Abstract
Converging evidence from neuroimaging and neuropsychological studies indicates that heavy marijuana use is associated with cingulate dysfunction. However, there has been limited human data documenting in vivo biochemical brain changes after chronic marijuana exposure. Previous proton magnetic resonance spectroscopy studies have demonstrated reduced basal ganglia glutamate and dorsolateral prefrontal cortex N-acetyl aspartate levels in adult chronic marijuana users. Similar studies have not been reported in adolescent populations. The present study used proton magnetic resonance spectroscopy to determine whether reductions in glutamate, N-acetyl aspartate and/or other proton metabolite concentrations would be found in the anterior cingulate cortex (ACC) of adolescent marijuana users compared with non-using controls. Adolescent marijuana users (N = 17; average age 17.8 years) and similarly aged healthy control subjects (N = 17; average age 16.2 years) were scanned using a Siemens 3T Trio MRI system. Proton magnetic resonance spectroscopy data were acquired from a 22.5 mL voxel positioned bilaterally within the ACC. Spectra were fitted using commercial software and all metabolite integrals were normalized to the scaled unsuppressed water integral. Analysis of variance and analysis of covariance was performed to compare between-group metabolite levels. The marijuana-using cohort showed statistically significant reductions in anterior cingulate glutamate (−15%, p < 0.01), N-acetyl aspartate (−13%, p = 0.02), total creatine (−10%, p < 0.01) and myo-inositol (−10%, p = 0.03). Within-voxel tissue-type segmentation did not reveal any significant differences in gray/white matter or cerebrospinal fluid content between the two groups. The reduced glutamate and N-acetyl aspartate levels in the adolescent marijuana-using cohort are consistent with precedent human 1H MRS data, and likely reflect an alteration of anterior cingulate glutamatergic neurotransmission and neuronal integrity within these individuals. The reduced total creatine and myo-inositol levels observed in these subjects might infer altered ACC energetic status and glial metabolism, respectively. These results expand on previous functional MRI data reporting altered cingulate function in individuals with marijuana-abuse.
Keywords: Chronic marijuana use, adolescent, proton magnetic resonance spectroscopy, anterior cingulate cortex
1.0 Introduction
Marijuana is the most commonly used illicit substance among adolescents in the United States, with annual prevalence of use estimated at approximately 11, 24 and 32% for 8th, 10th and 12th graders, respectively (Johnston et al. 2008). It is thought that heavy marijuana abuse during adolescence might lead to the perturbation of neuronal maturation processes occurring during this key developmental phase, giving rise to neurobiological alterations that are propagated through to adult brain circuits (Rubino and Parolaro, 2008) . Those changes ultimately can lead to altered decision-making capacities, emotional processing and cognitive performance (Realini et al. 2009). In addition, the marijuana-induced neurobiological changes may represent a risk for the development of psychosis in vulnerable individuals (Semple et al. 2005).
Converging evidence from neuroimaging and neuropsychological studies in adult human subjects indicates that chronic marijuana use is closely associated with cingulate dysfunction. Functional magnetic resonance imaging (fMRI) studies in adult chronic marijuana smokers have demonstrated that changes in anterior cingulate cortex (ACC) activation are associated with altered affective response (Gruber et al. 2009) and inhibitory processing (Gruber and Yurgelun-Todd, 2005). A positron emission tomography (PET) imaging study performed in combination with a Stroop task reported ACC hypoactivity in 25-day abstinent chronic marijuana users, thus inferring persistent deficits in executive cognitive functioning (Eldreth et al. 2004). Furthermore a study by Bolla and colleagues (Bolla et al. 2002) found that Stroop performance was related to frequency of cannabis use while a more recent investigation reported that subjects with a history of cannabis use produced significantly more errors on incongruent trials of the Stroop test (Battisti et al. 2010).
The functional alterations occurring within the ACC of chronic marijuana users are most likely to be accompanied by neurochemical changes. Δ9-tetrahydrocannabinol (Δ9-THC), the primary psychoactive compound within marijuana, activates the cannabinoid type-1 (CB1) receptor (Devane et al. 1988), a Gi/Go-protein-coupled receptor enriched within neurons throughout the central nervous system. Many CB1 receptors are located in glutamate neuron axon terminals where, through the inhibition of Ca2+ currents and modulation of K+ channel gating, they inhibit glutamate release (Hoffman et al. 2010). The binding of cortical glutamate to the N-methyl D-aspartate receptor (NMDAr) subtype peaks during early adolescence with a significant and rapid reduction with age (Insel et al. 1990). Therefore, glutamate plays a critical role in neurochemical remodeling throughout adolescence, and the perturbation of glutamatergic developmental processes are a potential consequence of chronic marijuana abuse. The in vivo quantification of these neurochemical differences would provide unique and complementary information to the emerging behavioral, structural and functional neuroimaging data.
Proton (1H) magnetic resonance spectroscopy (MRS), a non-invasive measurement technique that enables the detection of a wide range of neurometabolites and amino acid neurotransmitters in vivo, has been used to investigate biochemical brain changes resulting from chronic marijuana exposure in adult populations. One previous 1H MRS study revealed reduced basal ganglia glutamate, choline and myo-inositol in adult chronic marijuana users compared to healthy matched controls (Chang et al. 2006). A separate 1H MR spectroscopic imaging (MRSI) study reported reduced dorsolateral prefrontal cortex N-acetyl aspartate concentration in recreational adult marijuana smokers, possibly reflecting neuronal impairment or dysfunction in those subjects (Hermann et al. 2007). The critical question of whether or not similar neurochemical differences exists in adolescent chronic marijuana users remains unanswered. The aim of the present study was to employ 1H MRS for investigating neurometabolite levels in the ACC of adolescent marijuana users compared with non-using controls. Based on the precedent functional neuroimaging data and 1H MRS findings from adult chronic marijuana users, we hypothesized reduced ACC glutamate and N-acetyl aspartate concentrations in adolescent marijuana users compared to controls.
