Abstract
Background
Chronic insomnia is a common health problem with substantial consequences in older adults. Cognitive behavioral treatments are efficacious but not widely available. The aim of this study was to test the efficacy of brief behavioral treatment for insomnia (BBTI) vs an information control (IC) condition.
Methods
A total of 79 older adults (mean age, 71.7 years; 54 women [70%]) with chronic insomnia and common comorbidities were recruited from the community and 1 primary care clinic. Participants were randomly assigned to either BBTI, consisting of individualized behavioral instructions delivered in 2 intervention sessions and 2 telephone calls, or IC, consisting of printed educational material. Both interventions were delivered by a nurse clinician. The primary outcome was categorically defined treatment response at 4 weeks, based on sleep questionnaires and diaries. Secondary outcomes included self-report symptom and health measures, sleep diaries, actigraphy, and polysomnography.
Results
Categorically defined response (67% [n=26] vs 25% [n=10]; χ2=13.8) (P<.001) and the proportion of participants without insomnia (55% [n=21] vs 13% [n=5]; χ2=15.5) (P<.001) were significantly higher for BBTI than for IC. The number needed to treat was 2.4 for each outcome. No differential effects were found for subgroups according to hypnotic or antidepressant use, sleep apnea, or recruitment source. The BBTI produced significantly better outcomes in self-reported sleep and health (group × time interaction, F5,73=5.99, P<.001), sleep diary (F8,70= 4.32, P<.001), and actigraphy (F4,74=17.72, P<.001), but not polysomnography. Improvements were maintained at 6months.
Conclusion
We found that BBTI is a simple, efficacious, and durable intervention for chronic insomnia in older adults that has potential for dissemination across medical settings.
Insomnia is defined by difficulty falling asleep, difficulty staying asleep, nonrestorative sleep, and waking symptoms such as fatigue, impaired concentration, and mood disturbance.1,2 The prevalence of insomnia is approximately 5% to 20% in the general adult population3 and 20% to 30% in primary care medical settings. 4,5 Insomnia is commonly comorbid with physical and mental disorders6 and chronic, persisting for a year or longer in 74% of individuals.7 The health and functional consequences of insomnia include reduced quality of life, increased health care utilization and costs, disability, and risk for psychiatric disorders and cardiovascular disease.8,9 Insomnia is especially relevant for older adults, given its high prevalence (estimated at 15% –35%), persistence, and association with falls and hip fractures.3,10 Older adults are prescribed hypnotic agents disproportionately frequently and for disproportionately long-term use and are more likely than other populations to experience adverse drug effects.3,11–14
Pharmacologic and behavioral treatments for chronic insomnia have approximately equivalent efficacy.15,16 Each has specific merits and drawbacks. Hypnotic agents approved by the US Food and Drug Administration, including benzodiazepine receptor agonist (BZRA) drugs, are widely available, easy to use, and have rapid and sustained efficacy.15,17,18 However, BZRA safety concerns include dependence and abuse, cognitive impairment, and increased risk of falls and hip fractures, particularly in older adults.14,19,20 Behavioral and psychological techniques include sleep education, restriction of time in bed, stimulus control (strengthening associations between bed and sleep), and addressing anxiety-provoking beliefs about sleep.21 These treatments, particularly multicomponent cognitive behavioral therapy for insomnia (CBTI), are often preferred by patients22 and have consistent short-term and long-term efficacy23,24 with few apparent adverse effects. However, widespread use of CBTI is limited by the number of specialty-trained clinicians and by the duration, intensity, and initial cost of 6 to 8 individual treatment sessions. Moreover, most efficacy trials of pharmacologic and behavioral treatments have studied patients with primary insomnia and excluded the larger group of patients with substantial medical and psychiatric comorbidities, which includes many older adults.
The present study was conducted to test a behavioral treatment of insomnia that offers potential for widespread use. For a behavioral treatment to be relevant in general medical settings, it must be brief, acceptable to patients, deliverable by nurses or other allied health professionals, and efficacious over a short time interval in patients with typical comorbidities. We studied older adults because of the prevalence and consequences of insomnia and the potential safety benefits of a nondrug treatment in this group. The specific aim of this study was to test the short-term efficacy and 6-month durability of brief behavioral treatment for insomnia (BBTI) vs an information control (IC) intervention among older adults with insomnia.
METHODS
OVERVIEW
After recruitment, screening, and baseline assessments, participants were randomly assigned to BBTI or IC, and primary outcomes were assessed after 4 weeks. Outcomes included questionnaires, sleep diary, actigraphy, and polysomnography (PSG). The University of Pittsburgh Biomedical institutional review board approved the study. All participants provided written informed consent and were financially compensated. An earlier publication reported on a subset of self-reported outcomes in 35 participants.25
PARTICIPANTS
A convenience sample of 82 older adults with chronic insomnia was recruited from a single primary care practice (n=21) or from the community via advertisements (n=61). Participants met criteria for primary insomnia in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision) (DSM-IV-TR)2 and for general insomnia disorder in the International Classification of Sleep Disorders(Second Edition) (ICSD-2),1 verified by self-report questionnaires and structured clinician interviews. These criteria specify a sleep complaint lasting for at least 1 month; adequate opportunity and circumstances for sleep; and significant distress or daytime impairment. To enhance generalizability and clinical relevance, we did not apply quantitative self-report or objective sleep criteria for sleep latency, wakefulness, or total sleep time, or the DSM-IV-TRexclusion criteria for medical or psychiatric disorders. Therefore, our participants would be considered to have “comorbid insomnia.”26 Exclusion criteria were dementia; untreated psychiatric, substance use, or other sleep disorder; recent hospitalization; ongoing chemotherapy or other cancer treatment; and life expectancy less than 6 months. Individuals with treated depressive, anxiety, or sleep disorders were not excluded. Previously diagnosed and treated sleep apnea was not an exclusion, but the final sample did not include any such participants.
