Abstract
The major DNA repair pathway for coping with spontaneous forms of DNA damage, such as natural hydrolytic products or oxidative lesions, is base excision repair (BER). In particular, BER processes mutagenic and cytotoxic DNA lesions such as non-bulky base modifications, abasic sites, and a range of chemically distinct single-strand breaks. Defects in BER have been linked to cancer predisposition, neurodegenerative disorders, and immunodeficiency. Recent data indicate a large degree of sequence variability in DNA repair genes and several studies have associated BER gene polymorphisms with disease risk, including cancer of several sites. The intent of this review is to describe the range of BER capacity among individuals and the functional consequences of BER genetic variants. We also discuss studies that associate BER deficiency with disease risk and the current state of BER capacity measurement assays.
Keywords: DNA repair capacity, base excision, pathway assay, polymorphism, disease susceptibility
Overview of Base Excision Repair: Connection to Disease
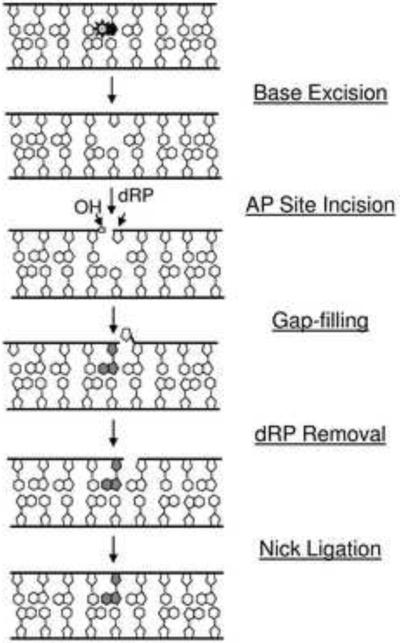
Base excision repair (BER) is the major DNA repair pathway for most spontaneous, alkylative, and oxidative DNA lesions. The process (Figure 1), which aims to remove and replace a damaged nucleotide, is typically initiated by one of several substrate-selective DNA glycosylases, which recognize and excise a range of base modifications such as uracil, 8-oxoguanine (8-oxo-dG), and 3-methyladenine among others [1, 2]. The resulting apurinic/apyrimidinic (AP) site, which can also be formed at high frequency by spontaneous (i.e., non-enzymatic) hydrolysis of the N-glycosidic bond, is then incised by an AP endonuclease, APE1 (aka APEX1 or REF1) in mammals [3–5]. The 5'-deoxyribose phosphate strand break product is subsequently removed and the missing nucleotide gap is filled, two steps that are most frequently executed by DNA polymerase β (POLβ) in mammals [6]. The remaining nick is sealed by a DNA ligase, which in mammals is typically ligase 3α (LIG3α) in complex with x-ray cross-complementing protein 1 (XRCC1) [7]. If executed as shown in Figure 1, the process is termed short-patch BER, since it involves the incorporation of only a single nucleotide. However, there are other sub-branches of BER – involving specialized single-strand break processing enzymes or the long-patch (2–13 nucleotides) proliferating cell nuclear antigen (PCNA)-dependent synthesis machinery – that will not be described in detail herein, but are reviewed in depth elsewhere [8–11]. Table 1 has been included as a quick reference for several key proteins of BER and the related process of single-strand break repair that will be mentioned herein.
Figure 1.
Mammalian BER pathway. Denoted are the five major enzymatic steps of a typical BER response. The substrate base lesion is indicated by the sunburst. OH = hydroxyl, dRP = 5'-deoxyribose phosphate. The shaded nucleotide designates the repair synthesis product. See text for further details.
Table 1.
Several key proteins of base excision repair (BER) and its related pathway, single-strand break repair.
