Abstract
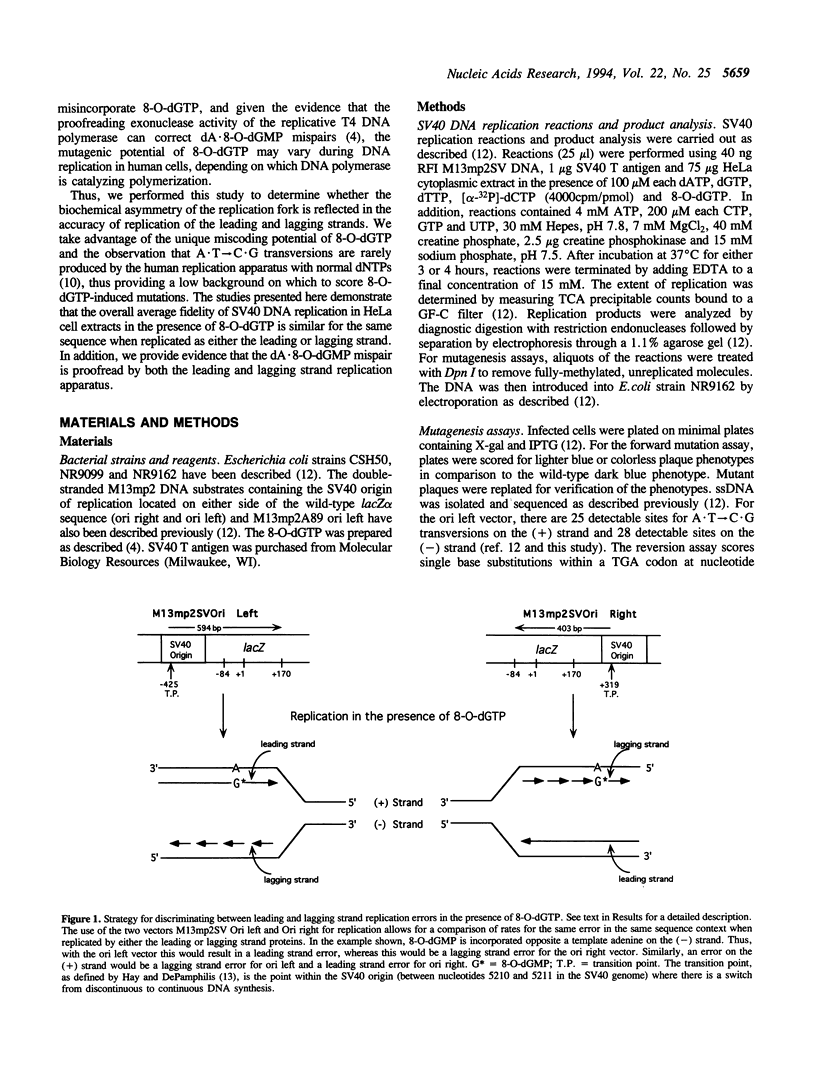
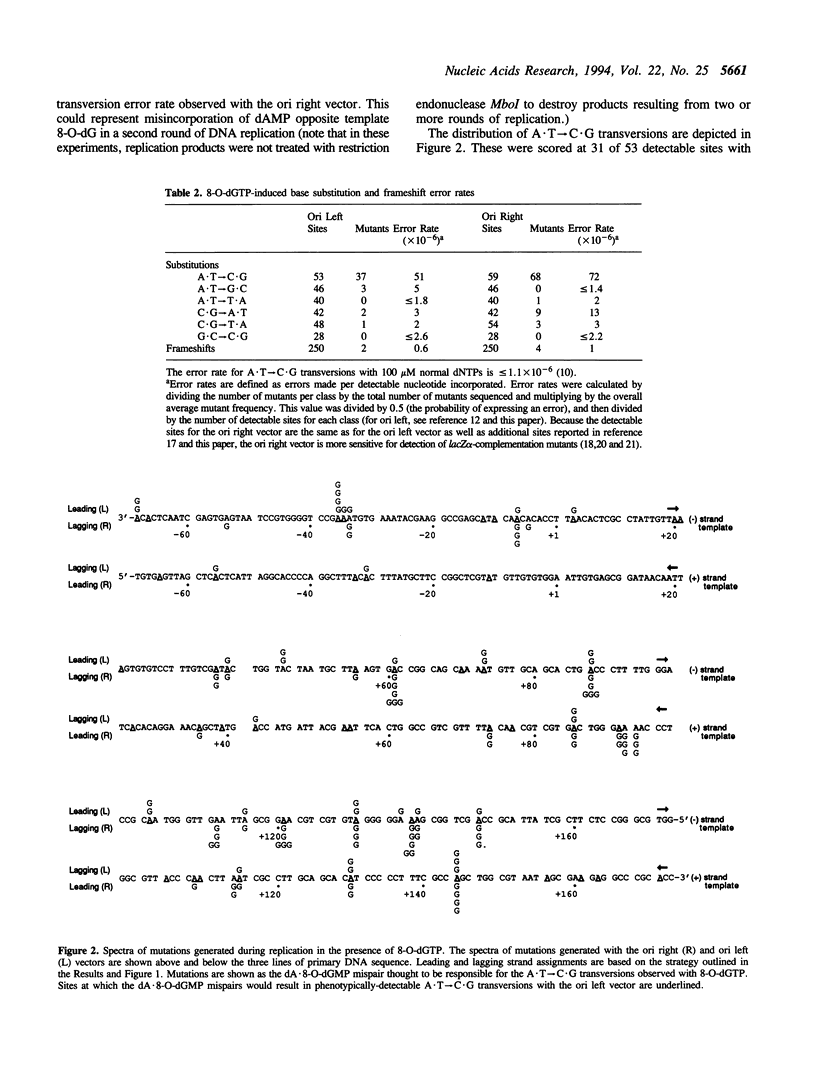
A product of oxidative metabolism, 8-oxodeoxyguanosine triphosphate (8-O-dGTP), readily pairs with adenine during DNA replication, ultimately causing A.T-->C.G transversions. This study utilized 8-O-dGTP as a probe to examine the fidelity of the leading and lagging strand replication apparatus in extracts of HeLa cells. Simian virus (SV) 40 T antigen-dependent DNA replication reactions were performed with two M13mp2 vectors with the SV40 origin located on opposite sides of the lacZ alpha sequence used to score replication errors. The presence of 8-O-dGTP at equimolar concentration with each of the 4 normal dNTPs resulted in a > 46-fold increase in error rate for A.T-->C.G transversion over that observed in the absence of 8-O-dGTP. A similar average error rate was observed on the (+) and (-) strands in both vectors, suggesting that the fidelity of replication by leading and lagging strand replication proteins is similar for the dA.8-O-dGMP mispair. Replication fidelity in the presence of 8-O-dGTP was reduced on both strands when an inhibitor of exonucleolytic proofreading (dGMP) was added to the reaction. These data suggest that the majority of dA.8-O-dGMP mispairs are proofread by both leading and lagging strand replication proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bambara R. A., Jessee C. B. Properties of DNA polymerases delta and epsilon, and their roles in eukaryotic DNA replication. Biochim Biophys Acta. 1991 Jan 17;1088(1):11–24. doi: 10.1016/0167-4781(91)90147-e. [DOI] [PubMed] [Google Scholar]
- Boyer J. C., Thomas D. C., Maher V. M., McCormick J. J., Kunkel T. A. Fidelity of DNA replication by extracts of normal and malignantly transformed human cells. Cancer Res. 1993 Jul 15;53(14):3270–3275. [PubMed] [Google Scholar]
- Cheng K. C., Cahill D. S., Kasai H., Nishimura S., Loeb L. A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G----T and A----C substitutions. J Biol Chem. 1992 Jan 5;267(1):166–172. [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Grollman A. P., Moriya M. Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet. 1993 Jul;9(7):246–249. doi: 10.1016/0168-9525(93)90089-z. [DOI] [PubMed] [Google Scholar]