2.0 Materials and methods
2.1 Subject selection
The local Institutional Review Board of the University of Utah approved this study (IRB), which met the criteria for investigations in human subjects based on the Helsinki Accord http://ohsr.od.nih.gov/guidelines/helsinki.html. Seventeen adolescent marijuana (MJ) and seventeen similarly aged healthy control (HC) subjects were recruited from the greater Salt Lake area via local advertisements and by word of mouth. The MJ subjects were unaware of specific criteria (e.g. lifetime marijuana use) or the purpose of the study at the time of enrollment. All subjects initially underwent a phone screen to obtain preliminary information on MJ use, and MJ subjects were enrolled if they reported having smoked at least 100 times in the previous year. Table 1 presents the demographic data and relevant clinical variable information for both cohorts, including Hamilton rating scale for depression (HAM-D) and Hamilton rating scale for anxiety (HAM-A) data. Healthy controls had no DSM-IV Axis I diagnosis based on structured and clinical interviews and had no first-degree family history of bipolar disorder or psychosis. Exclusion criteria for all subjects included major sensorimotor handicaps (e.g. deafness, blindness, paralysis); full scale IQ <70; history of claustrophobia, autism, schizophrenia, anorexia nervosa or bulimia, other drug dependence/abuse or alcohol dependence/abuse (during 2 months prior to scan), active medical or neurological disease, history of ECT; metal fragments or implants; and current pregnancy or lactation. All subjects provided written assent, and their parents (or legal guardians) provided written informed consent for their adolescent's participation. All adolescents, including HC, under the age of 18 completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL) (Kaufman et al. 1997) whereas participants 18 and older completed the Structured Clinical Interview for DSM-IV Patient Version (SCID-P). All HC and MJ subjects underwent a one step urine drug test (iScreen™, San Diego, CA) on the day of scanning to test for the presence of cocaine, methamphetamine, tetrahydrocannabinol, opiates and benzodiazepines. Subjects who tested positive for any other illicit substance were excluded from the study. Following successful enrollment into the study a urine sample was retained and sent for quantitative 9-carboxy-tetrahydrocannabinol analysis using LC-MS/MS (ARUP Laboratories, Salt Lake City, UT). The high and low-end limits-of-detection for that analysis were 1000 and 4 ng/mL, respectively. In addition, information regarding age of first MJ use, age of regular use, and frequency of use was obtained on all participants. Total lifetime MJ use was calculated by averaging the number of smokes per week multiplied by duration of use.
Table 1.
Demographics of the HC and MJ subject groups including clinical variable information for the MJ cohort.
| HC N = 17 | MJ N = 17 | |
|---|---|---|
| Gender: male/female | 8/9 | 15/2 |
| Age (yrs): mean ± SD / range | 16.2 ± 2.1 / 13 – 19 | 17.8 ± 1.1 / 16 – 19 |
| Age of first use (yrs): mean ± SD / range | Not applicable | 15 ± 1.5 / 12 – 17 |
| Age of regular use (yrs): mean ± SD / range | Not applicable | 15.8 ± 0.8 / 14 – 17 |
| Total MJ use (number of smokes): mean ± SD / range | Not applicable | 1367 ± 1292 / 235 – 5250 |
| Cannabinoid count (ng/mL): mean ± SD / range | Not applicable | 399 ± 381 / 16 – 1000 * |
| HAM-D: mean ± SD / range | 0.2 ± 0.4 / 0 – 1 | 1.8 ± 3.3 / 0 – 11 |
| HAM-D: mean ± SD / range | 1.5 ± 2.4 / 0 – 7 | 1.9 ± 2.1 / 0 – 7 |
Two subjects registered a negative (−ve) urine cannabinoid count immediately prior to scanning, whereas three subjects showed cannabinoid counts greater than the dynamic range of the test procedure (>1000 ng/mL). SD = standard deviation.
While all subjects screened negative for psychiatric history based on a phone screen, direct diagnostic interviews indicated that two MJ subjects reported a history of alcohol abuse but not dependence, one MJ subject had a history of alcohol abuse and dependence, and two subjects reported a history of depression and were taking antidepressants at the time of MRS.
2.2 Data acquisition
All 1H MRI/MRS measurements were performed using a 3.0 Tesla Siemens (Erlangen, Germany) MAGNETOM Trio™ whole-body MRI scanner. The Siemens-supplied VB15 software was used for all studies and no hardware upgrades were performed over the study's 8-month timeframe. In addition, HC and MJ cohorts were scanned in an interleaved fashion throughout the study period. A circularly polarized body radiofrequency (RF) coil was used for RF transmission and a manufacturer-supplied 12-channel phased array receive-only head coil was used for signal reception. Subjects were positioned supine within the head coil and foam pads were used to optimally fixate the subject's head. The phased array RF coil was used in a four-cluster configuration with each cluster comprised of three individual coils. This resulted in signal reception and data outputted from two anteriorly-oriented and two posteriorly-oriented coil clusters.