| Gene Name (Alternative Name(s)) | Encoded Protein and Relevant Function(s) |
|---|---|
| MUTYH(MYH) | E. coli MutY homolog; DNA glycosylase that excises A opposite 8-oxo-dG |
| UNG | Uracil DNA glycosylase |
| OGG1 | 8-oxo-dG DNA glycosylase; AP β-lyase |
| NEIL1 | E. coli Nei-like DNA glycosylase; excises oxidized pyrimidines, such as thymine glycol, formamidopyrimidine and 5-hydroxyuracil; AP β,δ-lyase |
| NEIL2 | E. coli Nei-like DNA glycosylase; excises 5-hydroxyuracil and oxidized derivatives of cytosine, particularly in bubble structures; AP β,δ-lyase |
| NEIL3 | E. coli Nei-like DNA glycosylase; excises oxidized purines, such as spiroiminodihydantoin, guanidinohydantoin, and formamidopyrimidines, particularly in bubble structures |
| NTHL1 (NTH1) | E. coli Nth-like DNA glycosylase; excises oxidized pyrimidine residues, such as thymine glycol, and has AP lyase activity |
| MPG (AAG) | Methylpurine DNA glycosylase |
| TDG | Thymine DNA glycosylase; excises T opposite G following hydrolytic deamination of 5-methylcytosine |
| SMUG1 | Single-strand selective monofunctional uracil DNA glycosylase; excises U, mainly from U:G mispairs, and oxidized uracil derivatives, such as 5-hydroxymethyluracil |
| APE1 (APEX1, REF1) | AP endonuclease; 3' to 5' exonuclease and 3'-phosphodiesterase |
| POL β | Gap-filling DNA polymerase |
| XRCC1 | Non-enzymatic, single-strand break scaffold protein |
| APTX (Aprataxin) | 5'-AMP DNA hydrolase |
| TDP1 | 3'-tyrosyl DNA phosphodiesterase; excises 3'-topoisomerase intermediates and 3'-phosphoglycolates |
| PNKP (PNK) | DNA polynucleotide 5'-kinase and 3'-phosphatase |
| LIG1 | DNA ligase |
| LIG3 | DNA ligase |
| FEN1 | 5'-flap structure-specific endonuclease; 5' to 3' exonuclease on nicked or gapped double-stranded DNA |
| PCNA | Proliferating cell nuclear antigen; processivity clamp for replicating DNA polymerases |
See text for further details.
Defects in BER have been genetically linked to cancer risk, immunological dysfunction, and neurodegenerative disease (Table 2). For instance, in a sub-set of individuals that develop multiple adenomatous colon polyps or colorectal cancer, mutations have been found in the E. coli MutY homolog DNA glycosylase (MUTYH) gene [12]. This autosomal recessive form of familial adenomatous polyposis (FAP) is now more commonly referred to as MYH-associated polyposis (MAP) [13, 14]. The human MYH protein excises adenine bases from DNA when inappropriately paired with guanine, cytosine, or 8-oxo-dG, a major base lesion often used as a biomarker for oxidative stress [15]. Several missense mutations have been identified in the MUTYH gene [16], and the few that have been functionally characterized, e.g., Tyr176Cys and Gly393Asp (which account for ~75% of the MAP alleles in Caucasians), result in a protein with at best poor DNA glycosylase activity [17]. This defect in DNA repair capacity presumably gives rise to the elevated G:C to T:A transversions seen in MAP patients, due to erroneous replication of unrepaired A:8-oxo-dG substrates [13, 14].
Table 2.
Connections of mutations in genes related to BER or an associated sub-pathway with human genetic disorders.
| Gene | Disorder | Key Points |
|---|---|---|
| MUTYH | MAP: MYH-associated polyposis |
|
| UNG | HIGM5: Hyper-IgM Syndrome Type V |
|
| APTX | AOA1: Ataxia with Oculomotor Apraxia type 1 |
|
| TDP1 | SCAN1: Spinocerebellar Ataxia with Axonal Neuropathy |
|
| PNKP | MCSZ: Microcephaly and Seizures |
|
See text for details.
Mutations in the gene encoding the major uracil DNA glycosylase (UNG) have been linked to a primary immunodeficiency disorder called Hyper-IgM Syndrome Type V (HIGM5) [18, 19]. The HIGM syndromes are a heterogeneous group of genetic disorders typified by antibody defects, immunodeficiency, and susceptibility to opportunistic infections. The symptoms of HIGM5 are similar to those of HIGM2, a disease that stems from mutations in the AICDA gene. The AICDA encoded protein, activation-induced deaminase (AID), is a DNA cytosine deaminase that generates uracil bases essential for somatic hypermutation and class switch recombination, the molecular processes behind antibody diversification in B cells [20, 21]. While the major role of UNG is typically to cope with uracil residues that arise in genomic DNA via spontaneous cytosine deamination or misincorporation during chromosome replication – events that can lead to mutagenesis or cellular dysfunction – the protein has an essential role in promoting the AID-initiated response as well [22]. The HIGM5-associated UNG mutations described to date either introduce a premature stop codon that generates a dysfunctional truncated protein or change amino acid residue 251 from phenylalanine to serine (Phe251Ser), resulting in an abnormal mitochondrial translocation of the glycosylase [19]. Such mutational events lead to the accumulation of uracil bases in the nuclear genome and underlie the IgG and IgA deficiency that stems from the inability of B cells to carry out AID-directed isotype switching during the immune response [23, 24].
