS and the suppression of cardiac sympathetic outflow.
LL-VNS and Neural Remodeling in the Stellate Ganglion
In addition to the reduced SGNA, we also observed a significant reduction of TH-positive nerve structures in the LSG and an increase of TH-negative ganglion cells. While it is known that remodeling changes in the LSG occurs during myocardial infarction,28 the plasticity of LSG during VNS has not been previously reported. We report in the present study significant plasticity of LSG, and that the changes are associated with reduced TH-positive nerve structures. However, the significant histological changes inside the LSG were observed in Group 1 dogs one week after cessation of the pacing, during which SGNA had already normalized. A possible explanation is that the remaining ganglion cells may have increased their activities to compensate for the neural remodeling caused by LL-VNS. When used clinically to suppress seizure disorder, the LL-VNS is administered intermittently. However, the seizures occurring during the VNS OFF time are also affected.29, 30 This “carry-over” effect of chronic LL-VNS is also seen in Group 1 dogs of the present study, i.e., continued partial SGNA suppression for more than 24 hrs. Structural neural remodeling may explain these carry-over effects of LL-VNS.
Chronic LL-VNS Suppresses Paroxysmal Atrial Tachyarrhythmias
There has been no clear clinical evidence linking therapeutic vagal stimulation with increased incidence of AF. In anesthetized dogs, vagal stimulation with moderate intensities (producing ≤40% sinus cycle length prolongation) can be used to deliver therapeutic benefits without the risk of atrial arrhythmogenesis.31 Also in anesthetized dogs, LL-VNS is shown to prevent AF inducibility.12 The authors attribute the effect to the inhibition of nerve activity of major ganglionated plexi, including the SLGP.13 The present study for the first time demonstrates that chronic LL-VNS can reduce the frequency of atrial tachyarrhythmias in ambulatory dogs. However, the antiarrhythmic mechanism of LL-VNS lies in the significant suppression of SGNA. The SLGPNA was reduced, but the reduction was statistically insignificant. This discrepancy may have stemmed from the study design of the present study, in which long-term data were acquired to allow investigating the chronic effects of LL-VNS. It is possible that LL-VNS acutely suppresses SLGPNA, but this effect is not sustained. Furthermore, the ability to record nerve activity in ambulatory animals enables us to record nerve activity without the need of anesthetic agents, which are specifically designed to inhibit nerve activities.
Clinical Implications
More than 50,000 patients worldwide have been implanted with the left cervical LL-VNS system for suppression of epilepsy and depression.30 Its application has generally been safe, with reversible bradyarrhythmias related to vagal stimulation therapy reported to be extremely rare at a frequency of about 0.1% of implanted devices.32 More recently, in a pilot human study,33 chronic right cervical vagal stimulation was also shown to be feasible, safe and beneficial for HF patients. Therefore, LL-VNS may constitute a safe non-pharmacological approach to control cardiac arrhythmias such as drug refractory PAT and PAF in which suppression of cardiac sympathetic outflow is desired. It is also possible that LL-VNS may help control ventricular arrhythmias in long QT syndrome or catecholaminergic ventricular tachycardia without the need for surgical sympathetic denervation.34, 35 In addition, LL-VNS might also apply to certain clinical conditions (e.g. palmar hyperhidrosis36) in which hyperactivity of the stellate ganglion may be responsible for the clinical manifestations of the diseases.
Study limitations
We used the left, rather than the right,8,33 cervical vagus nerve for LL-VNS for three reasons. Firstly, the left vagus nerve has fewer projections to the sinoatrial node.37 Left-sided stimulation may be less likely to cause heart rate changes. Bradycardia induced by vagal stimulation may lead to reactivation of SGNA that is undesirable in this study. A second reason is that left-sided stimulation is less likely to promote atrial arrhythmogenesis at the same intensity of stimulus when compared with the right.38 Third, as stated above, left LL-VNS has been widely used clinically and has proven to be safe. However, further studies are warranted to provide information about potential differences in the suppression of SGNA and atrial anti-arrhythmic effects for left versus right sided stimulation. In addition, atrial cardiac ganglionated plexi are reported to contain three types of neurons - efferent sympathetic, efferent parasympathetic and afferent ones.39 These neurons might co-localize with each other. Our recording methods could not differentiate one type of nerve activity from the other. A third limitation is that we only recorded from the LSG and the left vagus nerve. The autonomic interactions from the right side remain to be determined.