- Hay R. T., DePamphilis M. L. Initiation of SV40 DNA replication in vivo: location and structure of 5' ends of DNA synthesized in the ori region. Cell. 1982 Apr;28(4):767–779. doi: 10.1016/0092-8674(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Hendrickson E. A., DePamphilis M. L. Sequence specificity for the initiation of RNA-primed simian virus 40 DNA synthesis in vivo. J Mol Biol. 1984 May 15;175(2):131–157. doi: 10.1016/0022-2836(84)90471-6. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Dean F. B., Kwong A. D., Lee S. H. The in vitro replication of DNA containing the SV40 origin. J Biol Chem. 1990 Oct 25;265(30):18043–18046. [PubMed] [Google Scholar]
- Kouchakdjian M., Bodepudi V., Shibutani S., Eisenberg M., Johnson F., Grollman A. P., Patel D. J. NMR structural studies of the ionizing radiation adduct 7-hydro-8-oxodeoxyguanosine (8-oxo-7H-dG) opposite deoxyadenosine in a DNA duplex. 8-Oxo-7H-dG(syn).dA(anti) alignment at lesion site. Biochemistry. 1991 Feb 5;30(5):1403–1412. doi: 10.1021/bi00219a034. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Exonucleolytic proofreading. Cell. 1988 Jun 17;53(6):837–840. doi: 10.1016/s0092-8674(88)90189-4. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Lee H. S., Park M. K., Hwang E. S., Park E. M., Kasai H., Chung M. H. Identification of 8-hydroxyguanine glycosylase activity in mammalian tissues using 8-hydroxyguanine specific monoclonal antibody. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1545–1551. doi: 10.1006/bbrc.1993.2427. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki H., Sekiguchi M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature. 1992 Jan 16;355(6357):273–275. doi: 10.1038/355273a0. [DOI] [PubMed] [Google Scholar]
- Michaels M. L., Miller J. H. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J Bacteriol. 1992 Oct;174(20):6321–6325. doi: 10.1128/jb.174.20.6321-6325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo J. Y., Maki H., Sekiguchi M. Hydrolytic elimination of a mutagenic nucleotide, 8-oxodGTP, by human 18-kilodalton protein: sanitization of nucleotide pool. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11021–11025. doi: 10.1073/pnas.89.22.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov Y. I., Minnick D. T., Izuta S., Kunkel T. A. DNA replication fidelity with 8-oxodeoxyguanosine triphosphate. Biochemistry. 1994 Apr 19;33(15):4695–4701. doi: 10.1021/bi00181a029. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Izuta S., Thomas D. C., Kunkel T. A. Mispair-, site-, and strand-specific error rates during simian virus 40 origin-dependent replication in vitro with excess deoxythymidine triphosphate. J Biol Chem. 1994 Jan 21;269(3):1711–1717. [PubMed] [Google Scholar]