Recent data indicate a link between defects in single-strand break processing, a specialized sub-pathway of BER, and neurological abnormalities [10]. For instance, ataxia with oculomotor apraxia type 1 (AOA1) is an autosomal recessive disease characterized by early-onset and slowly progressive cerebellar ataxia, areflexia, and peripheral neuropathy that is caused by a mutation in the APTX gene encoding a protein named aprataxin [25, 26]. The main function of aprataxin appears to be to excise 5'-AMP groups that arise in DNA after abortive ligation reactions, which typically occur at sites of oxidative strand breaks where the 3'-terminus is nonligatable (e.g., 3'-phosphate groups) [27]. The disease-linked mutations in APTX range from nonsense (most notably Trp279X), frameshift, or missense changes of a conserved, functionally/structurally important residue, largely resulting in a gene product with inactive or impaired hydrolase function [28]. For individuals affected with the autosomal recessive disorder spinocerebellar ataxia with axonal neuropathy (SCAN1), a single homozygous mutation has been identified in the gene encoding tyrosyl-DNA phosphodiesterase 1 (TDP1) [29]. TDP1 functions to remove 3'-obstructive groups such as 3'-phosphoglycolates and trapped 3'-topoisomerase I intermediates from DNA strand break ends [30, 31]. The pathogenic mutation in TDP1 (A1478G) substitutes a conserved, active site histidine residue (His493) with arginine, severely diminishing the phosphodiesterase activity of the enzyme and creating a mutant protein that forms a prolonged covalent intermediate with DNA [32]. Additionally, a previously unknown autosomal recessive disease characterized by microcephaly, early-onset, intractable seizures and developmental delay (MCSZ) results from mutations in the polynucleotide kinase 3'-phosphatase (PNKP) gene [33]. PNKP, as the name suggests, phosphorylates DNA at 5'-hydroxyl termini and dephosphorylates 3'-phosphate termini to permit polymerization and/or ligation reactions [34, 35]. The PNKP mutations of MCSZ include a 17-bp duplication in exon 14, a 17-bp deletion, and missense substitutions, all of which lead to low protein production/stability and/or impaired protein activity [33]. It is worth pointing out that aprataxin, TDP1, and PNKP have each been shown to directly or indirectly interact with the single-strand break scaffold protein XRCC1, physically linking them to this sub-pathway of BER [10].
Despite the fact that AOA1, SCAN1 and MCSZ exhibit severe neurological defects, none of these disorders display cancer predisposition. This observation appears to suggest that strand break repair processes function primarily to maintain viability of certain cell types, yet do not play a major role in preserving genetic integrity, at least in the absence of exogenous challenges. Specifically, the tissue-selective nature of these diseases presumably stems from: (i) cells that are highly metabolically active, e.g, neurons, likely generate a greater number of lethal oxidative DNA strand breaks that require fully active repair mechanisms to avoid transcriptional arrest and apoptotic signaling and (ii) replicating cells, unlike differentiated neurons, utilize compensatory pathways, e.g., homologous recombination, to faithfully resolve any unrepaired strand break interruptions [36]. It is worth pointing out that the strictly neurological defects of these disorders are also similar in many ways to diseases that stem from mitochondrial dysfunction.
It has been argued that cancer cells must acquire a mutator phenotype, potentially arising from an impaired DNA damage response, to drive multistage carcinogenesis and to account for the multiple mutations characteristic of many cancers [37]. Recent studies have indicated that POLβ is mutated in a high percentage of tumors and that several of these mutations alter the coding sequence of the transcript [38]. Of the few tumor-associated POLβ variants characterized, the amino acid changes have been found to alter the ability of the protein to carry out accurate or efficient strand break resolution. For example, a substitution of Ile260Met or Lys289Met results in a mutant protein that exhibits an increased propensity to insert the incorrect nucleotide during gap repair [39, 40]. This replicative infidelity could potentially lead to a tumor-tumorassociated mutator phenotype. Indeed, expression of the POLβ variants Ile260Met and Lys289Met promotes cellular transformation in cell culture models [41, 42]. Whether amino acid changes in other BER proteins have a similar adverse outcome on repair accuracy, including a dominant-negative effect, is currently unknown. It is worth pointing out that an imbalance between the different steps of BER can result in genomic instability [43, 44] and more recently has been argued to be responsible for the somatic CAG repeat expansion observed in the progressive neurodegenerative genetic disorder Huntington disease [45].