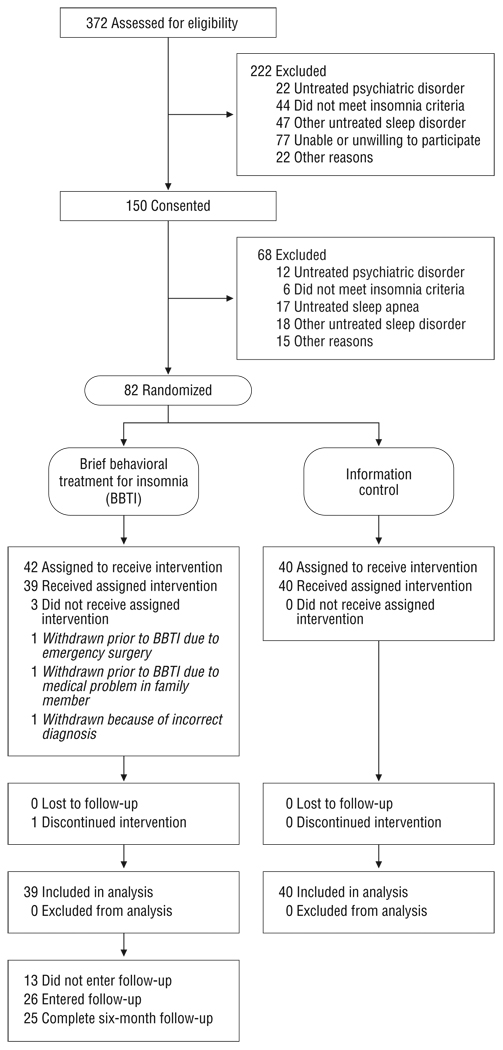
Potential subjects were evaluated with a telephone screening interview followed by in-person assessments. Sleep diaries; structured interviews; locally developed sleep, medical history, and medication surveys; and sleep and psychiatric symptom questionnaires were used for assessment and diagnosis. Figure 1 illustrates participant flow, and Table 1 summarizes pretreatment clinical characteristics.
Figure 1.
Study flowchart.
Table 1.
Baseline Demographic and Clinical Characteristicsa
| Characteristic | BBTI (n=39) |
IC (n=40) |
Statistical Findings | |
|---|---|---|---|---|
| Age, y | 72.5 (6.6) | 70.8 (7.8) | t77=1.04, P=.30 | |
| Female | 26 (67) | 28 (70) | , P=.75 | |
| White | 36 (92) | 38 (95) | Fisher exact, P=.68 | |
| Subjective socioeconomic status score (1, highest; 9, lowest) | 4.0 (1.6) | 3.4 (1.4) | t77=1.83, P=.07 | |
| Education | ||||
| ≤High school | 7 (18) | 7 (18) | Fisher exact, P=.96 | |
| Trade or technical school | 4 (10) | 3 (8) | ||
| College | 15 (38) | 18 (45) | ||
| Postgraduate | 13 (33) | 12 (30) | ||
| Medical and psychiatric status | ||||
| Chronic health conditions | 5.5 (2.6) | 5.5 (3.0) | t77=−0.06, P=.95 | |
| Current medicationsb | 5.3 (3.0) | 5.1 (3.1) | t77=0.30, P=.76 | |
| Currently taking sleep medication | 12 (31) | 17 (43) | , P=.28 | |
| Major depression (PHQ) | 0 | 0 | NA | |
| Other anxiety disorder (PHQ) | 1 (3) | 0 | NA | |
| Alcohol abuse (PHQ) | 0 | 0 | NA | |
| Currently taking antidepressant | 7 (18) | 2 (5) | Fisher exact, P=.09 | |
| Screening polysomnography | ||||
| Apnea-hypopnea indexc | 9.0 (5.4) (n=38) | 7.9 (5.5) (n=39) | t76=0.88, P=.38 | |
| Apnea-hypopnea index >5 | 28 of 39 (72) | 25 of 29 (64) | , P=.47 | |
| Desaturation indexd | 3.0 (3.3) (n=38) | 3.0 (3.3) (n=39) | t75=0.72, P=.47 | |
| Periodic limb movement arousal indexb | 6.0 (4.5) (n=38) | 5.6 (5.1) (n=39) | t75=0.51, P=.61 | |
Abbreviations BBTI, brief behavioral treatment for insomnia; IC, information control; NA, not applicable; PHQ, Patient Health Questionnaire.27
Unless otherwise indicated, data are reported as number (percentage) or mean (SD).
Square root (x+0.1) transformation used for statistical analysis.
Square root transformation used for statistical analysis.
Natural log (x+0.1) transformation used for statistical analysis.
INTERVENTIONS
Eligible participants were randomly assigned to BBTI or IC by permuted block design (block size, 4), stratified by age (≤75 vs >75 years) and sex. The BBTI consists of a 45- to 60-minute individual intervention session followed by a 30-minute follow-up session 2 weeks later and 20-minute telephone calls after 1 and 3 weeks (eAppendix; http://www.archinternmed.com). The BBTI emphasizes behavioral elements of insomnia treatment rather than the cognitive components present in CBTI. The BBTI includes sleep education and discussion of homeostatic and circadian mechanisms of human sleep regulation.28 This education provides the rationale for the 4 main interventions of BBTI: (1) reduce time in bed; (2) get up at the same time every day, regardless of sleep duration; (3) do not go to bed unless sleepy; and (4) do not stay in bed unless asleep. Napping is discouraged. These interventions derive from sleep restriction and stimulus control techniques, the efficacy of which has been well documented.16,29 Time in bed was limited to average self-reported sleep time plus 30 minutes, with a minimum of 6 hours.
The IC condition was intended to emulate the behavioral treatment information generally to available patients and practitioners and included instructions to read and review 3 publications from the American Academy of Sleep Medicine: Insomnia, 30 Sleep as We Grow Older,31 and Sleep Hygiene.32 The content of these publications overlaps substantially with BBTI but without individualized behavioral instructions. Two weeks later, IC participants received a 10-minute follow-up telephone call to encourage continued participation. Participants were referred back to the brochures for specific sleep-related questions.
