Abstract
Because of its large surface area and easy access for both delivery and monitoring, the skin is an attractive target for gene therapy for cutaneous diseases, vaccinations and several metabolic disorders. The critical factors for DNA delivery to the skin by electroporation (EP) are effective expression levels and minimal or no tissue damage. Here, we evaluated the non-invasive multielectrode array (MEA) for gene electrotransfer. For these studies we utilized a guinea pig model, which has been shown to have a similar thickness and structure to human skin. Our results demonstrate significantly increased gene expression 2 to 3 logs above injection of plasmid DNA alone over 15 days. Furthermore, gene expression could be enhanced by increasing the size of the treatment area. Transgene expressing cells were observed exclusively in the epidermal layer of the skin. In contrast to caliper or plate electrodes, skin EP with the MEA greatly reduced muscle twitching and resulted in minimal and completely recoverable skin damage. These results suggest EP with the MEA can be an efficient and non-invasive skin delivery method with less adverse side effects than other EP delivery systems and promising clinical applications.
Keywords: electroporation, gene delivery, guinea pig, skin, multielectrode array
1. Introduction
In the past two decades electroporation (EP) has received increased attention for its advantages compared to viral vectors for use in gene delivery. EP has been demonstrated to be an efficient non-viral in vivo gene delivery method by several independent research groups[1–5]. Diverse electrodes such as calipers, tweezers, needles, arrays and microneedle arrays have been designed and tested in different species[6–10]. Various electrical parameters have been studied for their expression efficiency and adverse effects[6, 11]. In vivo gene delivery by EP has been reported to achieve effective gene expression in various tissues and organs[12], such as liver[1], skin[13], muscle[14], brain[15], eye[16], lung[17], spleen[18], kidney[19], bladder[20], testis[21], artery[22], tumors[2].
Skin contains large numbers of potent antigen-presenting cells, Langerhans cells and dermal dendritic cells, as well as an abundant blood supply in the dermal layer of skin[23], which may help transgenic products distribute into distant organs through circulation[24]. These advantages make delivery of therapeutic genes to the skin very attractive, particularly, for i) the treatment of local diseases including skin cancer, chronic ulcer, burn, psoriasis; ii) vaccination against infectious diseases such as HIV, anthrax, malaria, as well as non-infectious diseases like cancer; iii) the correction of systemic or metabolic disorders like anemia in chronic kidney disease. Previous studies have shown that EP efficiently delivers plasmid DNA to the skin resulting in a 10–1000 fold increase of local and serum expression[24–27]. Skin EP delivery was successfully performed in rodent, porcine and non-human primate model systems[13, 24, 25]. Intradermal delivery of plasmid VEGF(165), FGF-2 or TGF-β by EP has been observed to promote wound healing in rat or mouse models[28–30]. Significant serum levels were achieved by EP delivery of both EPO and IL-12 plasmid DNA to the skin[24, 31–33]. A number of studies demonstrated that significant tumor regression could be achieved by electrically mediated delivery of plasmids expressing IFN-α, IL-12, IL-2, IL-15, IL-18, GM-CSF and other transgenes to cutaneous tumors (melanoma, squamous cell carcinoma)[6]. In our mouse melanoma model[32, 34], intratumoral EP of IL-12 plasmid resulted in complete tumor regression rates of 80%. Those mice were also resistant to subsequent tumor challenge. Moreover, our phase I human trial of IL-12 EP treatment of metastatic melanoma showed that distant untreated lesions could also regress, suggesting that not only had a local response been mounted against treated tumors but also a systemic memory response had been generated[35].
Current skin EP systems, utilize, for example, invasive needle electrodes as well as plate electrodes (calipers, forceps, etc.) and typically induce significant muscle twitching and discomfort and treatment can result in skin damage[25]. To overcome the pitfalls of these electrode designs, we developed a new non-invasive electrode known as multielectrode array (MEA). In previous studies[27], we reported that skin EP with the MEA could achieve comparable (in rat) or higher expression (in guinea pig) as compared to plate electrodes, while the applied voltage and muscle stimulation was greatly reduced. In the current study, we further modified the MEA to include flexible spring electrodes in the substrate to assure a full contact between all of the electrodes and the skin. We then characterized several critical aspects relevant to therapeutic applications. DNA delivery was tested in a guinea pig model, which has similar skin thickness and structure to human skin[36, 37]. Localized transgene expression and kinetics were assessed by the measurement of luciferase activity with an in vivo bioluminescence scan. The evaluation of the MEA has also included the correlation between expression and the size of the treated area, potential tissue damage, DNA distribution and localization of gene expressing cells.
2. Materials and methods
2.1 Animals
Female Hartley guinea pigs used in this study were 4 to 6 weeks old from Elm Hill Labs (Chelmsford, MA, USA). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Old Dominion University.
2.2 Plasmids
The reporter plasmids encoded luciferase (gWiz-Luc) and green fluorescent protein (gWiz-GFP), both from Aldevron (Fargo, ND, USA). Fluorescein-labeled plasmid MIR 7907 and Cy™3-labeled plasmid MIR7905 (Mirus Bio LLC, Madison, WI, USA) was used to observe DNA distribution.
2.3 DNA Injection and in vivo Electroporation
Prior to delivery, animals were anesthetized in an induction chamber charged with 3% isoflurane in O2 then fitted with a standard rodent mask and kept under general anesthesia during the procedure. Guinea pigs received intradermal (i.d.) injections of 50 µl or 200 µl plasmid DNA (2µg/µL dissolved in saline) on the left and right flank. Immediately after DNA administration, a MEA electrode with 4×4 2-mm-apart pins was placed over the injection site(s). Voltage was applied (each pair of electrodes was programmed to administer four pulses with total 72 pulses[27], electric field was 250 V/cm, pulse duration 150ms and 150ms delay). The electroporation parameters we chose here were based on our recently published study[38] in which we evaluated the effect of different electrotransfer parameters on trangene expression and skin damage using a similar designed MEA electrode in the guinea pig model. Electroporation was performed using the UltraVolt Model: Rack-2-500-00230 (Ultravolt, Inc. Ronkonkoma, NY, USA). The pulse parameters of 250 V/cm and 150 ms were found to give the highest expression with minimal damage to the skin. Increasing the field strength did not result in increased expression. For a single 200 µl injection or four 50 µl adjacent injections, four individual pulse applications were applied without change of pulse parameters.
2.4 Living Imaging of Luciferase Expression
At different selected time points after delivery, animals were anesthetized then administrated intradermally with the same DNA volume of D-luciferin with 7.5mg/mL in PBS buffer (Goldbio, St. Louis, MO, USA). Assessment of photonic emissions using the IVIS Spectrum system (Caliper Life Sciences, Hopkinton, MA, USA)) was performed 1.5 minutes after injection of D-luciferin. Background luminescence was determined by measuring luminescence from area without DNA injection.