Variability in BER Capacity
The above examples (summarized in Table 2) document a clear connection between pronounced defects in BER, or a related sub-pathway, and disease development. However, as evidenced with homozygous knockout mice, a severe deficiency in many of the key components of the BER pathway is likely to be incompatible with life [46], raising the following questions: “Do more subtle defects in BER exist within the human population?” and “Do these defects associate with disease susceptibility, at least in an exposure-dependent manner?”
As for the first question, early studies have indeed shown that DNA repair capacity differs among individuals. The pioneering assays used bleomycin (a radiomimetic agent) or γ-radiation applied in the G2 phase of the cell cycle to measure chromatid breaks and gaps as an indicator for repair efficacy [47–50]. These approaches have become known as the mutagen sensitivity or G2-phase chromosomal radiosensitivity assays, and over the past 30 years, have employed different test mutagens to appraise the varying DNA damage responses in a range of cell types, including peripheral blood lymphocytes, lymphoblastoid cell lines, skin fibroblast lines, white blood cells and buccal cells [51–54]. One of the typical strategies used genotoxins such as benzo[a]pyrene diol epoxide (a constituent in cigarette smoke) or ultraviolet light to assess nucleotide excision repair, a pathway designed to cope with bulky, helix-distorting DNA base adducts such as photodimers [53]. With the advent of detecting DNA double-strand breaks by quantifying the levels of γ-H2AX foci or measuring cellular sensitivity to low-dose-rate ionizing radiation, investigators reported a variability of around 1.5-fold in strand break repair efficiency among ataxia telangiactasia heterozygotes, retinoblastoma family members, and apparently normal individuals [55–57]. Further evidence for a disparity in DNA repair or a DNA damage phenotype emerges in family and twin studies, where heritability of radiation sensitivity associates with family members who are radiation sensitive or in families with breast cancer-affected relatives [52, 54].
Of the various repair capacity assays [58, 59], the Comet assay (or single cell gel electrophoresis assay) has most commonly been used to interrogate BER. This sensitive technique measures the steady-state level of endogenous DNA damage (i.e., alkaline-sensitive sites and single-strand breaks) in an individual eukaryotic cell, and can be designed to determine repair rate following exposure to a select genotoxin [60]. One study assessing endogenous DNA damage has revealed a continuum of damage that is highest among cancer cases (breast and thyroid), less in controls, and suggestively lowest in hyper-normal individuals (advanced age, no personal history of any cancer and no cancer reported in their families), with a Comet tail length ranging from 15.4 to 56.8 (an ~3.7-fold difference) among controls [61]. Case-control studies using the Comet assay with a radiation challenge or a combination of Comet and the human 8-oxo-dG DNA glycosylase (OGG1; known as Comet/Fragment Length Analysis using Repair Enzymes or FLARE) show that risks of bladder, kidney, esophageal, and lung cancer are elevated among those who exhibit higher levels of oxidative DNA damage [62–65]. Studies of cancer risk using the Comet assay are too numerous for full elaboration here, but some, such as the OGG1 Comet/FLARE assay have been developed expressly to evaluate oxidative metabolism, which has obvious relevance to BER. The overall conclusion from these studies suggests that normal human variation exists with respect to several measures of DNA damage, whether as baseline or constitutive damage, the amount of induced damage after a mutagen challenge, or the amount of repaired or unrepaired residual damage after the challenge. Estimates suggest that between 10 to 30% of the normal human population may be “sensitive” to various challenges, and thus, be defective in some aspect of DNA repair, possibly in the range of up to a 50% reduction [54], although such estimates are difficult to generalize across various assays and study populations.
Paz-Elizur et al. [66] recently established a robust and reproducible strategy for specifically assessing 8-oxo-dG repair activity in protein extracts prepared from human peripheral blood mononuclear cells (PBMCs). Since 8-oxo-dG is most commonly processed by OGG1, the assay was named “OGG”, although it was acknowledged that other enzymes maintain the ability to remove this base lesion [67]. Once the methods for cell handling and extract preparation, as well as the assay conditions were optimized, OGG activity was determined for whole cell extracts prepared from 120 healthy individuals. These investigations revealed an ~2.8-fold range in inter-individual variation for 8-oxo-dG repair, with no statistical difference between males and females, or between smokers and non-smokers [66]. This basic approach has also been applied to determine the association of OGG capacity with tumorigenesis and such studies suggest that low OGG activity may be a risk factor in lung and head and neck cancer [67].