Clinical Perspective.
The present study was conducted in ambulatory dogs with continuous recording of left stellate ganglion nerve activity (SGNA) and left vagus nerve activity (VNA) before, during and after low-level vagus nerve stimulation (LL-VNS) of the left cervical vagal nerve. We showed that LL-VNS can effectively suppress SGNA while not increasing or decreasing thoracic VNA. The most significant SGNA reduction occurred in the morning, when the sympathetic outflow was the highest. Immunohistochemical studies of the left stellate ganglion showed significant neural remodeling, including reduced sympathetic nerve structures, one week after cessation of LLVNS. We further demonstrated that LL-VNS can suppress paroxysmal atrial tachycardia (PAT) and paroxysmal atrial fibrillation (PAF) induced by intermittent rapid atrial pacing. A possible clinical implication is that LL-VNS can be used as a non-pharmacological approach to the control PAT and PAF through the suppression of cardiac sympathetic outflow. This method may also apply to other clinical conditions in which hyperactivity of the stellate ganglion and increased sympathetic outflow are responsible for the pathogenesis of the diseases. For example, previous studies have shown that the risk of sudden death is the highest in the morning. LL-VNS, which selectively suppresses sympathetic outflow in the morning, may be used to reduce the risk of sudden death. Other possible clinical applications include the suppression of ventricular tachyarrhythmias including those associated with long QT syndrome, catecholaminergic polymorphic ventricular tachycardia or structural heart diseases. It may also be effective in non-cardiac diseases caused by increased sympathetic outflow.
Supplementary Material
Acknowledgements
We thank Lei Lin, Jian Tan and Nicole Courtney for their assistance; Dr Xiaohong Zhou of Medtronic Inc and Mr Michael Bova of St Jude Medical Inc for donating the pacemaker equipment used in the study; and Amy Chang for her help in the preparation of the manuscript.
Funding Sources
This study was supported in part by NIH Grants P01HL78931, R01HL78932, R01HL71140, R21HL106554, a Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology (M.J.S.), a Korea Research Foundation Grant (KRF-2008-357-E00028) funded by the Korean Government (E.-K.C.) and a Medtronic-Zipes Endowment (P.-S.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject Codes: [130] Animal models of human disease; [132] Arrhythmias-basic studies
Disclosures
Medtronic Inc and St Jude Inc donated research equipment to our laboratory.
References
- 1.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial intarction risk stratification. Circulation. 1992;85:I-77–I-91. [PubMed] [Google Scholar]
- 2.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J.Clin.Invest. 2005;115:2305–2315. doi: 10.1172/JCI26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias : Implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 4.Mortara A, La Rovere MT, Pinna GD, Parziale P, Maestri R, Capomolla S, Opasich C, Cobelli F, Tavazzi L. Depressed arterial baroreflex sensitivity and not reduced heart rate variability identifies patients with chronic heart failure and nonsustained ventricular tachycardia: The effect of high ventricular filling pressure. Am Heart J. 1997;134:879–888. doi: 10.1016/s0002-8703(97)80011-7. [DOI] [PubMed] [Google Scholar]
- 5.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- 6.Vanoli E, De Ferrari GM, Stramba-Badiale M, Hull SS, Jr, Foreman RD, Schwartz PJ. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ.Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Popovic ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 8.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 9.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 10.Olgin JE, Takahashi T, Wilson E, Vereckei A, Steinberg H, Zipes DP. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. Journal of Cardiovascular Electrophysiology. 2002;13:475–481. doi: 10.1046/j.1540-8167.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopshire JC, Zhou X, Dusa C, Ueyama T, Rosenberger J, Courtney N, Ujhelyi M, Mullen T, Das M, Zipes DP. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 12.Li S, Scherlag BJ, Yu L, Sheng X, Zhang Y, Ali R, Dong Y, Ghias M, Po SS. Low-level vagosympathetic stimulation: A paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Scherlag BJ, Li S, Sheng X, Lu Z, Nakagawa H, Zhang Y, Jackman WM, Lazzara R, Jiang H, Po SS. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: Direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2010 doi: 10.1111/j.1540-8167.2010.01908.x. Epub. [DOI] [PubMed] [Google Scholar]