2.5 GFP Expression
Each excised sample was immediately frozen on dry ice. After visualization of GFP expression was observed and obtained by flurorescence stereoscope (Leica Model MZFL III, Leica, Heerbrugg, Switzerland), the specimens were embedded in tissue freeze media OCT compound (Electron Micriscopy Sciences, Hatfield, PA) and frozen at −80°C freezer. Several frozen sections (8 µm thickness) were cut from each sample. Each section was fixed in 25% Acetone + 75% Ethanol 20 minutes and then washed twice in PBS. Dry under dark and mount coverslip with VECTASHIELD® mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Sections were examined by Olympus BX51 fluorescent microscopy (Olympus, Tokyo, Japan) for the presence of GFP.
2.6 Histological Analysis
Each specimen was embedded, sectioned and fixed as mentioned above. Sections were dehydrated in 95% ethanol 30 seconds, stained in hematoxylin solution 5 minutes, rinsed with tap water 3 minutes, classified in 1% acid alcohol for 10 seconds, washed with running tap water for 1 minute, blued in 0.2% ammonia solution for 30 seconds, washed in running tap water for 3 minutes, rinsed in 95% alcohol, 10 dips, counterstained in eosin Y solution for 45 seconds, dehydrated through 95% alcohol, 2 changes of absolute alcohol, 10 dips each, cleared in 2 changes of xylene, 10 dips each, mounted with xylene based mounting medium. Sections were examined by Olympus BX51 microscopy.
2.7 Statistical Analysis
All values are reported as the mean ± SD. Analysis of luciferase activity was completed using a 2-tailed Student’s t-test when comparing two groups. Statistical significance was assumed at p<0.05. All statistical analysis was completed using the SigmaPlot 10.0.
3. Results
3.1 The level and duration of gene expression were significantly increased by intradermal DNA injection and non-invasive skin EP
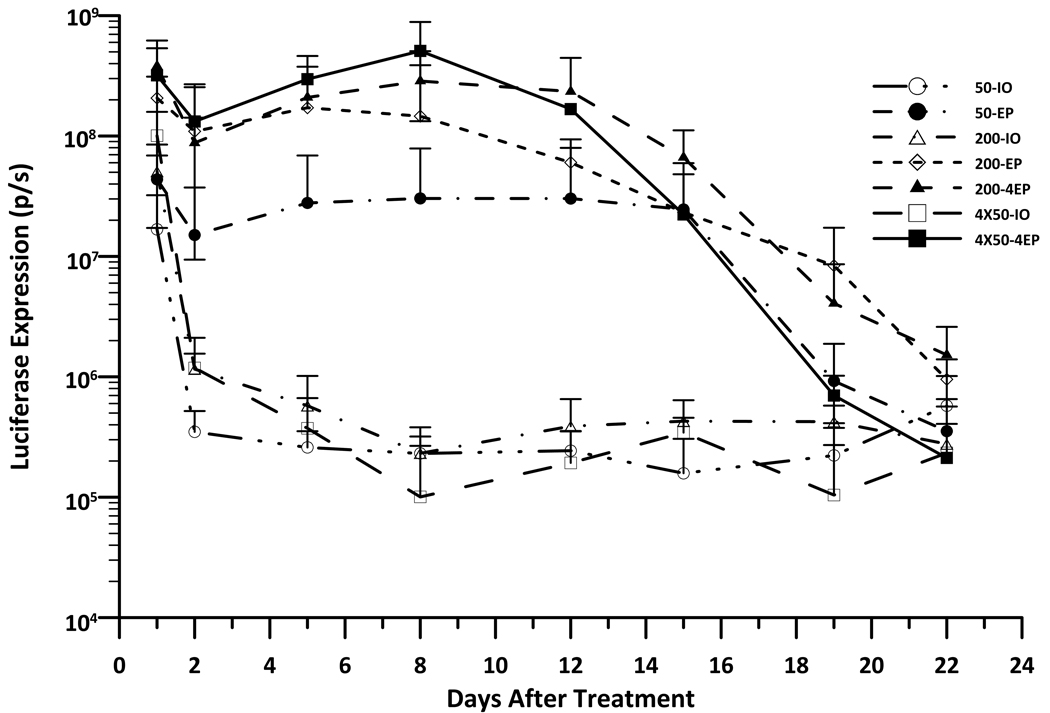
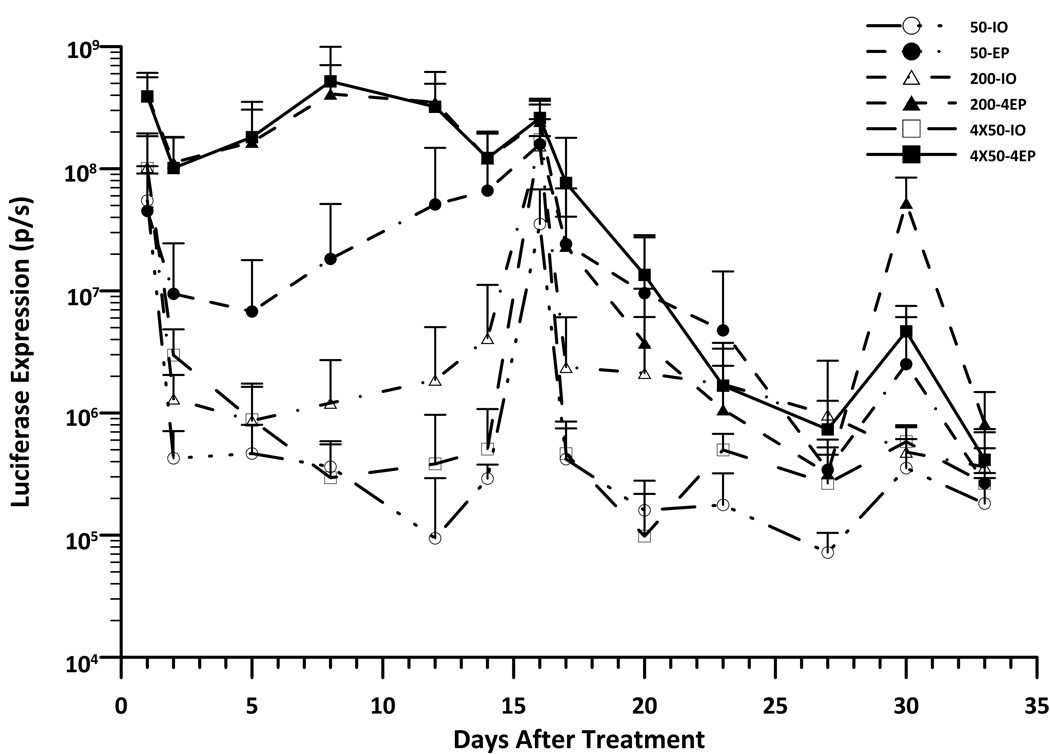
The correlation between the level and duration of gene expression to the size of the treated area when delivering by EP with the MEA was evaluated by in vivo bioimaging. As shown in Fig 1A, the maximum level of luciferase expression was achieved one day after delivery. While expression in the non-electroporated sites decreased dramatically by day 2 the expression of EP-treated sites was stable until day 15. The average levels of gene expression in the EP-treated groups were 2 to 3 logs higher than in the non-EP-treated groups from days 2 to 15. Among the different EP-treated groups, luciferase expression increased 3.7 to 6.3 fold in 200µL DNA with one EP application compared to 50µL DNA with one EP application from day 1 to 8 after delivery. However, the skin receiving 200µL DNA and four EP applications expressed the highest level of protein with a 4.5 to 15.8 fold increase in expression compared to 50µL DNA with one EP application from day 1 to day 12 (P< 0.05 for the most time points). (Table S1). At day 22 after delivery, the luciferase expression of EP-treated skin decreased to the level of DNA injection only, both of which were still slightly increased as compared to background. Given these findings, we wanted to address whether we could achieve long-term gene expression by repeated deliveries with MEA EP delivery. Based on the previously stated results, an one-time delivery would result in maximum gene expression within 24 hours and would remain relatively constant through day 15. Therefore, we