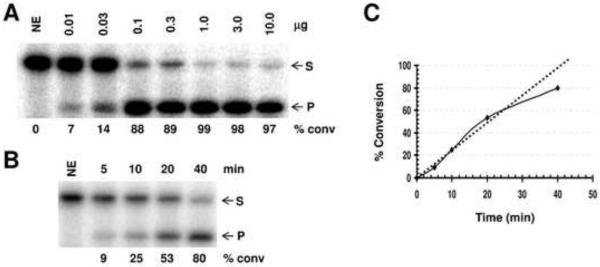
Using the platform established by Paz-Elizur and colleagues [66], we determined the extent of variability in the subsequent steps of BER among a set of EBV-transformed lymphoblastoid cell lines from nineteen cancer-free controls, two cancer patients, and two long-lived cancer-free individuals (hyper-normals) [61]. In brief, after culturing cells and preparing pellets for each line, whole cell extracts were generated using the mild freeze-thaw/hypotonic buffer strategy described for the OGG assay (see above). We then determined protein concentrations using the Bio-Rad Protein Assay, and verified these measurements via SDS-polyacrylamide gel electrophoresis followed by fluorescent protein staining with SYPRO Red®. Once extract concentrations were confidently determined, AP endonuclease, single nucleotide gap-filling, and nick ligation activities were measured using radiolabeled deoxyoligonucleotide substrates (see for instance [68, 69]). The fundamental approach is detailed in Figure 2 for the AP endonuclease assay (panels A thru C).
Figure 2.
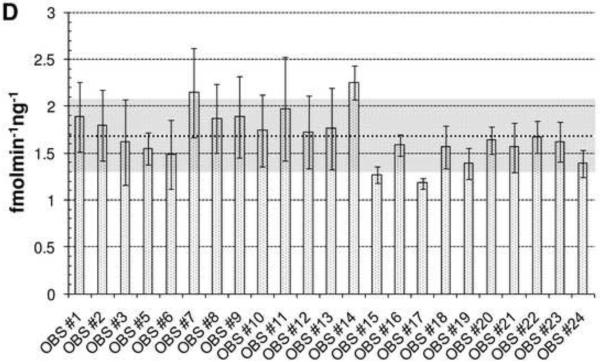
Total AP site incision activity of the whole cell extracts from the EBV-transformed lymphoblastoid cell lines. (A) Concentration-dependent conversion of an AP site-containing DNA substrate to the incised product. Shown is an image of a representative denaturing polyacrylamide gel. Following preparation of the whole cell extract and protein concentration determination (see text), incision reactions were performed using a 34 mer duplex deoxyoligonucleotide substrate harboring an abasic site analog (tetrahydrofuran) for 10 min at 37°C as essentially described [68, 69]. Reactions were stopped and resolved on a 15% polyacrylamide-urea denaturing gel to separate the 5'-32P-labeled intact 34 mer substrate (S) from the cleaved, labeled 15 mer product (P). Indicated are the amount of whole cell extract used in the reaction (μg) and the percentage of substrate converted to product, determined by standard phosphorimager analysis. NE = no extract control. (B) Time-dependent kinetics at 20 ng of whole cell extract. Shown is an image of a representative gel analysis at the indicated time points (min). See panel A for further details. (C) Plot of time-dependent reaction kinetics from incision results of panel B. (D) Total AP endonuclease capacity of the lymphoblastoid cell line extracts. Twenty ng of whole cell extract from the indicated cell line (designated by OBS #) was incubated with 0.5 pmol of labeled duplex AP-DNA substrate in TPE buffer containing 50 mM Tris-HCl (pH 7.1), 1 mM EDTA, 115 mM KCl, 20 μg of bovine serum albumine, and 2 pmol of polydA-polydT for 8 min at 37°C. Reactions were then analyzed by denaturing gel electrophoresis and standard phosphorimager analysis. Shown is the average and standard deviation of 3 independent incision reactions, reported as fmol of substrate converted to product per min per ng of total extract. The dashed line and shaded area denote the average and standard deviation, respectively, of all incision results obtained for all the extracts (i.e. the general range of assay variability). Note: OBS#4 did not efficiently grow to allow for cell harvesting and thus was not analyzed for BER-related biochemical activities.