Both interventions were delivered by a single master’s level mental health nurse practitioner (L.K.B.) with no prior experience in sleep medicine or behavioral interventions for insomnia. This choice was consistent with the balance between efficacy and effectiveness research we sought to achieve in this study. Further information regarding the interventions, training, participants’ treatment expectations, acceptance, integrity of treatment delivery, and outcomes is presented in the eAppendix.
MEASURES
Participants provided demographic information; completed retrospective self-report questionnaires, interviewer-administered questionnaires, and 2-week sleep diaries; and underwent actigraphy and in-home PSG studies both prior to treatment and 4 weeks after the start of the intervention. Participants who showed a favorable short-term response to the BBTI were recontacted after 6 months to complete questionnaires and sleep diaries. We did not systematically follow up with the BBTI nonresponders and IC participants because of our conceptual model: we assumed that in clinical practice, nonresponders would be offered other treatment such as medication. All IC participants were offered BBTI following the initial 4-week treatment period.
Demographic information included age, sex, race, highest educational level, and subjective socioeconomic status (SES) using the SES Ladder.33,34 Medical status was evaluated with a comorbidity questionnaire adapted from the Charlson Comorbidity Index,35,36 with an expanded range of health conditions. Current medications were grouped into 15 categories. Health-related quality of life was characterized with a single item on self-reported health from the Medical Outcomes Study 36-Item Short Form Health Survey.37,38 Neuropsychiatric status was characterized using the Patient Health Questionnaire,27 the 17-item Hamilton Rating Scale for Depression,39 the Hamilton Anxiety Rating Scale,40 and the Mini–Mental State Examination.41 Subjective sleep quality was characterized with the Pittsburgh Sleep Quality Index (PSQI)42 and sleepiness with the Epworth Sleepiness Scale.43
The Pittsburgh Sleep Diary44 is a daily self-report measure of bed time and rise time, sleep onset latency (SOL), wakefulness after sleep onset (WASO), and sleep quality (visual analog scale). Reported sleep parameters are used to calculate time in bed, total sleep time (TST), and sleep efficiency (SE, calculated as TST/time in bed × 100). The Pittsburgh Sleep Diary is sensitive to differences between patients with sleep disorder and controls44 and to behavioral treatment effects.25 Mean values for the final 2 weeks of the intervention period were used in outcome analyses.
Wrist actigraphy data (Minimitter Actiwatch-64; Minimitter, Bend, Oregon) were collected for 2 weeks with concurrent data from sleep diaries to determine objective sleep-wake patterns. One-minute epochs were analyzed with Actiware software, version 5.04 using sleep diary data to identify bed time and wake time. In cases where visual inspection showed an obvious discrepancy between diary sleep times and observed activity patterns, actigraphy bed and/or rise times were edited (fewer than 4% of daily records). Outcome variables included SOL, WASO, TST, and SE.
Polysomnography was conducted in participants’ homes at their usual sleep times using Compumedics Siesta (Compumedics Limited, Abbotsford, Victoria, Australia) monitors. One screening PSG was obtained to quantify apnea and periodic limb movements, and 2 additional consecutive nights were collected at both pretreatment baseline and after treatment. The eAppendix provides details of PSG recording and scoring; PSG outcome measures included SOL, WASO, TST, and SE averaged over 2 nights.
STATISTICAL ANALYSIS
All analyses were based on intention to treat. Three BBTI participants were excluded: 1 required back surgery before starting intervention; 1 withdrew prior to intervention owing to her spouse’s medical illness; and 1 was found not to meet inclusion criteria. Participant characteristics for the intervention groups were compared using ttests, χ2 tests, and Fisher Exact statistics. For the primary outcome analyses, 4 categorical outcomes were compared using χ2 tests: response (change in PSQI score of ≥3 points or increase in sleep diary SE of ≥10%); remission (response criterion plus final PSQI score of <5 and sleep diary SE of >85%, corresponding to “good sleep” values,42,45); partial response (improvement in PSQI or SE but worsening in the other measure); and nonresponse (change in PSQI of <3 points and increase in sleep diary SE of <10%).
There are no generally accepted criteria for response or remission in insomnia treatment studies.46 Our response criteria are supported by 3 types of data (eAppendix): (1) they correspond to approximately 1 standard deviation of the pretreatment values (Cohen deffect size of approximately 1.0); (2) they are consistent with mean change values in published clinical trials; and (3) they correspond to a change score of approximately −6 in the Insomnia Severity Index (ISI),47 which has been validated as a clinically significant change. As an additional measure of clinical significance, we ascertained whether each participant met DSM-IV-TR and/or ICSD-2 criteria for insomnia disorder after treatment using a structured interview and checklist. We calculated the number needed to treat and the absolute risk reduction associated with BBTI compared with IC using these categorical outcomes. We also conducted a series of subgroup analyses to confirm the general pattern of findings, using Fisher exact statistics (eTable 1 and eTable 2).
Secondary outcomes for 4 domains of continuous variables (general clinical and sleep measures, sleep diary, actigraphy, and PSG) were evaluated by repeated-measures multivariate analyses of variance (MANOVA). Factors were group (BBTI vs IC), time (pretreatment or posttreatment), and group × time interaction. Prior to the MANOVA, missing data were imputed from least squares mean values for each group using univariate mixed models (eAppendix). The MANOVA interaction effects were further explored with mixed models for individual outcomes (Tables 2, 3, 4, and 5). Although many sleep measures have skewed distributions, sensitivity analyses showed that significant interaction effects were unchanged with either raw or transformed data. We report individual outcomes in their original units to facilitate clinical interpretation. Statistical analyses were conducted using SAS software, version 9.2 (SAS Institute Inc, Cary, North Carolina).
Table 2.