aimed to attempt three deliveries at the same site and to produce longer-term expression. The delivery time points were selected to be day 0, day 15 and day 29. Our results from these experiments indicated that subsequent deliveries could not increase or even match gene expression of initial levels nor could it enhance the duration of the expression beyond the initial delivery time frame (Fig. 1B). While in all samples both EP and the plasmid injection only control had similar luciferase expression at one day post second delivery, the expression rapidly decreased and reached background levels by day 12 after the second delivery (Day 27). For the third delivery, both non-EP and EP treated sites could not reach high expression. The gene expression of all sites very rapidly dropped to the background level by day 4 after the third delivery (Day 33). The study was performed twice and reached the same conclusion.
Figure 1. Kinetic of gene expression in skin after i.d. DNA (gWiz-Luciferase) injection and non-invasive EP.
Delivery groups, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site. A, Time course of luciferase expression in guinea pig skin after 1 time delivery at d0. Bars represent mean ± SD. 4–5 sites were analyzed for each delivery. B, Time course of luciferase expression in guinea pig skin with 3 time deliverys, separately at d0, d15 and d29. Bars represent mean ± SD. 5–6 sites were analyzed for each delivery. p/s = photons/second.
3.2 Gene expression by skin EP delivery with the MEA was exclusively in the epidermal layer of the skin
Flurorescence stereoscopy and microscopy was used to observe the distribution of the gene transfected cells in the guinea pig skin after i.d. DNA injection and EP. Using flurorescence stereoscopy, no expression was observed in either the non-EP or EP treated sites at 1 hour post-delivery. However, green florescence protein (GFP) expression of non-EP skin was present at day 1, decreased rapidly to scattered dots by day 2, and no expression was observed by day 7 or 9(Fig. 2A, 50µL-IO). In the EP-treated skin, GFP expressing areas were larger than those of non-EP controls and the flurorescence intensity was maintained at similar levels till day 7 (Fig. 2A, 50µL-1EP or 200µL-4EP). At day 9, very few flurorescence-bright dots were observed in EP-treated skin. No fluorescence was observed in non-treated controls.
Figure 2. Distribution of gene-expressing cells after i.d. DNA (gWiz-GFP) injection and non-invasive EP.
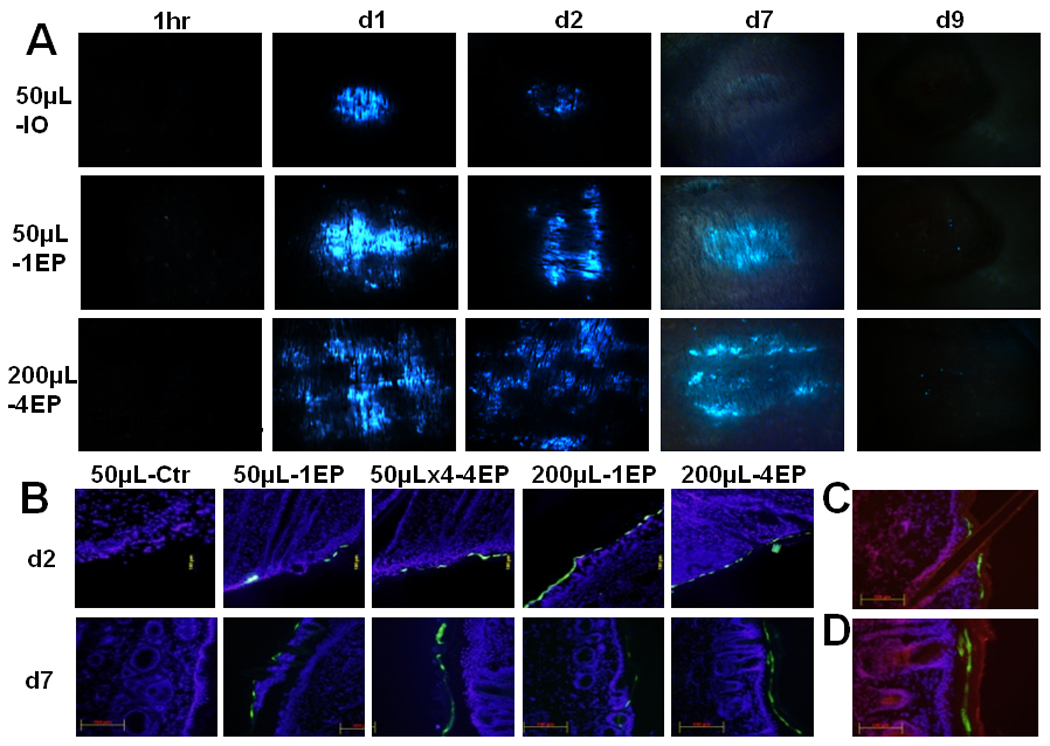
Skin samples were collected post-delivery, 1hour, day 1, day 2, day 7 or day 9. Samples were analyzed by immunoflurescence microscopy. Delivery group, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 50µLx4-4EP: 4 injection of 50µL DNA and each EP on the injection site; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs. A, One representative picture of 3 treated sites. (B, C, D) Total 6 cryosections (2 sections per sample) of each delivery were analyzed. Cell nuclei were blue-stained by DAPI. GFP-expressing cells were shown green. (C, D) Cell nuclei and stratum corneum was shown red-stained by propidium iodide. B, One representative section of each delivery was presented for post-delivery day 2 and day 7 (magnification = 100, scale bar=100µm). C, One representative section from post-delivery day 2 (magnification = 200). D, One representative section from post-delivery day 7 (magnification = 200, scale bar=100µm).