Static magnetic field (B0) shimming initially was performed over the whole head field-of-view using the FASTMAP method (Gruetter, 1993). Three-dimensional (3D) high-contrast and high-resolution T1-weighted MP-RAGE MR images were used to facilitate accurate MRS voxel positioning and for within-voxel tissue-type segmentation (TR/TE/TI = 2000/3.53/1100 ms; FOV = 256 × 256 × 224 mm; isotropic 1 mm in-plane resolution). A point-resolved spectroscopy (PRESS) sequence was used to acquire 1H-MRS spectra from a voxel measuring 3.0 cm × 2.5 cm × 3.0 cm with the smallest dimension along the anterior-posterior axis. The MRS voxel was obliquely placed along the sagital plane and positioned bilaterally to cover predominantly gray matter within the ACC. Additional local B0 shimming was performed over the MRS voxel using automated and manual shimming routines to ensure an unsuppressed water signal linewidth ≤9 Hz. Outer-volume suppression (OVS) was achieved using six saturation bands positioned at least 2-cm away from the MRS voxel faces and band saturation was achieved using hyperbolic secant adiabatic full passage RF pulses. A three-pulse water elimination through T1-effects (WET; Ogg et al. 1994) scheme was interleaved with the OVS module for global water suppression. For slice-selective excitation and refocusing, the transmitter frequency was positioned at 3.0 and 4.7 ppm for the water suppressed and water unsuppressed acquisitions, respectively. PRESS MRS acquisition parameters for each dataset were as follows: TR = 2000 ms, TE = 30 ms, number of excitations (NEX) = 128. An unsuppressed water reference spectrum also was acquired (TR/TE = 2000/30 ms; NEX = 4) and used for eddy current correction of metabolite spectra and normalization of metabolite peak areas.
2.2.1 Tissue segmentation
Whole brain tissue-type segmentation was performed on all MP-RAGE images using the FAST tool (Zhang et al. 2001) provided with the freely-available FMRIB software library (FSL; Smith et al. 2004). Home-written MATLAB (version R2009b, The MathWorks, Natick, MA) functions then were used to extract the 3D volume corresponding to the positioned MRS voxel in order to obtain within-voxel gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) tissue content for each subject. The gray matter fraction was calculated as the ratio to total brain matter i.e. 100 × GM/(WM+GM).
2.2.2 Data processing
All free induction decay (FID) data were stored as Siemens TWIX files and transferred to personal computer systems to be processed by home-written preprocessing functions written and developed using MATLAB. Signal weighting and signal lineshape/phase correction is coil-cluster specific and must be accounted for prior to signal combination. The home-written MATLAB functions performed these steps in an automated fashion and corrections were carried out as follows. The TWIX file header contains individual coil cluster-specific weighting function coefficients that are calculated during routine Siemens service protocols. The individual metabolite and unsuppressed water FIDs from a given coil cluster are multiplied by the relevant weighting factors prior to coil cluster recombination. Eddy current correction was performed using a previously reported time-domain method (Klose, 1990). Briefly, the water-suppressed metabolite FIDs from a given coil cluster were eddy current corrected by dividing each data point by a phase factor determined from the corresponding unsuppressed water data point. Extensive analysis of the in vivo datasets showed that cluster-specific eddy current correction alone yielded four sets of FIDs with identical phase (data not presented). Subsequently, this permitted the direct combination of the four FIDs prior to any spectral fitting.
2.2.3 Spectral quantification
Spectral fitting and signal quantification was performed using the commercially-available Linear Combination (LC) Model software (Provencher, 1993; version 6.1.4E). The PRESS spectra were fitted using a simulated basis set that was generated using density matrix theory as described in a previous publication (Choi et al. 2008). For the simulations, proton chemical shifts and J-coupling constant values were taken using precedent literature values (Govindaraju et al. 2000) and basis functions were generated for a total of fourteen metabolites including: alanine (Ala), aspartate (Asp), total creatine (tCr; creatine+phosphocreatine), γ-amino butyric acid (GABA), glutamine (Gln), glutamate (Glu), glycerophosphocholine (GPC), myo-inositol (Ins), lactate (Lac), N-acetyl aspartate (NAA), N-acetyl aspartyl glutamate (NAAG), phosphorylcholine (PCh), scyllo-inositol (sI) and taurine (Tau). Note that the GPC and PCh integrals were combined to provide a quantification estimate of total choline (tCho). The LC Model 'analysis window' was set to cover the 0.5 – 4.5 ppm range. LC Model reports Cramer-Rao lower bound (CRLB) estimates for each metabolite included within the basis set, which reflect the reliability of each spectral fit. Metabolite fits with CRLB <20% observed for both subject groups were included in the final analysis. The metabolite peak areas were corrected for the CSF-fraction using segmented MRI data and then normalized using the corresponding unsuppressed water signal integral (scaled: × 10−9). The calculated metabolite/water ratios are presented as mean ± standard deviation (SD).
2.3 Statistical analysis
One way analysis of variance (ANOVA) was used for comparing group mean metabolite levels using OriginPro (version 8.0; OriginLab Corporation, Northampton, MA). In addition, analysis of covariance (ANCOVA) for two independent samples was performed using the freely-available calculator provided at http://faculty.vassar.edu/lowry/ancova2L.html (last accessed 01/14/2011). ANCOVA was applied to test for potentially confounding demographic variables (age and sex), biological factors (within-voxel tissue content) and MRS-related technical parameters including the signal-to-noise ratio (SNR) and full-width at half-maximum (FWHM). The SNR and FWHM values were extracted directly from the LC Model output files for each subject scan.