General Clinical and Sleep Measures
| BBTI | IC | ||||
|---|---|---|---|---|---|
| Measurement Scale | Pretreatment/ Posttreatment Score, Mean (SD) |
Difference (SE) [95% CI] |
Pretreatment/ Posttreatment Score, Mean (SD) |
Difference (SE) [95% CI] |
BBTI−IC Difference, (SE) [95% CI], Cohen d Effect Size |
| Hamilton Rating Scale for Depression39 | 7.46 (0.39)/4.98 (0.48) | −2.48 (0.49) [−3.45 to −1.51] | 7.93 (0.39)/7.35 (0.47) | −0.58 (0.47) [−1.52 to 0.37] | −1.91 (0.68) [−3.26 to −0.56], 0.64 |
| Hamilton Anxiety Rating Scale40 | 5.39 (0.36)/3.61 (0.43) | −1.78 (0.38) [−2.54 to −1.02] | 5.88 (0.35)/5.10 (0.42) | −0.78 (0.36) [−1.50 to −0.05] | −1.00 (0.53) [−2.05 to 0.05], 0.43 |
| Pittsburgh Sleep Quality Index42 | 10.44 (0.48)/6.89 (0.48) | −3.55 (0.45) [−4.44 to −2.66] | 10.38 (0.47)/9.83 (0.47) | −0.55 (0.44) [−1.42 to 0.32] | −3.00 (0.62) [−4.24 to −1.76], 1.10 |
| Epworth Sleepiness Scale43 | 6.21 (0.60)/5.90 (0.56) | −0.31 (0.42) [−1.14 to 0.52] | 7.29 (0.60)/6.51 (0.55) | −0.78 (0.41) [−1.60 to 0.05] | 0.47 (0.59) [−0.71 to 1.64], 0.18 |
| SF-3637,38 | 72.51 (2.84)/76.36 (2.77) | 3.85 (1.76) [0.35 to 7.34] | 67.53 (2.81)/65.20 (2.73) | −2.33 (1.73) [−5.78 to 1.12] | 6.18 (2.47) [1.27 to 11.09], 0.57 |
Abbreviations: BBTI, brief behavioral treatment for insomnia; CI, confidence interval; IC, information control; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey.
Table 3.
Sleep Diary Measures
| BBTI | IC | ||||
|---|---|---|---|---|---|
| Characteristic | Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
BBTI−IC Difference (SE) [95% CI], Cohen d Effect Size |
| Bed time, min from midnight | −35.19 (8.33)/−19.87 (7.90) | 15.32 (5.49) [4.40 to 26.25] | −26.60 (8.18)/−32.41 (7.80) | −5.81 (5.35) [−16.47 to 4.84] | 21.14 (7.66) [5.88 to 36.40], 0.63 |
| Morning rise time, min from 7:00 AM | −24.12 (11.59)/−57.18 (10.21) | −33.06 (7.41) [−47.82 to −18.30] | −5.43 (11.39)/−19.10 (10.08) | −13.67 (7.22) [−28.05 to 0.71] | −19.39 (10.35) [−39.99 to 1.22], 0.43 |
| Time spent in bed, min | 448.42 (11.27)/396.05 (9.90) | −52.36 (7.64) [−67.58 to −37.14] | 456.65 (11.06)/447.60 (9.77) | −9.05 (7.45) [−23.89 to 5.78] | −43.31 (10.67) [−64.56 to −22.05], 0.93 |
| Sleep latency, min | 42.54 (4.49)/19.71 (3.17) | −22.83 (3.71) [−30.21 to −15.44] | 31.17 (4.40)/30.17 (3.10) | −1.00 (3.59) [−8.15 to 6.15] | −21.83 (5.16) [−32.11 to −11.55], 0.96 |
| Wake after sleep onset, min | 52.06 (5.51)/28.02 (5.16) | −24.04 (5.76) [−35.51 to −12.58] | 51.08 (5.31)/47.67 (5.03) | −3.41 (5.55) [−14.47 to 7.64] | −20.63 (8.00) [−36.56 to −4.71], 0.59 |
| Total sleep time, min | 342.57 (10.89)/339.47 (10.64) | −3.11 (8.07) [−19.18 to 12.97] | 355.04 (10.61)/358.39 (10.30) | 3.36 (7.77) [−12.11 to 18.82] | −6.46 (11.20) [−28.77 to 15.84], 0.13 |
| Sleep efficiency, % | 78.58 (1.57)/87.33 (1.53) | 8.75 (1.55) [5.66 to 11.84] | 81.05 (1.53)/82.25 (1.47) | 1.21 (1.50) [−1.77 to 4.19] | 7.54 (2.16) [3.25 to 11.83], 0.80 |
| Sleep quality, 0–100 | 51.03 (2.24)/64.39 (2.46) | 13.35 (2.63) [8.11 to 18.59] | 51.79 (2.18)/55.15 (2.43) | 3.36 (2.58) [−1.77 to 8.50] | 9.99 (3.68) [2.65 to 17.32], 0.62 |
Abbreviations: BBTI, brief behavioral treatment for insomnia; CI, confidence interval; IC, information control.
Table 4.
Actigraphy Measures
| BBTI | IC | ||||
|---|---|---|---|---|---|
| Characteristic | Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
BBTI−IC Difference (SE) [95% CI], Cohen d Effect Size |
| Sleep latency, min | 18.08 (2.04)/12.62 (4.48) | −5.47 (2.78) [−11.01 to 0.08] | 15.19 (2.15)/19.87 (2.41) | 4.70 (2.81) [−0.91 to 10.30] | −10.16 (3.96) [−18.05 to −2.28], 0.59 |
| Wake after sleep onset, min | 57.28 (4.43)/46.62 (3.99) | −10.65 (2.09) [−14.82 to −6.48] | 56.92 (4.48)/55.38 (3.97) | −1.54 (2.17) [−5.87 to 2.80] | −9.12 (3.02) [−15.13 to −3.11], 0.69 |
| Total sleep time, min | 379.65 (8.21)/338.16 (8.14) | −41.50 (6.49) [−54.42 to −28.57] | 381.64 (8.45)/370.44 (8.02) | −11.21 (6.68) [−24.52 to 2.10] | −30.29 (9.32) [−48.84 to −11.73], 0.74 |
| Sleep efficiency, % | 80.37 (1.20)/82.82 (1.16) | 2.45 (0.97) [0.52 to 4.37] | 81.42 (1.24)/79.99 (1.15) | −1.43 (1.00) [−3.41 to 0.56] | 3.88 (1.39) [1.11 to 6.64], 0.64 |
Abbreviations: BBTI, brief behavioral treatment for insomnia; CI, confidence interval; IC, information control.