To visualize the localization of gene expressing cells after non-invasive surface EP, cross-sections of the skin were labeled with DAPI and PI for flurorescence microscopy observation. Surprisingly, almost all GFP expressing cells from EP-treated skin were located in the epidermal layer at day 2 or day 7 (Fig. 2B). Gene-expressing cells at day 2 were cells with nuclei beneath the stratum corneal layer of the epidermis but by day 7 those GFP expressing cells had lost their nuclei and moved into the stratum corneum. For DNA injection alone, no expression was observed in the epidermal layer of skin at either day 2 or day 7 (Fig. 2C, 2D). Skin receiving plasmid injection only expressed the luciferase and GFP transgenes one day after delivery (Fig. 1 and Fig. 2A). GFP expressing cells were observed in the dermis for both DNA injection only and EP delivery groups after one day (Fig. S1). These transgene expressing cells were scattered in the areas surrounding the DNA injection site and occasionally were seen close to the epidermal layer. However, no expression was found in the epidermis for the DNA injection alone while GFP expression was observed there for the skin treated with EP after delivery day 1(Fig. S1).
3.3 Skin damage caused by noninvasive electroporation using MEA was limited and completely recoverable
For potential clinical applications, any skin damage including significant infiltration, necrosis and scar formation would limit the therapeutic applications of the MEA. Under our parameters for EP, no severe tissue damage, such as skin burning, ulceration or scar formation, was found from gross observation (Fig. 3A). Skin redness and prints of the MEA array did occur after EP delivery but were not present by day 5. Some hair loss was noted in the area of EP application. However, the hair loss was transient and hair grew back within one week after the delivery. Damage was also assessed histologically by hematoxylin and eosin (H & E) staining. In contrast to DNA injection alone, which did not present with any damage, focal cell vacuolization or degeneration in the epidermal layer was observed for all EP-treated skin (Fig. 3B). By day 7, this cell vacuolization was no longer present. Notably, most epidermal cells were morphologically normal after EP delivery. The statistically significant infiltration and necrosis, which were seen in the epidermal or dermal layer in our previous study with the 4 plate electrode[25], was not observed in this study.
Figure 3. Gross observation and histology of skin after i.d. DNA injection and non-invasive EP.
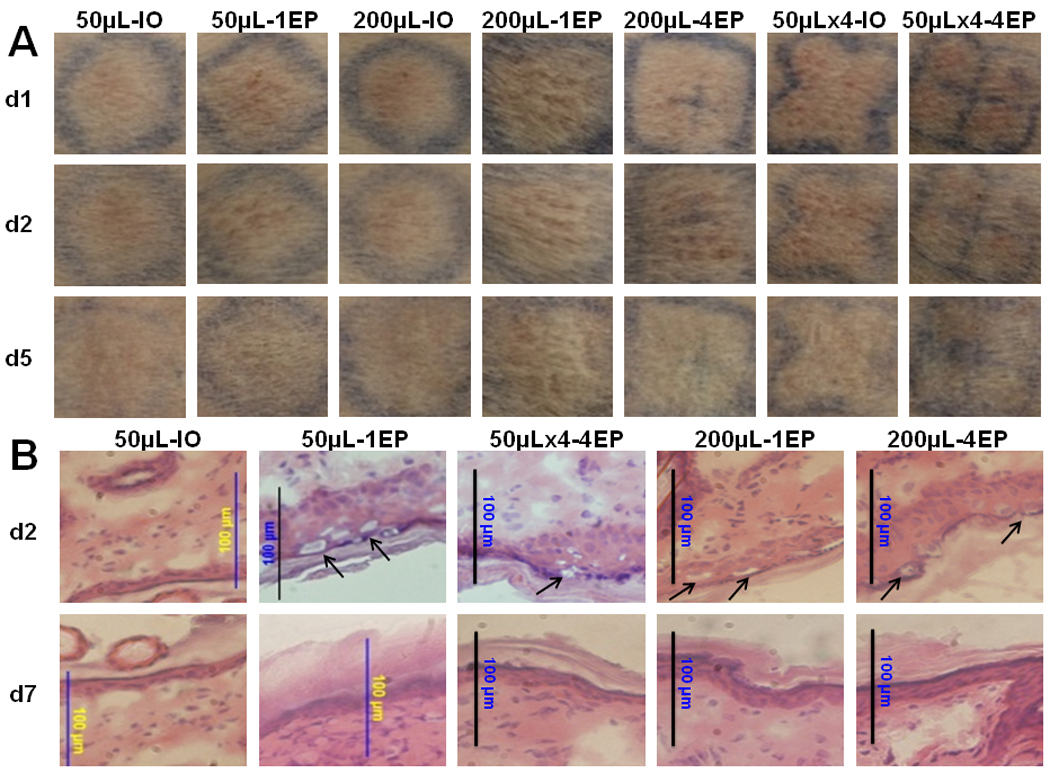
A, Skin observation after delivery. Pictures were taken at post-delivery day 1, day 2 and day 5. One representative picture of 4 to 5 sites was shown here. Delivery group, 50µL-IO: 50µL DNA without EP; 50µL- 1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site. B, Hematoxylin & eosin-stained skin samples. One representative of 3 treated sites was presented here for post delivery day 2 or day 7. Arrows are indicated the focal cell vacuolization. (magnification = 200, scale bar=100µm).
3.4 Skin EP with the MEA facilitated intradermal DNA diffusion into the epidermal direction
Although DNA was administered intradermally before EP, the transfected cells were exclusively indentified within the epidermis, not the dermis (Fig. 2). To elucidate the association between DNA distribution and gene expression, Fluorescein or Cy™3-labeled plasmid was administered either by i.d. injection alone or with EP using the MEA. The skin samples were harvested and analyzed by flurorescence stereoscopy one hour after delivery. While dense DNA-flurorescence with sharp margins was shown in injection alone samples (Fig. 4B, 50µL-IO), larger, dimmer peripheral DNA distribution was observed in the skin with EP delivery (Fig. 4C, 50µL-EP). Under flurorescence microscopy, DNA was distributed symmetrically from high concentration in the injection site to low concentration at both peripherial areas in the dermis (Fig. 4D). There was no labeled DNA which appeared close to the epidermis after DNA i.d. injection alone (Fig. 4D). However, EP changed this pattern. The relative scattered and spread distribution was seen from injection site to the epidermal direction. A few labeled DNA spots were observed in the epidermis (Fig. 4E).