- 14.Vanhoutte PM, Levy MN. Prejunctional cholinergic modulation of adrenergic neurotransmission in the cardiovascular system. Am J Physiol. 1980;238:H275–H281. doi: 10.1152/ajpheart.1980.238.3.H275. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, Zipes DP. Vagal modulation of adrenergic effects on canine sinus and atrioventricular nodes. Am.J.Physiol. 1983;244:H775–H781. doi: 10.1152/ajpheart.1983.244.6.H775. [DOI] [PubMed] [Google Scholar]
- 16.Tan AY, Zhou S, Ogawa M, Song J, Chu M, Li H, Fishbein MC, Lin SF, Chen LS, Chen PS. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008;118:916–925. doi: 10.1161/CIRCULATIONAHA.108.776203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi E-K, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin S-F, Chen P-S. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan BX, Ardell JL, Hopkins DA, Losier AM, Armour JA. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat.Rec. 1994;239:75–87. doi: 10.1002/ar.1092390109. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa M, Zhou S, Tan AY, Song J, Gholmieh G, Fishbein MCLH, Siegel RJ, Karagueuzian HS, Chen LS, in SF, Chen PS. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. Journal of the American College of Cardiology. 2007;50:335–343. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Shen MJ, Choi EK, Tan AY, Han S, Shinohara T, Maruyama M, Chen LS, Shen C, Hwang C, Lin SF, Chen PS. Patterns of baseline autonomic nerve activity and the development of pacing-induced sustained atrial fibrillation. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.11.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circulation Research. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 22.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–138. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- 23.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the framingham heart study population. American Journal of Cardiology. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhou S, Jung BC, Tan AY, Trang VQ, Gholmieh G, Han SW, Lin SF, Fishbein MC, Chen PS, Chen LS. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5:131–139. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin a in human heart: A new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 26.Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, Alloatti G. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2906–H2912. doi: 10.1152/ajpheart.01253.2006. [DOI] [PubMed] [Google Scholar]
- 27.Scherlag BJ, Nakagawa H, Jackman WM, Lazzara R, Po SS. Non-pharmacological, non-ablative approaches for the treatment of atrial fibrillation: Experimental evidence and potential clinical implications. J Cardiovasc Transl Res. 2011;4:35–41. doi: 10.1007/s12265-010-9231-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ.Res. 2004;95:76–83. doi: 10.1161/01.RES.0000133678.22968.e3. [DOI] [PubMed] [Google Scholar]
- 29.Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005–1012. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 30.Terry R. Vagus nerve stimulation: A proven therapy for treatment of epilepsy strives to improve efficacy and expand applications. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4631–4634. doi: 10.1109/IEMBS.2009.5332676. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Ilsar I, Sabbah HN, Ben DT, Mazgalev TN. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: Therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm. 2009;6:244–250. doi: 10.1016/j.hrthm.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Asconape JJ, Moore DD, Zipes DP, Hartman LM, Duffell WH., Jr Bradycardia and asystole with the use of vagus nerve stimulation for the treatment of epilepsy: A rare complication of intraoperative device testing. Epilepsia. 1999;40:1452–1454. doi: 10.1111/j.1528-1157.1999.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz PJ, De Ferrari GM. Vagal stimulation for heart failure: Background and first in-man study. Heart Rhythm. 2009;6:S76–S81. doi: 10.1016/j.hrthm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long qt syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–759. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N.Engl.J.Med. 2008;358:2024–2029. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 36.Edmondson RA, Banerjee AK, Rennie JA. Endoscopic transthoracic sympathectomy in the treatment of hyperhidrosis. Ann Surg. 1992;215:289–293. doi: 10.1097/00000658-199203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardell JL, Randall WC. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- 38.Yelich MR, Euler DE, Wehrmacher WH, Sinha SN, Randall WC. Parasympathetic influence on atrial vulnerability in the puppy. Am J Physiol. 1978;235:H683–H689. doi: 10.1152/ajpheart.1978.235.6.H683. [DOI] [PubMed] [Google Scholar]
- 39.Butler CK, Smith FM, Cardinal R, Murphy DA, Hopkins DA, Armour JA. Cardiac responses to electrical stimulation of discrete loci in canine atrial and ventricular ganglionated plexi. American Journal of Physiology. 1990;259:H1365–H1373. doi: 10.1152/ajpheart.1990.259.5.H1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.