Table 5.
Polysomnography Measures
| BBTI | IC | ||||
|---|---|---|---|---|---|
| Characteristic | Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
Pretreatment/ Posttreatment, Mean (SD) |
Difference (SE) [95% CI] |
BBTI−IC Difference (SE) [95% CI], Cohen d Effect Size |
| Sleep latency, min | 22.49 (4.81)/29.21 (4.81) | 6.72 (5.70) [−4.64 to 18.07] | 25.84 (4.69)/25.59 (4.74) | −0.25 (5.58) [−11.36 to 10.87] | 6.96 (7.98) [−8.93 to 22.85], 0.20 |
| Wake after sleep onset, min | 102.12 (8.77)/85.26 (8.49) | −16.86 (8.30) [−33.40 to −0.32] | 90.22 (8.58)/92.46 (8.38) | 2.23 (8.11) [−13.91 to 18.38] | −19.09 (11.61) [−42.20 to 4.02], 0.38 |
| Total sleep time, min | 350.12 (8.83)/324.82 (9.43) | −25.30 (10.17) [−45.55 to −5.05] | 351.12 (8.63)/333.31 (9.31) | −17.81 (9.95) [−37.63 to 2.00] | −7.49 (14.23) [−35.82 to 20.85], 0.12 |
| Sleep efficiency, % | 74.38 (1.82)/74.86 (1.67) | 0.48 (1.57) [−2.65 to 3.61] | 75.67 (1.78)/74.16 (1.64) | −1.51 (1.53) [−4.57 to 1.54] | 2.00 (2.20) [−2.38 to 6.37], 0.21 |
Abbreviations: BBTI, brief behavioral treatment for insomnia; CI, confidence interval; IC, information control.
RESULTS
GENERAL RESULTS
The most frequent reason for exclusion was clinical or PSG evidence of untreated apnea, other sleep disorder, or psychiatric disorder (Figure 1). Excluded subjects differed from included subjects in the percentage of men (53% [n=36] vs 32% [n=25]), but not in age, sex, race, medical comorbidities, or PSQI score (eTable 1). Treatment groups did not differ significantly in sociodemographic or clinical characteristics (Table 1). Participants tolerated the interventions well: 1 BBTI participant dropped out owing to an unrelated medical problem; 92% of BBTI participants (n=36) and 100% of IC participants (n=40) completed all scheduled in-person and telephone sessions, and no significant adverse effects were reported.
CATEGORICAL OUTCOMES
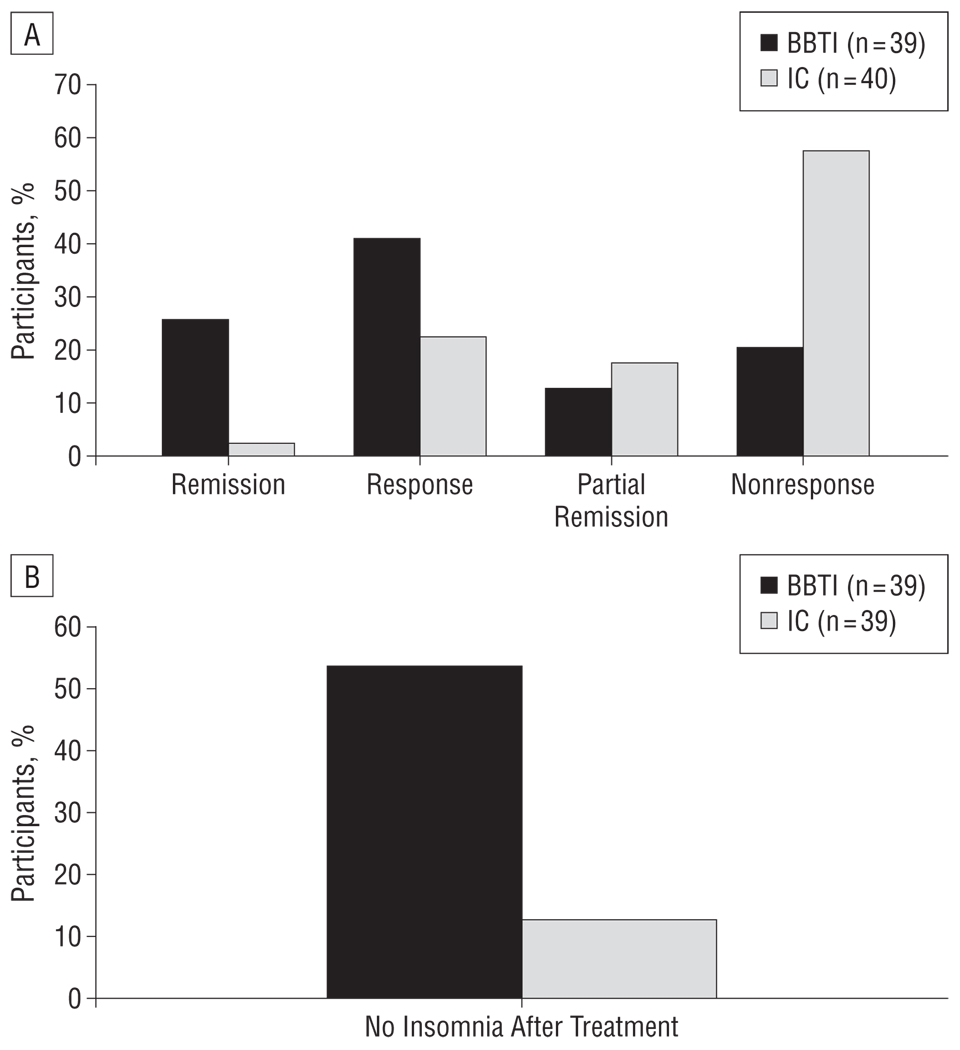
Using the 4 outcome categories, we found that the BBTI group had significantly better outcomes than the IC group (, P <.001) (Figure 2A). The BBTI participants also had significantly better outcomes as evaluated by 2 categories of remission or response vs partial response or nonresponse (χ2=13.8, P <.001). Using these 2 categories, we found that the number needed to treat was 2.4 (95% confidence interval [CI], 1.6–4.6), and the absolute risk reduction was 41.7% (95% CI, 21.7%–61.6%). The percentage of participants who no longer met criteria for insomnia disorder at the end of intervention was higher in the BBTI group (55% [n=21]) than in the IC group (13% [n=5]) (χ2=15.5, P<.001) (Figure 2B). The number needed to treat for this outcome was 2.4 (95% CI, 1.6–4.3) for BBTI vs IC, and the absolute risk reduction was 42.4% (95% CI, 23.5%–61.4%).