3.0 Results
3.1 Tissue segmentation
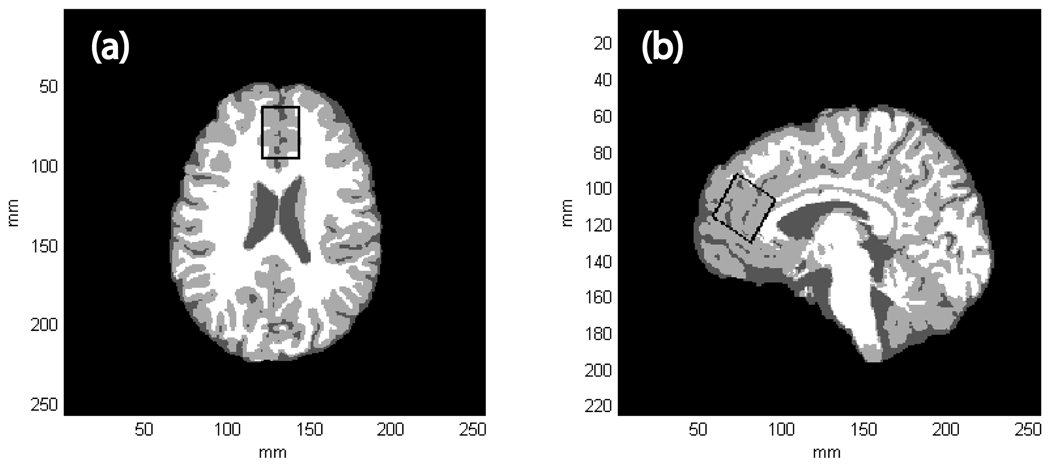
Figures 1(a) and 1(b) show axial and sagital tissue-segmented slices extracted from a 3D MPRAGE dataset recorded from a MJ subject. The black rectangle corresponds to the MRS voxel positioned within the ACC, which was obliqued along the sagital plane. Table 2 shows the group mean within-voxel tissue content for both subject cohorts and statistical analysis did not reveal any significant differences between the two cohorts.
Figure 1.
(a) A single tissue-segmented axial slice extracted from a 3D MPRAGE dataset recorded from a 19 year-old female MJ subject. WM, GM and CSF are represented by white, light gray and dark gray pixels, respectively. (b) The corresponding single tissue-segmented sagital slice extracted from the same MPRAGE dataset. The black rectangle depicts the positioning of the MRS voxel within the ACC, which was obliqued along the sagital dimension. For this subject, GM, WM and CSF tissue fractions were estimated to be 67, 23 and 10%, respectively.
Table 2.
Within-voxel GM fraction and CSF content expressed as group mean % fraction ±SD.
| Tissue type | HC | MJ | Statistics |
|---|---|---|---|
| GM | 71 ± 5 | 72 ± 4 | F(1,32) = 0.90, p = 0.34 |
| CSF | 9 ± 5 | 8 ± 4 | F(1,32) = 0.40, p = 0.54 |
3.2 Spectral and statistical analysis
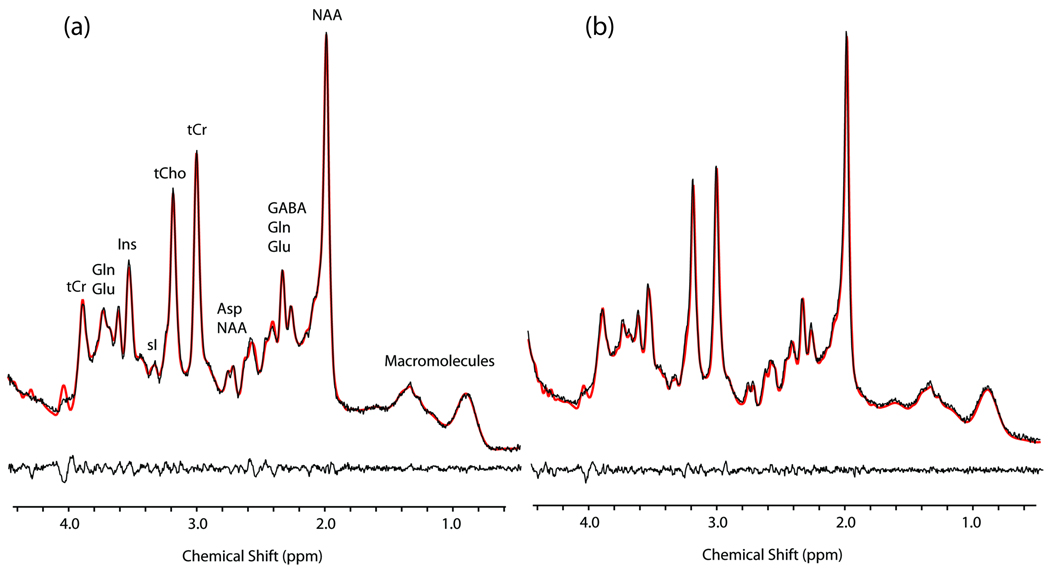
Figure 2 shows representative LC-Model fitted 1H MRS spectra recorded from a HC and a MJ subject (see figure legend for spectral assignments and additional details). The group mean (±SD) SNR and FWHM parameters were (MJ 43 ± 11; HC 49 ± 8, F(1,32) = 3.1, p = 0.1) and (MJ 5.3 ± 1.1 Hz; HC 4.9 ± 0.7 Hz, F(1,32) = 1.82, p = 0.2), respectively. Table 3 shows the outputted CRLB values for 6 individual metabolites, with estimated mean CRLB values of <20% reported for both subject groups. These metabolites were Asp, tCr, Glu, Ins, NAA and tCho.
Figure 2.
LC-Model fitted PRESS 1H-MRS data recorded from a 13 year-old HC subject (a) and a 19 year-old MJ subject (b). The black line spectra correspond to the ‘raw’ 1H-MRS data with the LC-Model fits overlaid as red line spectra. The residual spectra (raw data minus the LC-Model fit) are displayed below each spectrum. The signal assignments displayed in (a) are based on (Govinduraju et al. 2000) and can be directly translated to spectrum (b).
Table 3.