Figure 4. DNA distribution in the skin after i.d. DNA injection and non-invasive EP.
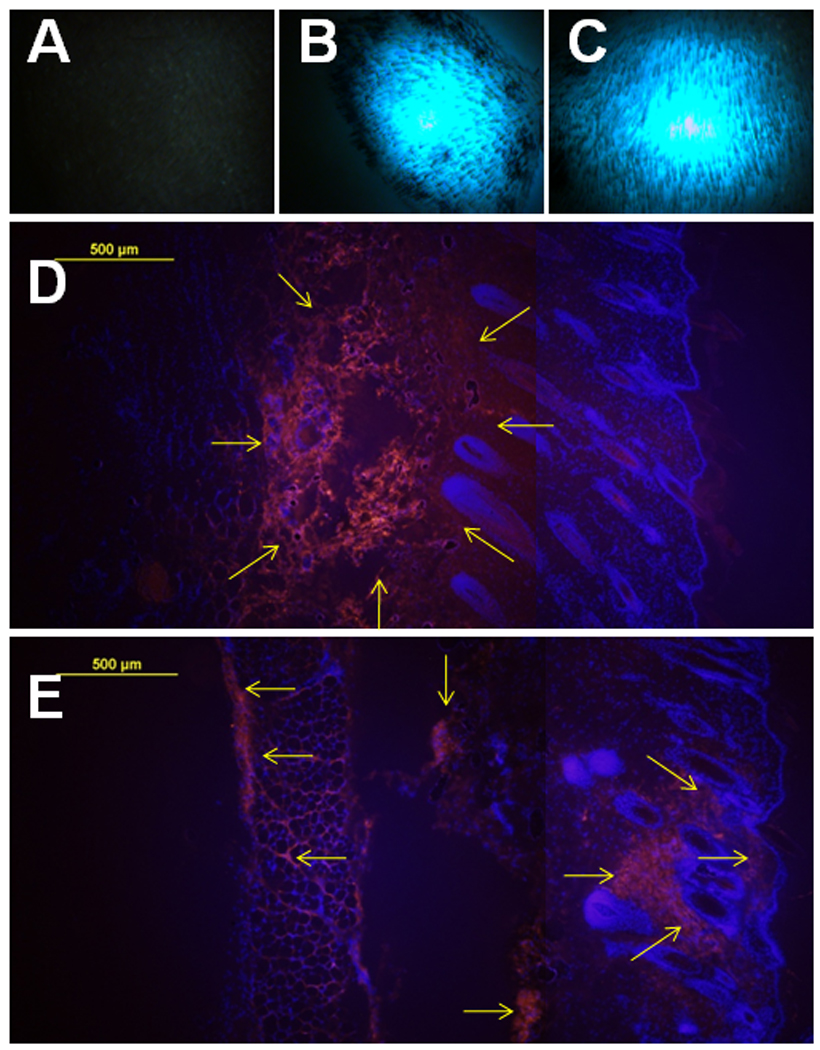
A, B, C, Skin observation by flurorescence stereoscope after delivery with Fluorescein-labeled plasmid. Pictures were taken at post-delivery 1 hour. One representative picture of 2 sites was shown here. Delivery group: A, control; B, 50µL-IO: 50µL DNA without EP; C, 50µL-1EP: 50µL DNA with 1 EP on the injection site. D, E, total 4 cryosections (2 sections per sample) of each delivery were analyzed. Cell nuclei were blue-stained by DAPI. Cy™3-labeled DNA was shown red as indicated by arrows. D, One representative section of 50µL-IO was presented. E, One representative section of 50µL-EP was presented (magnification = 100, scale bar=100µm).
4. Discussion
While many studies focus on the application of skin EP for superficial cancers [6, 39], a few studies have demonstrated that significant serum levels of products could be obtained by EP gene transfer to skin[24, 31, 34]. Considering the easy access and large area of the skin, the expression level could be potentially increased by increasing the area treated to achieve the effective protein concentration in serum. Indeed, luciferase expression could be significantly enhanced by increasing the delivery area. Here we demonstrated that local protein expression levels can be increased by an average 7.8 fold (d1 to d12, p<0.01) by quadrupling the size of the treated area (200µL-4EP compared to 50µL-1EP). It could, however, be interpreted as marginal electric field effect because four pulse deliveries were applied adjacently. The marginal areas were exposed to repeated electrical field, so more cells could have been transfected and/or more DNA transferred into the same cells. To achieve more protein product locally or systemically, we can simply apply multiple injections and pulse deliveries or expand the MEA without any change of EP parameters, for example the current 4×4 array electrodes could be expanded to a 7×7 array to assure a 4-fold increase of size.
One of the critical aspects for skin EP is the duration of expression after electrogene transfer. The kinetics of luciferase expression in mice has been studied by several groups[24–26, 40–42]. A significant increase in gene expression was obtained by skin EP with plate electrodes in two weeks[24–26, 40]. Different expression patterns were reported, which may be due to different electrodes and/or parameters of EP chosen by the different groups. EP with needle electrodes showed increased expression for longer than 3 weeks[41, 42], most likely because needles can achieve deeper penetration of electrical field or may facilitate DNA diffusion from the injection site into the adjacent dermis or even muscle layers[42, 43]. Interestingly, in guinea pig, luciferase expression in the epidermis reached the first peak at day 1, then slightly dropped at day 2 and slowly reached the second peak at day 8. The significant expression after EP can last up to 15 days. If EP delivery method targets to epidermal layer of the skin as in this study, the duration of transgenic expression very likely depends on the epidermal turn over.
Multiple EP treatment applications were often utilized to treat cancer in animal models or clinical trials[25, 32, 34, 35]. In this study, multiple deliveries were designed to achieve long-term expression and assess the feasibility of skin EP for protein replacement. Unfortunately, luciferase expression patterns after the second and third deliveries were shown to be completely different as compared to the first delivery. No definite interval of high expression was observed after the second and third deliveries. The presence of anti-luciferase IgG antibodies was discovered in the guinea pig serum after three EP deliveries and is most likely the cause of the change in expression patterns (Fig. S1). Vandermeulen et al also demonstrated that high titers of anti-luciferase IgG antibody were induced by multiple intra-pinna electroporations (one priming and two boosts) in mice[44]. These results indicate that since luciferase is an exogenous protein capable of eliciting an immune response, it is not a good reporter for multiple deliveries or long term expression studies in guinea pigs. On the other hand, the capability to induce an immune reaction to a weak antigen by skin EP is helpful for researchers to design an effective vaccination against infectious diseases or cancer [10, 44–51].