Our results indicate that for AP site incision, the twenty-three individuals examined exhibited an ~1.9-fold inter-individual variation, from 1.18 fmolmin−1ng−1 for OBS#14 to 2.25 fmolmin−1ng−1 for OBS#17, with a mean of 1.68 for all of the individuals (Figure 2D). However, for each study participant (other than OBS#17), the average and standard deviation at least partially overlapped with the average and standard deviation of the sample set (Figure 2D, dashed line and shaded area, respectively). Generally consistent with our results herein, Redaelli et al. [70], using a less sensitive plasmid-based assay and protein extracts prepared from lymphocytes, observed a range of AP endonuclease activity of ~2.5-fold among 10 healthy individuals. Rossi et al., using the same plasmid-based assay and peripheral blood lymphocytes, found no obvious age-related decline in AP site incision capacity among 23 women [71] and no association of AP endonuclease activity with early onset sporadic breast cancer [72]. As for gap-filling (Figure 3A) and nick ligation (Figure 3B), we observed an ~1.3-fold and 3.4-fold inter-individual variation, respectively. The greater disparity in nick ligation likely stems at least in part from the low overall activity and the corresponding limited sensitivity of the assay. Like the AP endonuclease profile, the average repair capacity of each individual for gap-filling and nick sealing generally fell within the experimental variability of the population (Figure 3, shaded area). It is worth pointing out that in experiments not detailed herein, we found nick ligation to be the rate limiting step for complete uracil-initiated BER using extracts prepared in the manner described above.
Figure 3.
Single-nucleotide gap-filling (A) and nick ligation (B) capacity of the twenty-three EBV-transformed lymphoblastoid cell line extracts. (A) Single-nucleotide gap-filling. The linear range of activity for this biochemical repair step was found to occur at 1 μg of whole cell extract and 10 min of reaction time at 37°C. Three deoxyoligonucleotides (15P, pG18, and 34G) were annealed to form a one nucleotide gap substrate, with the upstream primer strand 5'-32P-labeled [68, 69]. Gap-filling reactions consisted of 10 μ1 mixture containing 1 μg of protein extract, 0.5 mM dCTP, and 1 pmol of labeled DNA substrate in TPE buffer, and the percentage of primer extended by a single-nucleotide was monitored following high resolution denaturing gel electrophoresis and standard phosphorimager analysis. Shown is the average and standard deviation of 3 independent reactions, reported as fmol of substrate converted to product per min per μg of total extract. The dashed line and shaded area denote the average and standard deviation, respectively, of all gap-filling results obtained for all the extracts (i.e. the general range of assay variability). (B) Nick ligation. The linear range of activity for this biochemical repair step was found to occur at 4 μg of whole cell extract and 30 min of reaction time. Three deoxyoligonucleotides (15P, pC19, and 34G) were annealed to form a 3'-hydroxyl and 5'-phosphate ligatable DNA nick, with the upstream primer strand 5'-32P-labeled [68, 69]. Reaction mixture (10 μ1) containing 4 ug of protein extract, 1 mM ATP, and 1 pmol of labeled DNA substrate in TPE buffer was incubated for 15–30 min at 37°C, and the percentage of full-length ligated product was determined following denaturing gel electrophoresis and standard phosphorimager analysis. Shown is the average and standard deviation of 3–6 independent reactions, reported as fmol of substrate converted to product per min per μg of total extract. The dashed line and shaded area denote the average and standard deviation, respectively, of all nick ligation results obtained for all the extracts (i.e. the general range of assay variability).
Expounding upon the results of Figures 2 and 3 briefly, the two cancer patients (OBS#1, breast and ovarian, and #15, prostate and other) do not have any features related to their various BER efficiencies that stand out relative to the controls or the two hypernormals (OBS#2 and #3), other than OBS#1 displays slightly lower AP endonuclease activity than nearly all of the other study participants, although overlapping with the shaded area. While an intriguing observation, significant follow-up work is required to address any possible role of APE1 deficiency in this individual's disease. Comparing the various BER biochemical results with the previous Comet assay measurements also did not reveal any compelling relationship (data not shown). Obviously, what is described herein is simply a pilot study to establish proof-of-principle for the methodologies, and much larger experiments are required in the future to evaluate (i) the reliability of the approach, (ii) the effects of different cell types or cellular transformation on the readouts, and of course (iii) any possible association with disease risk. Related to point (ii), a recent study found that while the OGG assay is robust and reproducible when used on PBMCs (intra-assay coefficient of variation = 8%), a high intra-culture variability was observed with EBV-transformed lymphoblastoid cell lines (intra-culture coefficient of variation = 16.8%) [73].