The LC-Model derived group mean (±SD) CRLB values for six fitted metabolites where the estimated mean values were <20% for both subject cohorts.
| Metabolite | HC | MJ |
|---|---|---|
| Asp | 14 ± 3 | 14 ± 3 |
| tCho | 2 ± 0.2 | 2.3 ± 0.5 |
| tCr | 2 ± 0.2 | 2 ± 0.4 |
| Glu | 5.2 ± 0.7 | 5.9 ± 0.8 |
| Ins | 3.1 ± 0.7 | 3.5 ± 0.5 |
| NAA | 2.3 ± 0.5 | 2.7 ± 0.6 |
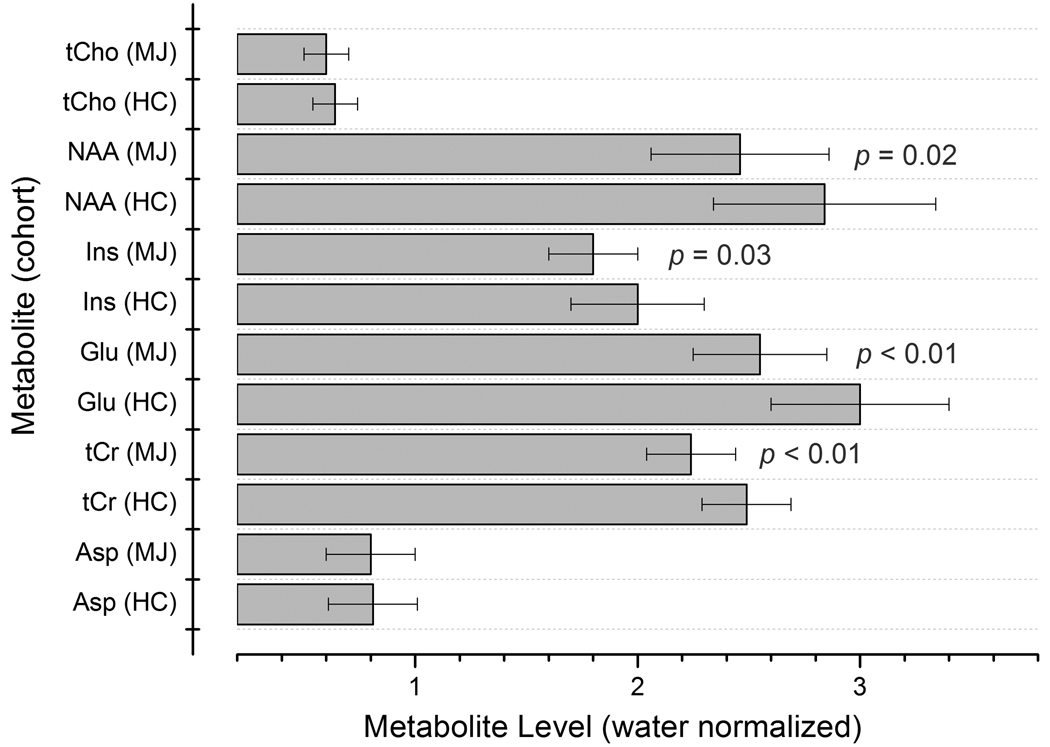
Figure 3 displays a bar chart showing the group mean metabolite/water ratios for Asp, tCr, Glu, Ins, NAA and tCho. The MJ cohort showed significantly decreased ACC Glu (MJ 2.55 ± 0.30; HC 3.00 ± 0.39, F(1,32) = 12.5, p <.01), tCr (MJ 2.24 ± 0.23; HC 2.49 ± 0.19, F(1,32) = 9.3, p < 0.01), Ins (MJ 1.80 ± 0.20; HC 2.00 ± 0.30, F(1,32) = 5.3, p = 0.03) and NAA (MJ 2.46 ± 0.40; HC 2.84 ± 0.50, F(1,32) = 5.8, p = 0.02) water normalized levels. Significant differences were not observed for ACC Asp (MJ 0.80 ± 0.20; HC 0.81 ±0.20, F(1,32) = 0.05, p = 0.8) or tCho (MJ 0.60 ± 0.10; HC 0.64 ± 0.10, F(1,32) = 3.5, p = 0.1) levels. Importantly, the raw water signal integral (arbitrary units) was found to be consistent between the two subject cohorts (MJ 2.85 ±0.11; HC 2.8 ± 0.11, F(1,32) = 0.9, p = 0.35).
Figure 3.
Metabolite/water ratios measured for 6 metabolites in the HC and MJ cohorts. Group mean values are shown and the error bars represent SD.
For the most part, ANCOVA tests performed to co-vary for the effects of age, sex, SNR, FWHM, and GM fraction had negligible effect on HC and MJ mean metabolite levels (data not shown). However, co-varying for sex increased the significance of reduced ACC Glu levels detected in MJ subjects (F(1,31) = 16.2, p < 0.001) whereas co-varying for SNR decreased the significance of the NAA findings (F(1,31) = 2.1, p = 0.2). Finally, correlation analysis carried out to investigate the relationship between metabolite levels and total lifetime use of marijuana, age of onset of marijuana use and cannabinoid concentration at time of scan, did not reach statistical significance for any metabolite. In addition, for each metabolite no correlation was observed between HAM-D and metabolite concentration.
4.0 Discussion
To the best of our knowledge, this is the first study to use in vivo 1H MRS to detect changes in ACC neurometabolites and neurotransmitters in adolescent marijuana abusers compared with non-using control subjects. The major findings of the present study were significantly reduced ACC Glu (−15%), NAA (−13%), Ins (−10%) and tCr (−10%) levels in the adolescent MJ population.
The widely believed physiologic stability of the tCr methyl proton (CH3) resonance across age, sex and a variety of brain pathologies has lead to its frequent use as an internal reference for normalization of 1H MRS data. However, decreased tCr levels have been observed in several tumor types (Howe and Opstad, 2003) and following ischemic stroke (Munoz Maniega et al. 2008), and a recent report demonstrated differential tCr levels in individuals with bipolar disorder, schizophrenic patients and healthy control subjects (Ongur et al. 2009). Hence, for the present cross-sectional MRS study we initially measured the ACC tCr levels in both subject groups using the tissue water signal as an internal reference standard. That analysis revealed significantly decreased water normalized tCr levels in the MJ cohort although comparable water signal integrals and MRS voxel GM, WM and CSF fractions calculated for both groups. Hence, for the present study it was crucial that all measured metabolite levels were normalized to tissue water.