Figure 2.
Categorical treatment outcomes. A, Outcomes for participants assigned to the brief behavioral treatment for insomnia (BBTI) and IC groups (χ2=13.8, P<.001). See the “Methods” section for definitions. B, Percentages of participants in each group who no longer met the Diagnostic and Statistical Manual of Mental Disorders(Fourth Edition, Text Revision)2 and/or International Classification of Sleep Disorders(Second Edition)1 criteria for insomnia after treatment (χ2=15.5, P<.001). See the “Methods” section for details.
OUTCOMES BY DOMAIN
The MANOVA for general clinical and sleep measures showed a significant treatment group × time interaction (F5,73=5.99, P<.001), as well as significant group (F5,73=2.34, P=.05), and time (F5,73=2.34, P<.001) main effects (Table 2). Mixed models indicated significant effects of BBTI and significant differences between BBTI and IC in change scores for depression, sleep quality, and general health. Effect sizes were moderate. No group difference was found for change in anxiety or sleepiness. The MANOVA for the sleep diary domain also indicated significant interaction (F8,70=4.32, P<.001) and time effects (F8,70=6.65, P<.001) but no significant effect for group (F8,70=1.67, P=.12) (Table 3). The BBTI group had later bedtimes, improved sleep quality, and improved SOL, WASO, and SE compared with the IC group, with moderate to large effect sizes. The TST and morning rise time did not show differential effects of the 2 interventions. Significant differential treatment effects were found for actigraphy (MANOVA interaction, F4,74=17.72, P<.001; time, F4,74=15.37, P<.001; and group, F4,74=2.13, P=.09) (Table 4). We found that the BBTI group had a significantly greater reduction in actigraphy-based WASO and SOL and a significantly greater increase in SE compared with the IC group using mixed models. The BBTI group also had a significantly greater reduction in actigraphically measured TST from pretreatment to posttreatment. However, the significant MANOVA interaction was still seen after excluding TST (interaction, F3,75=5.85, P=.001). No differential treatment effects were noted for PSG (MANOVA interaction, F4,74=1.20, P=.32; time, F4,74=3.95, P=.006; group F4,74=0.47, P=.76) (Table 5). Sensitivity analyses using natural log and square root transformations in univariate mixed model analyses revealed an identical pattern of significant results.
6-MONTH FOLLOW-UP
Follow-up data were available for 25 BBTI participants (9 remitters, 12 responders, 3 partial remitters, and 1 nonresponder who did not desire other treatment). Ofthese,40% (n=10) met criteria for remission, 44% (n=11) met criteria for response,12%(n=3) met criteria for partial response, and 1 met criteria for nonresponse. Sixty-four percent no longer met DSM-IV-TR or ICSD-2 criteria for insomnia disorder (16 of 25). We also examined changes of at least 1 category in either direction from posttreatment to 6-month follow-up. Twenty percent of participants improved (n=5), 56% were unchanged (n=14), and 24% worsened (n=6). Sleep diary mean (SD) TST significantly increased from the end of short-term treatment to 6-month follow-up (340 [52.6] vs 386 [80.5] minutes, t22=−3.90, P<.001).
COMMENT
Older adults with chronic insomnia treated with BBTI showed clinically and statistically significant improvement in sleep outcomes at 4 weeks compared with participants treated with IC. The superiority of BBTI was seen for outcomes based on categorically defined criteria, presence of insomnia disorder, number needed to treat, retrospective clinical ratings, sleep diary, and actigraphy but not PSG. Various clinical subgroups did not show differential effects, and treatment gains were maintained at 6 months. The BBTI delivered by a nurse clinician may be an efficacious and practical treatment for chronic insomnia in older adults.
Differences in specific inclusion and exclusion criteria and study instruments make it difficult to compare the magnitude of treatment effects across behavioral treatment studies of insomnia. The improvements seen with BBTI appear similar to those observed with traditional CBTI in middle-aged populations, although somewhat smaller in magnitude,16,23 and comparable in magnitude to those reported for CBTI and other behavioral treatments in older adults.24,45,48 For instance, a meta-analysis of CBTI and behavioral interventions for insomnia in older adults reported mean effects sizes of 0.60, 0.51, 0.19, 0.38, and 0.73 for diary outcomes of sleep quality, SOL, TST, SE, and WASO, respectively.21 Corresponding values in the current study were 0.62, 0.96, 0.13, 0.80, and 0.59, respectively. Like most CBTI studies, we found an initial reduction in TST concurrent with improvements in SE and other sleep ratings. The TST increased at 6-month follow-up, when initial sleep restriction was relaxed. The magnitude of BBTI effects is also similar to that found for BZRA medications.49 We did not observe the significant treatment effects on PSG observed in some behavioral treatment studies.45,50,51 This could result from the shorter duration of BBTI compared with CBTI or from differences in participant age, inclusion criteria, sampling strategies and biases, acute treatment duration, and the use of laboratory vs in-home PSG.52,53 Actigraphy and in-home PSG relied in part on self-reported data to identify bed time, which could lead to some inaccuracy in these “objective” measures. In our study as in most others, both sleep disturbances and treatment improvement were larger for self-reports than for PSG measures in patients with insomnia23,54; this discrepancy may be a fundamental characteristic of insomnia.55
Currently, there are no universally accepted criteria for categorical treatment outcomes in insomnia studies. 46 Our criteria included a general measure of sleep quality (PSQI) and a widely used summary sleep diary metric in behavioral treatment studies of insomnia (SE). The magnitude of change used to define response corresponded to a large effect size, was consistent with changes reported in published studies, and corresponded to the minimally important difference in the ISI (eAppendix). Other categorical treatment outcomes, such as 50% reduction in sleep diary values50 or changes in the ISI score to “normal” values, have been used in other studies.51 Our acute response and remission rates (41% [n=16] and 26% [n=10]) were lower than those found in studies using the ISI as an outcome measure in a 6-week CBTI study (59.5 and 39%)51 but similar to those reported in a study of older adults using 85% diary SE as a criterion.45 Our study may have underestimated treatment effects because we did not alter the 1-month reporting frame of the PSQI for posttreatment outcomes. However, confidence in our outcomes is increased by convergent findings when we used the presence or absence of insomnia diagnosis as a criterion.