Similar extract and biochemical approaches can be employed to evaluate repair of additional base lesions (oxidative, alkylative, or hydrolytic), as well as the processing of non-conventional single-strand break termini (e.g., 3'-phosphates or -phosphoglycolates). A key consideration before proceeding, however, is the robustness of the activity of interest, particularly relative to the nuclease contaminants that will undoubtedly be present in a whole cell protein extract. In addition, such an approach is limited in that it interrogates only a single event during BER, and not the pathway as a whole like methods such as the Comet assay.
Besides the configuration of the repair capacity assay itself, another important consideration is the selection of the source or cell type for the analysis. For instance, it has been reported that monocytes (a specific blood cell population), unlike the dendritic cells derived from them or peripheral blood lymphocytes, are defective in BER, due largely to reduced XRCC1 and LIG3α protein levels [74]. Furthermore, since generalizing within individuals with respect to differences in variation between tissues has not been done extensively, inferences to target organs from results with PBMCs or lymphoblastoid cell lines, for example, are currently tenuous. To more directly weigh surrogate tissue against the target organ, Paz-Elizur et al. [75] compared protein extract activities from PBMCs and lung tissue in their study of lung cancer. The authors found an R2 of 0.858 (p = 0.003) between OGG repair capacity in PBMCs and lung tissue removed during surgery in the same patients (n = 7). Other investigators have compared PBMCs with bronchial epithelial cells in 10 subjects and found a correlation co-efficient of 0.83 (p = 0.003) with respect to the activity of DNA-dependent protein kinase, a protein that operates in non-homologous end-joining [76]. While the list of examples touched upon herein is not exhaustive, it does underscore the importance of understanding the relationship between easily obtainable surrogate samples, such as peripheral blood, and the organ of interest.
As for the second question proposed at the beginning of this section, how differences in BER capacity correlate with human disease is still unclear. However, it is noteworthy that mice haploinsufficient (i.e., heterozygous) in BER exhibit increased vulnerability to tumor development (see for example [77]). We re-visit in the final section the topics of BER capacity assays and their potential value in determining disease risk.
Sequence Variation in BER Factors
Mohrenweiser and colleagues reported in 1998 that extensive natural variation occurs in genes that encode DNA repair proteins [78]. Since that original publication, it has become recognized that humans are in fact quite heterogeneous in their DNA repair genetic composition [79, 80]. However, whether this genetic variability affects BER capacity has only been crudely evaluated (see next section). We have compiled a list of reported nonsynonomous variants (arising from missense mutations) that have been found both in seemingly healthy individuals and within disease tissue in several key BER proteins (Table 3). Keep in mind that Table 3 does not include: (i) all genes that have been associated with BER [e.g. the compensatory DNA polymerases ι, λ, ε or δ, the accessory factor replication factor C (RFC), or poly(ADP-ribose) polymerase 1 (PARP1/ADPRT1)]; (ii) nucleotide changes that do not alter the coding properties of the transcript (i.e., synonomous), but may affect transcript stability; or (iii) nucleotide differences observed in the untranslated regions or promoter regulatory elements that may affect gene expression. For a more comprehensive and up-to-date list of BER nucleotide changes visit http://egp.gs.washington.edu/welcome.html and http://www.ncbi.nlm.nih.gov/SNP.
Table 3.
Nonsynonomous substitutions in select BER proteins.