1H MRS and MR spectroscopic imaging (MRSI) methods have been used to investigate the effects of chronic marijuana abuse on brain metabolites in adult populations (Chang et al. 2006; Hermann et al. 2007) although none of those previous studies reported reduced ACC tCr levels in marijuana users. The reduced tCr concentration observed in the present study is likely to reflect altered ACC energetic status within the adolescent MJ group, although due to the composite nature of the tCr proton peak it remains unclear as to whether the observed reduction stems from modulation of creatine and/or phosphocreatine. Additional phosphorous (31P) MRS studies performed in similar adolescent MJ and HC cohorts will be critical for investigating this potential creatine/phospocreatine imbalance.
The reduced ACC Glu (−15%) level detected in the adolescent MJ population is one of the most significant findings from the present study. This observation is consistent with the decreased basal ganglia Glu levels (−12%) previously reported in adult marijuana users (Chang et al. 2006) although similar single voxel ACC 1H MRS measurements have not been reported. ACC glutamatergic tone clearly is altered in the adolescent MJ population although, as highlighted below, we cannot draw unequivocal conclusions based on the reduced ACC Glu levels observed in the present study. Glu is stored primarily in the vesicles within neurons and is the major excitatory neurotransmitter within the mammalian brain. In addition, Glu is the precursor of GABA (major inhibitory neurotransmitter) and NAAG (a dipeptide neurotransmitter). As a neurotransmitter, Glu is released into the synaptic cleft and rapidly transported into astroglia where it is enzymatically converted into Gln. Subsequently, Gln is shuttled back into neurons for conversion to Glu. However, decreased Glu levels might stem from neuronal impairment (Gln->Glu conversion) and/or glial dysfunction with decreased Glu uptake or compromised re-shuttling of Gln back into neurons. The decrease in ACC Glu in our MJ population is intriguing and may be related to observed behavioral changes in MJ users. It is known that CB1 receptors are located presynaptically on neurons and that activation of these receptors, such as through the active compound in cannabis, Δ9-THC, leads to suppression of glutamate and GABA neurotransmitter release (Hajos and Freund, 2002). Glutamate and GABA are the major excitatory and inhibitory amino acid neurotransmitters in the central nervous system and therefore modulation of the presynaptic release of these neurotransmitters will lead to changes in neuronal synaptic plasticity. It is likely that these changes in synaptic plasticity will be associated with altered neurocognitive function in MJ users, including those mediated by the cingulate network. However, additional spectroscopic information regarding ACC Gln and GABA concentration is needed to provide key information from which to draw stronger conclusions. The CRLB values observed for Gln and GABA in the present study were deemed unreliable for inclusion into our final data analysis. As such we currently are investigating alternative 1H MRS acquisition and processing strategies for improving Gln and GABA quantification (Schulte and Boesiger, 2006; Shulte et al. 2006).
The reduced ACC NAA (−13%) observed in the MJ population is also in agreement with precedent literature. Reduced NAA levels are also supported by a previous 1H MRSI study, which showed significant reduction of dorsolateral prefrontal cortex NAA levels (−11%) in adult male recreational marijuana users when compared to healthy controls (Hermann et al. 2007). NAA is a putative marker for neuronal viability and the present study suggests diminished neuronal and axonal integrity (loss and/or dysfunction) within the ACC of adolescent MJ users. Given that cannabis use has been reported as a potential risk factor for developing psychosis in predisposed individuals (Semple et al. 2005), it is of note that the reduced ACC NAA levels in adolescent MJ users is similar to the reduced frontal lobe and ACC NAA concentration previously reported in schizophrenic patients (Buckley et al. 1994; Cecil et al. 1999; Ende et al. 2000).
Furthermore we detected a significant reduction in ACC Ins levels (−10%). Ins is localized mainly within astrocytes and thus widely believed to be a glial marker (Brand et al. 1993). Reduced prefrontal cortex Ins levels have been previously detected using 1H MRS in conditions known to be characterized by significant glial loss such as major depressive disorder (Coupland et al. 2005). The reduced Ins levels detected in the present study might be a consequence of glial loss and/or glial dysfunction.
Previous 1H MRS studies have detected age-related metabolite concentration changes in healthy adult subjects (Brooks et al. 2001; Sailasuta et al. 2008; Chang et al. 2009) although the literature documenting metabolite changes throughout adolescence is sparse. One 1H MRS study tracked NAA levels throughout childhood and adolescence demonstrating a rise in gray matter NAA/Cho ratios with a maximum level reached at approximately 10 years of age and a decline thereafter (Horska et al. 2002). Similar developmental changes are likely to occur for Glu, Ins and tCr and comprehensive cross-sectional and longitudinal 1H MRS studies performed in healthy adolescent populations will be critical to help place the findings from the present study into further context.
ANCOVA analysis performed to check for the effects of potentially confounding demographic, technical and biological variables identified mostly subtle effects on the statistical significance of decreased metabolite levels in the MJ population compared to controls, although co-varying for sex enhanced the significance of the Glu findings. Note that, although similarly aged, the HC and MJ subjects were not sex-matched. Therefore, to further assess the potential issue of sex-dependence on metabolite levels, ANOVA was repeated with all females removed from the two subject cohorts (MJ N = 15; HC N = 8). The results from these tests revealed lower Glu (F(1,21) = 21, p < 0.001), NAA (F(1,21) = 7.2, p = 0.01), tCr (F(1,21) = 17, p < 0.001) and Ins (F(1,21) = 4.7, p = 0.04) in MJ subjects further supporting MJ-induced neurochemical alterations independent of sex effects. Also, co-varying for SNR removed the significance of reduced NAA levels in the MJ population. This observation can be rationalized as the LC Model software estimates SNR based on the largest resonance detected in the analysis window (0.5 – 4.5 ppm), thus utilizing the 2.0 ppm NAA resonance for all SNR calculations in the present study. We observed a lower estimated SNR for the MJ cohort that approached statistical significance (p = 0.1) and strongly believe that this further supports reduced NAA in this population. It follows that covarying NAA concentration for SNR essentially violates the ANCOVA analysis and leads to erroneous and unreliable adjusted means. Correlation analysis did not approach statistical significance for any metabolite level versus clinical variable data although our current MJ sample size is relatively small (N = 17). We are planning to enroll additional MJ subjects for similar MRS studies and aim to increase the number of samples with lower ages for first and regular MJ use.