Our findings can also be placed in the context of other studies aimed at the dissemination of behavioral insomnia treatments. The magnitude of observed sleep diary changes was comparable to magnitudes reported for an abbreviated form of CBTI in a primary care,56 group CBTI delivered in primary care practices,57,58 and CBTI provided to patients with medical comorbidities.59 Brief, nurse-administered behavioral and cognitive behavioral treatments appear to be feasible and efficacious for older adults with comorbid insomnia. Other novel forms of behavioral treatment delivery such as Internet programs may also be efficacious.60 Thus, a range of options is now available to administer behavioral treatments for insomnia across a range of clinical settings.
Although BBTI shares many features with other behavioral insomnia treatments, some particular features make it an especially attractive option. First, it has a strong behavioral focus, which may avoid some of the perceived stigma associated with “psychological” treatments in medical settings. Second, it is overtly linked to a physiologic model of sleep regulation,28 which provides a sound empirical rationale for both patients and physicians. Third, it provides patients with a workbook and specific written prescriptions for sleep behaviors. Fourth, it is simple enough to be taught in a short amount of time to nurses, who are often responsible for behavioral health management in primary care offices. Finally, it appears to have comparable efficacy to established treatments. Thus, BBTI possesses efficacy, efficiency, and acceptability—3 characteristics of a successful “entry level” treatment in a stepped care approach to behavioral management of insomnia.61
Strengths of this study included convergent self-report, observer-rated, and physiologic outcomes and a sample that is generalizable to practice settings. Limitations included a control condition that was not matched for therapist time, the use of a single therapist for both conditions, or a limited follow-up interval. The control condition was selected to represent an ecologically valid comparison for primary care settings, where sleep and insomnia are infrequently addressed and behavioral treatment is rarely available. The use of a single therapist was likely to be less problematic for a control condition that, by design, consisted of self-education. Finally, longer follow-up in both intervention groups might have been informative. Our follow-up strategy was based on our conceptual model of insomnia treatment in primary care: if behavioral treatment cannot be delivered in a brief format with rapid results, patients are likely to proceed to pharmacologic treatment. Finally, 68% of our insomnia patients had an apnea-hypoxia index higher than 5, raising the possibility that treatment for apnea could further enhance outcomes.
In summary, BBTI produced statistically and clinically meaningful improvements that were sustained for 6 months. Future studies should examine the feasibility of educating nurses and other health professionals in BBTI and the effectiveness of BBTI delivered in actual practice settings on symptom-based, functional, and health care economic outcomes.
Acknowledgments
Funding/Support: This work was supported by NIH grants AG020677, AG000972, and RR024153 (University of Pittsburgh Clinical and Translational Science Award).
Role of the Sponsor: The sponsors had no role in the design and conduct of the study or the preparation, review, or approval of the manuscript.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00177203
Author Contributions: Dr Buysse had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design:Buysse, Germain, Moul, and Monk. Acquisition of data:Buysse, Germain, Moul, Franzen, Brar, and Fletcher. Analysis and interpretation of data:Buysse, Germain, Moul, Franzen, Fletcher, Begley, Houck, Mazumdar, Reynolds, and Monk. Drafting of the manuscript:Buysse, Germain, Franzen, Fletcher, Begley, Houck, Reynolds, and Monk. Critical revision of the manuscript for important intellectual content:Moul, Franzen, Brar, and Mazumdar. Statistical analysis:Begley, Houck, and Mazumdar. Obtained funding:Buysse, Reynolds, and Monk. Administrative, technical, and material support:Germain, Moul, Franzen, Fletcher, and Monk. Study supervision:Germain and Moul.
Financial Disclosure: Dr Buysse has served as a paid consultant and/or has received compensation for continuing medical education activities indirectly sponsored by Actelion, Cephalon, Eli Lilly, Eisai, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Philips, Purdue Pharma, Sanofi-Aventis, Sepracor, Servier, Somnus Therapeutics, Takeda, and Transcept. Dr Reynolds has received pharmaceutical supplies for his National Institutes of Health (NIH)-sponsored work from Forest Laboratories, Bristol-Myers Squibb, Eli Lilly, and Pfizer.
Publisher's Disclaimer: Disclaimer: This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging (NIA) or the National Center for Research Resources (NCRR).
Previous Presentations: Portions of this work were presented as abstracts and posters at the Associated Professional Sleep Societies SLEEP Meetings; June 17–22, 2006; Salt Lake City, Utah; June 7–12, 2008; Baltimore, Maryland; and June 6–11, 2009; Seattle, Washington.
Online-Only Material: The eAppendix, eTable 1, and eTable 2 are available at http://www.archinternmed.com.
Additional Contributions: The staff at the Neuroscience Clinical and Translational Research Center of the University of Pittsburgh Clinical and Translational Research Center conducted the polysomnographic studies. The physicians and staff at Partners in Health, where many of the participants were recruited and studied, provided valuable assistance. Finally, we thank the participants for allowing us to work with them on this study.