- Roberts J. D., Kunkel T. A. Fidelity of a human cell DNA replication complex. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7064–7068. doi: 10.1073/pnas.85.19.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. D., Nguyen D., Kunkel T. A. Frameshift fidelity during replication of double-stranded DNA in HeLa cell extracts. Biochemistry. 1993 Apr 20;32(15):4083–4089. doi: 10.1021/bi00066a033. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Thomas D. C., Kunkel T. A. Exonucleolytic proofreading of leading and lagging strand DNA replication errors. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3465–3469. doi: 10.1073/pnas.88.8.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakumi K., Furuichi M., Tsuzuki T., Kakuma T., Kawabata S., Maki H., Sekiguchi M. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J Biol Chem. 1993 Nov 5;268(31):23524–23530. [PubMed] [Google Scholar]
- Shibutani S., Takeshita M., Grollman A. P. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991 Jan 31;349(6308):431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Kunkel T. A. Replication of UV-irradiated DNA in human cell extracts: evidence for mutagenic bypass of pyrimidine dimers. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7744–7748. doi: 10.1073/pnas.90.16.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Nguyen D. C., Piegorsch W. W., Kunkel T. A. Relative probability of mutagenic translesion synthesis on the leading and lagging strands during replication of UV-irradiated DNA in a human cell extract. Biochemistry. 1993 Nov 2;32(43):11476–11482. doi: 10.1021/bi00094a002. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Roberts J. D., Sabatino R. D., Myers T. W., Tan C. K., Downey K. M., So A. G., Bambara R. A., Kunkel T. A. Fidelity of mammalian DNA replication and replicative DNA polymerases. Biochemistry. 1991 Dec 24;30(51):11751–11759. doi: 10.1021/bi00115a003. [DOI] [PubMed] [Google Scholar]
- Waga S., Bauer G., Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J Biol Chem. 1994 Apr 8;269(14):10923–10934. [PubMed] [Google Scholar]
- Waga S., Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994 May 19;369(6477):207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- Zhang S. S., Grosse F. Accuracy of DNA primase. J Mol Biol. 1990 Dec 5;216(3):475–479. doi: 10.1016/0022-2836(90)90370-2. [DOI] [PubMed] [Google Scholar]