4.1 Study limitations
As described above, our MJ sample included some individuals with a history of co-morbid alcohol abuse or dependence and two subjects with a history of depression who were taking antidepressants at the time of MRS scanning. The presence of comorbidity and/or history of comorbidity within the MJ cohort could be potential confounds in the present study, and additional studies should examine the relationship between pure marijuana users and those with co-morbid diagnoses. However, it should be noted that the prevalence of co-morbid mood disorder in individuals with substance abuse has been reported to be extremely high (Grella et al. 2001; Lubman et al. 2007; Guillem et al. 2009). For example, high prevalence rates of co-morbid psychiatric disorders were found in a study by Lubman and colleagues (Lubman et al. 2007) who found that 49% of those studies met criteria for current mood disorder, with 68% reporting a lifetime history. This is consistent with a more recent study showing that among 90 cannabis users, current mood disorder was present in 48% in the last 12 months (Guillem et al. 2009).
Inaccurate statements about the self-reported age of first MJ use, age of regular MJ use and total MJ use cannot be ruled out. The degree to which inaccurate self-report measures may have affected our correlation analysis is difficult to determine. The cannabinoid urine toxicology test had a relatively low positive cutoff (4 ng/mL), although due to drug clearance effects we cannot use the data to unequivocally rule out history of cannabis use within our HC population. Any regular marijuana in this group would seem unlikely as they were queried on history of all substance use in an open ended manner. Future work will employ hair analysis for verifying cannabis smokers and excluding potential HC subjects.
The voxel size parameters were selected to optimize SNR per unit time and we acknowledge the partial volume effects that result. The major objective of the present study was to evaluate potential metabolic abnormalities in gross ACC volume but future studies might aim to acquire 1H MRS data from more ACC sub-regions. MRS voxel segmentation based on high-resolution MPRAGE image data showed a relatively small spread of GM and WM tissue content within the two cohorts, suggesting similarly positioned voxels across all studies.
Between-group differences in metabolite spin-spin (T2) and spin-lattice (T1) relaxation times might account for the differential metabolite/water ratio observed in the present study. This section briefly discusses these effects for tCr and water although the arguments can be directly translated to the reductions observed for Glu, Ins and NAA. Based on previously reported 3.0 Tesla tCr CH3 T2 (164 ms; Choi et al. 2006) and T1 (1100 ms; Traber et al. 2004) relaxation times, our calculations show that a 34% reduction in tCr T2 (104 ms) or a 30% increase in tCr T1 (1440 ms) would account for the reduced tCr/water levels reported in the present study. Although the metabolite values were corrected for CSF fraction it is useful to consider differing CSF and tissue relaxation times and how varying tissue fractions might alter calculated metabolite/water ratios. Based on water unsuppressed multi-TE (30 – 1000 ms) PRESS ACC data recorded from 10 subject s in our laboratory, we calculated the mean CSF and tissue T2 relaxation times as 437 ±80 and 69 ±2ms, respectively. Hence, differential CSF fractions would lead to variable denominator values across subjects and that this could account for variable metabolite/water ratios. Using our estimated CSF and tissue water T2 values we calculate that an increase in CSF fraction from 9 to 51% is required to account for the 15% Glu reduction detected in the adolescent MJ subjects. For the present study this is highly unlikely owing to the closely similar CSF fractions between the HC and MJ cohorts. We performed a similar analysis of CSF fractional changes in relation to longitudinal relaxation times using precedent CSF (4163 ms; Lin et al. 2001) and tissue (1100 ms; Wansapura et al. 1999) T1 values. That analysis suggested that, assuming a starting CSF fraction of 9%, an unfeasible 23% increase of tissue content is required to account for the 15% Glu reduction in MJ subjects. While these water effects are unlikely to have significantly influenced our findings our protocol has been modified to measure and account for these effects on an individual subject basis.
4.2 Study strengths
The novel findings from the present study provide unique insights into the neurochemical alterations occurring within adolescent marijuana abusers, and the results are complementary to the body of neuropsychological and neuroimaging data emerging from these populations. Substantial effort was made to enroll well-characterized subject cohorts. Only three of the seventeen MJ subjects presented with depression or a history-of-depression. Given the co-morbidity of alcohol use and marijuana it is noteworthy that only two of the seventeen MJ subjects met diagnostic criteria for an alcohol use disorder.
From a technical perspective, all of the MRS datasets acquired and processed in the present study were of high-quality, characterized by a high SNR and low signal linewidths. Spectral fitting employed state-of-the-art spectral simulation methods that generated individual metabolite basis spectra based on the actual PRESS sequence used for the in vivo studies. Only those metabolites with suitably low LC Model-reported CRLB values (indicative of reliable spectral fits) were used for between-group comparisons. In addition, we ran numerous ANCOVA analyses to test for the possibility of confounding demographic, biological or technical variables in order to parse-out the observed metabolic alterations detected in the MJ population.