The distribution of transfected cells by EP is dependent on both the skin differences between the animals as well as the electrodes employed. Our results show that uniform epidermal expression in guinea pig skin can be obtained by EP with the MEA. The study of intradermal DNA EP with the caliper electrode demonstrated that the transfected cells were present at the dermis in mouse while at the epidermis in xenograft human skin[40, 44]. Moreover, EP with tweezer electrodes resulted in transgenic expression in the lower dermal region of rabbit skin[52]. However, EP with needle array electrodes could result in transfected cells in the dermis, epidermis, hypodermis even around the muscle layer, but mainly in the panniculus carnosus muscle layer of the mice[42, 43] or dermis of the pig[53]. For plate electrodes, the electrical field went through all layers of skin between the two plates[54]. For the needle electrodes, the electrical field was confined between the two (array) needles in the skin[54]. However, the electric field generated by the MEA is designed to decrease the depth of penetration thereby reducing muscle contraction. We observed significantly reduced muscle twitching when using the MEA as compared to the 4 plate electrodes or needle electrodes.
It is necessary to point out that non-invasive electrodes such as plates and the MEA do not directly affect DNA distribution after i.d. administration. On the other hand, the needle electrodes may penetrate the injection site and facilitate DNA diffusion into the surrounding area. This is a potential explanation for the spread of expression usually observed by EP with needle arrays[42, 53]. The histological characterization of skin also plays a role in the distribution of transgenic expression. With the same plate electrodes or i.d. DNA injection only, both Zhang’s and Hengge’s groups demonstrated that gene expressing cells in the dermis for mouse skin but in the epidermis for xenografted human skin[40, 55]. The epidermal expression in guinea pig by the MEA may also be associated with its similarity to human skin structure[36, 37].
Consistent with our previous report[27], EP with the MEA could greatly reduce the adverse effects of needle or plate electrodes while comparable or higher expression levels were achieved. Minimal skin damage was observed grossly as well as histologically and complete recovery after EP was observed. Tissue damage such as the dermal necrosis or burning seen in previous studies done by our group[25] and others[56] was not observed in this study. When multiple deliveries with the MEA were applied to the same sites, skin redness and hair loss was slightly increased for both DNA injection alone and EP, but completely healed by day 5 (Fig. S2). These results were consistent with our previous finding in mice where skin damage was increased by repeated gene delivery with plate electrodes[26]. Both studies suggest that repeated application of EP pulses at the same site should be avoided.
Based on the DNA distribution and gene expression we can see there are two types of expression for non-invasive EP skin delivery with the MEA in guinea pigs. One is local expression around DNA injection site with the duration of 1–2 days. Another is epidermal expression distant from DNA injection site with the duration of 15 days. The first pattern is obviously independent of EP because it occurred in both DNA injection alone and EP treated locations (Fig. 1a, 1b and 2a). The latter pattern is specifically related to MEA EP because it did not occur with DNA injection alone. The two patterns of transgenic expression may explain why the luciferase expression with EP dropped slightly at day 2. It is possible that Day 1 expression with EP included the component related to non-EP dependent expression and that waned rapidly. Further histological analysis of DNA distribution (Fig. 4D. 4E) and gene expression location (Fig. 2B and S3) demonstrated that MEA EP first facilitates DNA diffusion from the dermal layer into the epidermal layer and then electrotransfer of DNA into epidermal cells.
5. Conclusion
Efficient gene delivery can be obtained by skin electroporation with a non-invasive multielectrode array. The high expression can be maintained for up to 15 days after single skin EP with MEA. The gene expression level can be easily multiplied by increasing the delivery area without any change of EP parameters. Skin EP with MEA was found to target the epidermal cells for gene transfer. In contrast to plate electrodes, skin EP with MEA significantly reduced muscle twitching and resulted in minimal and completely recoverable skin damage. However, multiple EPs with MEA are not recommended to apply in the same site because of the potential of skin damage. Further studies will focus on whether we can translate these findings into vaccination, cancer immunogene therapy or long-term endogenous gene expression for protein deficiencies.
Supplementary Material
Note: *1, Delivery groups, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site. *2, Bold number indicates p<0.05 or 0.01 by Student’s t-test.
Guinea pig sera were collected before and after DNA EP treatments. ELISA plate was coated with 20 µg/ml luciferase protein overnight. 1:100 diluted samples were used to detect Ab level by HRP-anti-GP IgG secondary Abs. GP107 to 112, individual animal was treated with DNA w/o EP(s). Number in the figure indicates mean value of all 6 guinea pigs.
Skin observation after treatment. Pictures were taken at post-2nd treatment day 1, day 2 and day 5. One representative picture of 4 to 5 sites was shown here. Treatment group, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site.
Skin samples were collected post-treatment day 1. Samples were analyzed by immunoflurescence microscopy. Treatment group, 50µL-IO: 50µL DNA injection alone; 50µL-EP: 50µL DNA with 1 EP on the injection site. Total 6 cryosections (2 sections per sample) of each treatment were analyzed. Cell nuclei were blue-stained by DAPI. GFP-expressing cells were green and indicated by arrows. One representative picture of 3 treated sites. A, C for 50µL-IO. A, epidermis and dermis. C, dermis. B, D for 50µL-EP. B, epidermis and dermis. D, dermis. (magnification = 40, scale bar=500µm).
Acknowledgements
This research was supported in part by a research grant from the National Institutes of Health R01 EB005441 and by the Frank Reidy Research Center for Bioelectrics at Old Dominion University.
The authors would like to thank Lifang Yang for her help in the cryosection preparation and H&E staining, and Division of Pharmacology of Department of Physiological Sciences at East Virginia Medical School for providing Microm HM 505E Cryostat.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was done at Frank Reidy Research Center for Bioelectrics, Old Dominion University, Norfolk, Virginia, USA, 23508
Conflict of interest
With respect to duality of interest and financial disclosures, Dr. R. Heller is an inventor on patents which cover the technology that was used in the work reported in this manuscript. In addition, Dr. R. Heller owns stock and stock options in Inovio Pharmaceutical Corporation and has an ownership interest in RMR Technologies.