REFERENCES
- 1.American Academy of Sleep Medicine. The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Darien, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) 4th ed. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22 suppl 2:S359–S365. [PubMed] [Google Scholar]
- 5.Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154(10):1417–1423. doi: 10.1176/ajp.154.10.1417. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Germain A, Moul DE. Diagnosis, epidemiology and consequences of insomnia. Prim Psychiatry. 2005;12(8):37–44. [Google Scholar]
- 9.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53(6):955–962. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 11.Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, PA: Elsevier Saunders; 2005. [Google Scholar]
- 12.Aparasu RR, Mort JR, Brandt H. Psychotropic prescription use by community-dwelling elderly in the United States. J Am Geriatr Soc. 2003;51(5):671–677. doi: 10.1034/j.1600-0579.2003.00212.x. [DOI] [PubMed] [Google Scholar]
- 13.Bloom HG, Ahmed I, Alessi CA, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. J Am Geriatr Soc. 2009;57(5):761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159(1):5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26(7):793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 18.Krystal AD, Erman M, Zammit GK, Soubrane C, Roth T. ZOLONG Study Group. Long-term efficacy and safety of zolpidem extended-release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study. Sleep. 2008;31(1):79–90. doi: 10.1093/sleep/31.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin RD, Hasin DS. Sedative use and misuse in the United States. Addiction. 2002;97(5):555–562. doi: 10.1046/j.1360-0443.2002.00098.x. [DOI] [PubMed] [Google Scholar]
- 20.Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 21.Hamai Y, Fujii T, Yamashita T, et al. Evidence for an elevation in serum interleukin-2 and tumor necrosis factor-alpha levels before the clinical manifestations of preeclampsia. Am J Reprod Immunol. 1997;38(2):89–93. doi: 10.1111/j.1600-0897.1997.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 22.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15(4):302–305. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 23.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998–2004) Sleep. 2006;29(11):1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 24.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 25.Germain A, Moul DE, Franzen PL, et al. Effects of a brief behavioral treatment for late-life insomnia: preliminary findings. J Clin Sleep Med. 2006;2(4):403–406. [PubMed] [Google Scholar]
- 26.National Institutes of Health. NIH state-of-the-science conference statement on manifestations and management of chronic insomnia in adults. [Accessed November 6, 2010]; http://consensus.nih.gov/2005/insomniastatement.pdf.
- 27.Kroenke K, Spitzer R. The PHQ-9: a new depression and diagnostic severity measure. Psychiatr Ann. 2002;32(9):509–521. [Google Scholar]
- 28.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T, Dement WE, editors. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, PA: W.B. Saunders Co; 2000. pp. 377–390. [Google Scholar]
- 29.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–1180. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 30.American Academy of Sleep Medicine. Insomnia: The Inability to Fall Asleep or Stay Asleep. Westchester, IL: American Academy of Sleep Medicine; 2000. [Google Scholar]
- 31.American Academy of Sleep Medicine. Sleep as We Grow Older: Learning How Aging Affects Sleep. Westchester, IL: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 32.American Academy of Sleep Medicine. Sleep Hygiene: Behaviors That Promote Sound Sleep. Westchester, IL: American Academy of Sleep Medicine; 2002. [Google Scholar]
- 33.Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–592. doi: 10.1037//0278-6133.19.6.586. [DOI] [PubMed] [Google Scholar]
- 34.Adler N, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot MG. Social status and health: a comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc Sci Med. 2008;66(5):1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 38.Ware JE, Snow KK, Kosinski M. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: Health Institute, New England Medical Center; 1993. [Google Scholar]
- 39.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 41.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 42.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 43.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3(2):111–120. [PubMed] [Google Scholar]
- 45.Morin CM, Colecchi C, Stone J, Sood RK, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 46.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 48.Lichstein KL, Riedel BW, Wilson NM, Lester KW, Aguillard RN. Relaxation and sleep compression for late-life insomnia: a placebo-controlled trial. J Consult Clin Psychol. 2001;69(2):227–239. doi: 10.1037//0022-006x.69.2.227. [DOI] [PubMed] [Google Scholar]
- 49.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, III, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278(24):2170–2177. [PubMed] [Google Scholar]
- 50.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 51.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edinger JD, Fins AI, Sullivan RJ, Jr, et al. Sleep in the laboratory and sleep at home: comparisons of older insomniacs and normal sleepers. Sleep. 1997;20(12):1119–1126. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 53.Edinger JD, Glenn DM, Bastian LA, et al. Sleep in the laboratory and sleep at home II: comparisons of middle-aged insomnia sufferers and normal sleepers. Sleep. 2001;24(7):761–770. doi: 10.1093/sleep/24.7.761. [DOI] [PubMed] [Google Scholar]
- 54.Buysse DJ, Germain A, Hall ML, et al. EEG spectral analysis in primary insomnia: NREM period effects and sex differences. Sleep. 2008;31(12):1673–1682. doi: 10.1093/sleep/31.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manconi M, Ferri R, Sagrada C, et al. Measuring the error in sleep estimation in normal subjects and in patients with insomnia. J Sleep Res. 2010;19(3):478–486. doi: 10.1111/j.1365-2869.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 56.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–182. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 57.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39(1):45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 58.Espie CA, MacMahon KM, Kelly HL, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. 2007;30(5):574–584. doi: 10.1093/sleep/30.5.574. [DOI] [PubMed] [Google Scholar]
- 59.Rybarczyk B, Stepanski E, Fogg L, Lopez M, Barry P, Davis A. A placebo-controlled test of cognitive-behavioral therapy for comorbid insomnia in older adults. J Consult Clin Psychol. 2005;73(6):1164–1174. doi: 10.1037/0022-006X.73.6.1164. [DOI] [PubMed] [Google Scholar]
- 60.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry. 2009;66(7):692–698. doi: 10.1001/archgenpsychiatry.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espie CA. “Stepped care”: a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32(12):1549–1558. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]