5.0 Conclusions
In conclusion, the reduced Glu and NAA levels in the MJ cohort are consistent with precedent adult human 1H MRS data, and likely reflect an impairment of ACC glutamatergic neurotransmission and neuronal viability within these individuals. The reduced tCr and Ins levels observed in the MJ subjects might infer altered ACC energetic status and glial metabolism, respectively. These results expand on previous fMRI data reporting altered cingulate function in individuals with marijuana-abuse. We currently are working on developing and implementing methodology to enable the concomitant and reliable measurement of Glu, Gln and GABA for application to similar adolescent populations.
Acknowledgments
Disclosures
Dr. Yurgelun-Todd is a consultant to Kyowa Hakko, Eli Lilly and Janssen Pharmaceutica. Dr. Renshaw is a consultant to Kyowa Hakko, Novartis and Roche and receives research support from Roche and GlaxoSmithKline. This study was supported by NIH 1R01 DA020269 (DYT) and NIH K24DA015116 (PFR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew P. Prescot, Email: andrew.prescot@utah.edu.
Allison E. Locatelli, Email: allison.locatelli@utah.edu.
Perry F. Renshaw, Email: PERRY.RENSHAW@hsc.utah.edu.
Deborah A. Yurgelun-Todd, Email: Deborah.Yurgelun-Todd@hsc.utah.edu.
References
- Battisti RA, Roodenrys S, Johnstone SJ, Pesa N, Hermens DF, Solowij N. Chronic cannabis users show altered neurophysiological functioning on Stroop task conflict resolution. Psychopharmacology. 2010;212:613–624. doi: 10.1007/s00213-010-1988-3. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brooks JC, Roberts N, Kemp GJ, Gosney MA, Lye M, Whitehouse GH. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb Cortex. 2001;11:598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, Redmond O, Stack JP, Ennis JT, Waddington JL. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical, neurodevelopmental, and cognitive correlates. Biol Psychiatry. 1994;36:792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Lenkinski RE, Gur RE, Gur RC. Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999;20:131–140. doi: 10.1016/S0893-133X(98)00063-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Jiang CS, Ernst T. Effects of age and sex on brain glutamate and other metabolites. Magn Reson Imaging. 2009;27:142–145. doi: 10.1016/j.mri.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Bhardwaj PP, Seres P, Kalra S, Tibbo PG, Coupland NJ. Measurement of glycine in human brain by triple refocusing 1H-MRS in vivo at 3.0T. Magn Reson Med. 2008;59:59–64. doi: 10.1002/mrm.21450. [DOI] [PubMed] [Google Scholar]
- Choi C, Coupland NJ, Bhardwaj PP, Kalra S, Casault CA, Reid K, Allen PS. T2 measurement and quantification of glutamate in human brain in vivo. Magn Reson Med. 2006;56:971–977. doi: 10.1002/mrm.21055. [DOI] [PubMed] [Google Scholar]
- Coupland NJ, Ogilvie CJ, Hegadoren KM, Seres P, Hanstock CC, Allen PS. Decreased prefrontal Myo-inositol in major depressive disorder. Biol Psychiatry. 2005;57:1526–1534. doi: 10.1016/j.biopsych.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389–395. doi: 10.1016/s0920-9964(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser YI, Joshi V, Rounds-Bryant J. Drug treatment outcomes for adolescents with comorbid mental and substance use disorders. Journal of Nervous and Mental Disease. 2001;189:384–392. doi: 10.1097/00005053-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105:139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Guillem E, Pelissolo A, Vorspan F, Bouchez-Arbabzadeh S, Lepine JP. Sociodemographic profiles, addictive and mental comorbidity in cannabis users in an outpatient specific setting. Encephale-Revue De Psychiatrie Clinique Biologique Et Therapeutique. 2009;35:226–233. doi: 10.1016/j.encep.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, Mann K. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–1289. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horska A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, Barker PB. In vivo quantitative proton MRSI study of brain development from childhood to adolescence. J Magn Reson Imaging. 2002;15:137–143. doi: 10.1002/jmri.10057. [DOI] [PubMed] [Google Scholar]
- Howe FA, Opstad KS. 1H MR spectroscopy of brain tumours and masses. NMR Biomed. 2003;16:123–131. doi: 10.1002/nbm.822. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med. 1990;14:26–30. doi: 10.1002/mrm.1910140104. [DOI] [PubMed] [Google Scholar]
- Lin C, Bernstein M, Huston J, Fain S. In vivo and in vitro measurements of T1 relaxation at 3.0T. Proc 9th meeting ISMRM; Glasgow. 2001. p. 1391. [Google Scholar]
- Lubman DI, Allen NB, Rogers N, Cementon E, Bonomo Y. The impact of co-occurring mood and anxiety disorders among substance-abusing youth. J Affect Disord. 2007;103:105–112. doi: 10.1016/j.jad.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S, Cvoro V, Armitage PA, Marshall I, Bastin ME, Wardlaw JM. Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke. 2008;39:2467–2469. doi: 10.1161/STROKEAHA.107.507020. [DOI] [PubMed] [Google Scholar]
- Ogg RJ, Kingsley PB, Taylor JS. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104:1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res. 2009;172:44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286:S108–S113. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Sailasuta N, Ernst T, Chang L. Regional variations and the effects of age and gender on glutamate concentrations in the human brain. Magn Reson Imaging. 2008;26:667–675. doi: 10.1016/j.mri.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte RF, Boesiger P. ProFit: two-dimensional prior-knowledge fitting of J-resolved spectra. NMR Biomed. 2006;19:255–263. doi: 10.1002/nbm.1026. [DOI] [PubMed] [Google Scholar]
- Schulte RF, Lange T, Beck J, Meier D, Boesiger P. Improved two-dimensional J-resolved spectroscopy. NMR Biomed. 2006;19:264–270. doi: 10.1002/nbm.1027. [DOI] [PubMed] [Google Scholar]
- Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. J Magn Reson Imaging. 2004;19:537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9:531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]