References
- 1.Heller R, Jaroszeski M, Atkin A, Moradpour D, Gilbert R, Wands J, Nicolau C. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996;389(3):225–228. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- 2.Nishi T, Yoshizato K, Yamashiro S, Takeshima H, Sato K, Hamada K, Kitamura I, Yoshimura T, Saya H, Kuratsu J, Ushio Y. High-efficiency in vivo gene transfer using intraarterial plasmid DNA injection following in vivo electroporation. Cancer Res. 1996;56(5):1050–1055. [PubMed] [Google Scholar]
- 3.Sugimura K, Harimoto K, Kishimoto T. [In vivo gene transfer methods into bladder without viral vectors] Hinyokika Kiyo. 1997;43(11):823–827. [PubMed] [Google Scholar]
- 4.Rols MP, Delteil C, Golzio M, Dumond P, Cros S, Teissie J. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat Biotechnol. 1998;16(2):168–171. doi: 10.1038/nbt0298-168. [DOI] [PubMed] [Google Scholar]
- 5.Mir LM. Nucleic acids electrotransfer-based gene therapy (electrogenetherapy): past, current, and future. Mol Biotechnol. 2009;43(2):167–176. doi: 10.1007/s12033-009-9192-6. [DOI] [PubMed] [Google Scholar]
- 6.Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Ther. 2006;17(9):890–897. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 7.Rabussay D. Applicator and electrode design for in vivo DNA delivery by electroporation. Methods Mol Biol. 2008;423:35–59. doi: 10.1007/978-1-59745-194-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Cemazar M, Golzio M, Sersa G, Rols MP, Teissie J. Electrically-assisted nucleic acids delivery to tissues in vivo: where do we stand? Curr Pharm Des. 2006;12(29):3817–3825. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- 9.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 10.Daugimont L, Baron N, Vandermeulen G, Pavselj N, Miklavcic D, Jullien MC, Cabodevila G, Mir LM, Preat V. Hollow microneedle arrays for intradermal drug delivery and DNA electroporation. J Membr Biol. 2010;236(1):117–125. doi: 10.1007/s00232-010-9283-0. [DOI] [PubMed] [Google Scholar]
- 11.Gothelf A, Gehl J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr Gene Ther. 2010;10(4):287–299. doi: 10.2174/156652310791823443. [DOI] [PubMed] [Google Scholar]
- 12.Mir LM, Moller PH, Andre F, Gehl J. Electric pulse-mediated gene delivery to various animal tissues. Adv Genet. 2005;54:83–114. doi: 10.1016/S0065-2660(05)54005-7. [DOI] [PubMed] [Google Scholar]
- 13.Glasspool-Malone J, Somiari S, Drabick JJ, Malone RW. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2(2):140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- 14.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16(9):867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 15.Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103(4):865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa H, Takano M, Matsumoto N, Sawada H, Ide C, Mimura O, Dezawa M. Effect of GDNF gene transfer into axotomized retinal ganglion cells using in vivo electroporation with a contact lens-type electrode. Gene Ther. 2005;12(4):289–298. doi: 10.1038/sj.gt.3302277. [DOI] [PubMed] [Google Scholar]
- 17.Dean DA. Electroporation of the vasculature and the lung. DNA Cell Biol. 2003;22(12):797–806. doi: 10.1089/104454903322625000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tupin E, Poirier B, Bureau MF, Khallou-Laschet J, Vranckx R, Caligiuri G, Gaston AT, Duong Van Huyen JP, Scherman D, Bariety J, Michel JB, Nicoletti A. Non-viral gene transfer of murine spleen cells achieved by in vivo electroporation. Gene Ther. 2003;10(7):569–579. doi: 10.1038/sj.gt.3301914. [DOI] [PubMed] [Google Scholar]
- 19.Terada Y, Hanada S, Nakao A, Kuwahara M, Sasaki S, Marumo F. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney. Kidney Int. 2002;61(1 Suppl):S94–S98. doi: 10.1046/j.1523-1755.2002.0610s1094.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Iwashita H, Otani M, Masunaga K, Inadome A. Delivery of DNA into bladder via electroporation. Methods Mol Biol. 2008;423:249–257. doi: 10.1007/978-1-59745-194-9_18. [DOI] [PubMed] [Google Scholar]
- 21.Muramatsu T, Shibata O, Ryoki S, Ohmori Y, Okumura J. Foreign gene expression in the mouse testis by localized in vivo gene transfer. Biochem Biophys Res Commun. 1997;233(1):45–49. doi: 10.1006/bbrc.1997.6361. [DOI] [PubMed] [Google Scholar]
- 22.Martin JB, Young JL, Benoit JN, Dean DA. Gene transfer to intact mesenteric arteries by electroporation. J Vasc Res. 2000;37(5):372–380. doi: 10.1159/000025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maricq HR, Darke CS, Archibald RM, Leroy EC. In vivo observations of skin capillaries in workers exposed to vinyl chloride. An English-American comparison. Br J Ind Med. 1978;35(1):1–7. doi: 10.1136/oem.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gothelf A, Eriksen J, Hojman P, Gehl J. Duration and level of transgene expression after gene electrotransfer to skin in mice. Gene Ther. 2010;17(7):839–845. doi: 10.1038/gt.2010.35. [DOI] [PubMed] [Google Scholar]
- 25.Heller LC, Jaroszeski MJ, Coppola D, McCray AN, Hickey J, Heller R. Optimization of cutaneous electrically mediated plasmid DNA delivery using novel electrode. Gene Ther. 2007;14(3):275–280. doi: 10.1038/sj.gt.3302867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heller LC, Jaroszeski MJ, Coppola D, Heller R. Comparison of electrically mediated and liposome-complexed plasmid DNA delivery to the skin. Genet Vaccines Ther. 2008;6:16. doi: 10.1186/1479-0556-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heller R, Cruz Y, Heller LC, Gilbert RA, Jaroszeski MJ. Electrically mediated delivery of plasmid DNA to the skin, using a multielectrode array. Hum Gene Ther. 2010;21(3):357–362. doi: 10.1089/hum.2009.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraro B, Cruz YL, Baldwin M, Coppola D, Heller R. Increased perfusion and angiogenesis in a hindlimb ischemia model with plasmid FGF-2 delivered by noninvasive electroporation. Gene Ther. 2010;17(6):763–769. doi: 10.1038/gt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009;17(4):651–657. doi: 10.1038/mt.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee PY, Chesnoy S, Huang L. Electroporatic delivery of TGF-beta1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Invest Dermatol. 2004;123(4):791–798. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 2001;8(23):1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- 32.Lucas ML, Heller L, Coppola D, Heller R. IL-12 plasmid delivery by in vivo electroporation for the successful treatment of established subcutaneous B16.F10 melanoma. Mol Ther. 2002;5(6):668–675. doi: 10.1006/mthe.2002.0601. [DOI] [PubMed] [Google Scholar]
- 33.Gothelf A, Hojman P, Gehl J. Therapeutic levels of erythropoietin (EPO) achieved after gene electrotransfer to skin in mice. Gene Ther. 2010;17(9):1077–1084. doi: 10.1038/gt.2010.46. [DOI] [PubMed] [Google Scholar]
- 34.Lucas ML, Heller R. IL-12 gene therapy using an electrically mediated nonviral approach reduces metastatic growth of melanoma. DNA Cell Biol. 2003;22(12):755–763. doi: 10.1089/104454903322624966. [DOI] [PubMed] [Google Scholar]
- 35.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, Heller R. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. 2008;26(36):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mershon MM, Mitcheltree LW, Petrali JP, Braue EH, Wade JV. Hairless guinea pig bioassay model for vesicant vapor exposures. Fundam Appl Toxicol. 1990;15(3):622–630. doi: 10.1016/0272-0590(90)90046-m. [DOI] [PubMed] [Google Scholar]
- 37.Sueki H, Gammal C, Kudoh K, Kligman AM. Hairless guinea pig skin: anatomical basis for studies of cutaneous biology. Eur J Dermatol. 2000;10(5):357–364. [PubMed] [Google Scholar]
- 38.Ferraro B, Heller LC, Cruz YL, Guo S, Donate A, Heller R. Evaluation of delivery conditions for cutaneous plasmid electrotransfer using a multielectrode array. Gene Ther. 2010 doi: 10.1038/gt.2010.171. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heller LC, Heller R. Electroporation gene therapy preclinical and clinical trials for melanoma. Curr Gene Ther. 2010;10(4):312–317. doi: 10.2174/156652310791823489. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Nolan E, Kreitschitz S, Rabussay DP. Enhanced delivery of naked DNA to the skin by non-invasive in vivo electroporation. Biochim Biophys Acta. 2002;1572(1):1–9. doi: 10.1016/s0304-4165(02)00270-2. [DOI] [PubMed] [Google Scholar]
- 41.Byrnes CK, Malone RW, Akhter N, Nass PH, Wetterwald A, Cecchini MG, Duncan MD, Harmon JW. Electroporation enhances transfection efficiency in murine cutaneous wounds. Wound Repair Regen. 2004;12(4):397–403. doi: 10.1111/j.1067-1927.2004.012409.x. [DOI] [PubMed] [Google Scholar]
- 42.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4(9):e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Z, Wu X, Song N, Cao Y, Liu W. Electroporation-mediated plasmid gene transfer in rat incisional wound. J Dermatol Sci. 2007;47(2):161–164. doi: 10.1016/j.jdermsci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Vandermeulen G, Richiardi H, Escriou V, Ni J, Fournier P, Schirrmacher V, Scherman D, Preat V. Skin-specific promoters for genetic immunisation by DNA electroporation. Vaccine. 2009;27(32):4272–4277. doi: 10.1016/j.vaccine.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Roos AK, Moreno S, Leder C, Pavlenko M, King A, Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13(2):320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25(10):1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandermeulen G, Staes E, Vanderhaeghen ML, Bureau MF, Scherman D, Preat V. Optimisation of intradermal DNA electrotransfer for immunisation. J Control Release. 2007;124(1–2):81–87. doi: 10.1016/j.jconrel.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Roos AK, Eriksson F, Walters DC, Pisa P, King AD. Optimization of skin electroporation in mice to increase tolerability of DNA vaccine delivery to patients. Mol Ther. 2009;17(9):1637–1642. doi: 10.1038/mt.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brave A, Gudmundsdotter L, Sandstrom E, Haller BK, Hallengard D, Maltais AK, King AD, Stout RR, Blomberg P, Hoglund U, Hejdeman B, Biberfeld G, Wahren B. Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation. Vaccine. 2010;28(51):8203–8209. doi: 10.1016/j.vaccine.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brave A, Nystrom S, Roos AK, Applequist SE. Plasmid DNA vaccination using skin electroporation promotes poly-functional CD4 T-cell responses. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.109. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51.Hirao LA, Draghia-Akli R, Prigge JT, Yang M, Satishchandran A, Wu L, Hammarlund E, Khan AS, Babas T, Rhodes L, Silvera P, Slifka M, Sardesai NY, Weiner DB. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J Infect Dis. 2011;203(1):95–102. doi: 10.1093/infdis/jiq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Medi BM, Hoselton S, Marepalli RB, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits. I: efficacy. Int J Pharm. 2005;294(1–2):53–63. doi: 10.1016/j.ijpharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Drabick JJ, Glasspool-Malone J, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001;3(2):249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 54.Corovic S, Pavlin M, Miklavcic D. Analytical and numerical quantification and comparison of the local electric field in the tissue for different electrode configurations. Biomed Eng Online. 2007;6:37. doi: 10.1186/1475-925X-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hengge UR, Walker PS, Vogel JC. Expression of naked DNA in human, pig, and mouse skin. J Clin Invest. 1996;97(12):2911–2916. doi: 10.1172/JCI118750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babiuk LA, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21(7–8):649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: *1, Delivery groups, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site. *2, Bold number indicates p<0.05 or 0.01 by Student’s t-test.
Guinea pig sera were collected before and after DNA EP treatments. ELISA plate was coated with 20 µg/ml luciferase protein overnight. 1:100 diluted samples were used to detect Ab level by HRP-anti-GP IgG secondary Abs. GP107 to 112, individual animal was treated with DNA w/o EP(s). Number in the figure indicates mean value of all 6 guinea pigs.
Skin observation after treatment. Pictures were taken at post-2nd treatment day 1, day 2 and day 5. One representative picture of 4 to 5 sites was shown here. Treatment group, 50µL-IO: 50µL DNA without EP; 50µL-1EP: 50µL DNA with 1 EP on the injection site; 200µL-IO: 200µL DNA without EP; 200µL-1EP: 200µL DNA and 1 EP; 200µL-4EP: 200µL DNA and 4 EPs; 50µLx4-IO: 4 injections with 50µL DNA without EP; 50µLx4-4EP: 4 injections with 50µL DNA and each EP on the injection site.
Skin samples were collected post-treatment day 1. Samples were analyzed by immunoflurescence microscopy. Treatment group, 50µL-IO: 50µL DNA injection alone; 50µL-EP: 50µL DNA with 1 EP on the injection site. Total 6 cryosections (2 sections per sample) of each treatment were analyzed. Cell nuclei were blue-stained by DAPI. GFP-expressing cells were green and indicated by arrows. One representative picture of 3 treated sites. A, C for 50µL-IO. A, epidermis and dermis. C, dermis. B, D for 50µL-EP. B, epidermis and dermis. D, dermis. (magnification = 40, scale bar=500µm).