| Gene | Population Variants | Disease-Associated Variants |
|---|---|---|
| MUTYH | P18Le, V22Me, G25De, W103Ren, E155Q, A227Ve, R241We, L257Re, Q324H, A373Ve, Q335Hn, Q338He, G382D, N394Se, S487Pe, G500En, G503Ee, L526Mn, L529Me, R531Qn, R534Qe | See Table 2 and [99,100,147] |
| UNG | Q4Re | R88C, G143R |
| OGG1 | P27Te, P29Re, R46Q*, A85Se, R154H*e, R229Q*en, E230Qe, A288V*en, G308Ee, D322Nn, F324Se, S326C*n | G12E*, S31P*, A53T*, R131Q*, R169Q*, S232T*, I321T, T398S* |
| NEIL1 | S82C*en, G83D*en, C136R*e, A164Se, I182Me, S248Re, D252N*en, R339W, R339Qe | E181K*, P208S, K242R*, G245R*, R334G* |
| NEIL2 | P69Ae, T70Sen, R103Qen, R103We, V141Ie, R257Len, P304Ten | P123T, R164T |
| NEIL3 | R38Cen, S61Ge, V76Men, P117Ren, Q144Pe, M149Te, Q172Hen, L199Fe, L199Ve, R251He, H286Ren, C290Ye, R315Q, I346Ven, P366Le, T406Ie, N418Se, N438De, L443Pen, N448Ke, K466Te, H471Qeen, S473Le, C480Fe, R520Gen, A547Sen, H556Ren | D132V |
| NTHL1 | R21Wen, G31V, R33Ke, M102Le, Q141He, I176Te, S234Len, D239Ye, A264Te | None [107] |
| MPG | K17Qe, A21Ve, K22Qn, G24Ee, D45Ne, R55Ce, P59Le, Y66He, Y71Hn, Q88Re, R115Ce, R136Qe, A253Ve, A258Vn, A293Se, A298Sn | None [107] |
| TDG | I134Me, G199Sen, K201Te, V268Ge, K300Re, Y346De, G347Re, V367Le, V367Mn, A374Te, P379He, G381Ee, S396Ne | R66G |
| SMUG1 | G15Ven, C23Se, R105Wen, N244e | None [107] |
| APE1 | K35Qe, G39Ee, Q51Hen, I64Te, I64Ven, D148E*en, G241Re, P311Se, T313Ae, A317Ve | P112L, R237C |
| POL β | Q8R, Q31Ke, K52Ee, K54Te, R137Qe, G144Ae, T196Se, P242Ren | I260M, K289M and [38] |
| XRCC1 | R5He, R7Pe, V10Me, V72Aen, R107He, E157Ke, P161L*en, F173L, N183Se, R194W*en, A214Ve, R280H*en, K298Ne, T304Aen, P309Sen, R399Q*n, V381Me, S485Ye, E491Ke, L514Pe, H528Ye, R559Qe, R560We, Y576Ne, Y576S*en | ND |
| APTX | G44Se, I41Te, K167Ee, S158Ye | See Table 2 and text |
| TDP1 | E95De, P101Le, A134Te, D187Ge, R304Qe, R535He, T569Ae, P598Le | See Table 2 and text |
| PNKP | P20Sen, A63Ven, T103Ie, E113Ke, P136Le, R139He, R180Sen, Y196Nen, T217Se, V478Gen, R513We | See Table 2 and text |
| LIG1 | A17Ee, A24Ven, S47Fe, S51Ce, P52Le, R62Wen, D72Ge, S91Pe, R94He, P119Le, P203Le, R235We, G249Een, N267Sen, Y289Fe, S318Fe, V349Men, L355Fe, V369Ien, R409He, M480Ven, K487Re, E497Ge, T614Ien, R641Le, D647Ne, R672Ce, E673D, R677Len, S839Ne, Q892He, T918Pe | E566K, R771W |
| LIG3 | R30Ce, R100Ce, G190Ce, R224We, D349Ge, D679Ve, R780Hn, K811T, R867He, S887Fe, K898Te, P899S, T927Me | ND |
| FEN1 | P151Le, R192Qe, R211We, L218Qe, E357De, F376Le | See [82] |
| PCNA | S39Re | ND |
Population variants were identified as follows:
denotes variants from the NIEHS SNP program (http://egp.gs.washington.edu)
denotes variants from the NCBI/Entrez SNP resource (http://www.ncbi.nlm.nih.gov/SNP). Disease-associated variants are ones that have not been observed in the normal population.
Indicates those variants that have been functionally characterized (see text).
Italics designates variants that are found at 3% or higher frequency. None = genes that have been sequenced in at least one disease state and have not been found to have missense mutations (i.e. changes that lead to nonsynonomyous amino acid substitutions). ND = not determined (to our knowledge). See main text for additional information.
As gleaned from previous resequencing efforts, it appears that on average there are at least 4 amino acid variants per DNA repair gene, with an average allele frequency of ~5% (see for instance [79]). Analysis of the current sequence results also indicate that: (1) rare variants (allele frequency of <3%) make-up >75% of the total and (2) about 1/3rd of the amino acid changes are predicted to adversely impact structure/function based on computational scrutiny. Given the level of genetic complexity, it is evident that very few individuals are homozygous wild-type for all genes of a single DNA repair pathway. Determining how these complex genotypes ultimately affect repair capacity and disease susceptibility is the challenge going forward. Finally, it is noteworthy that differing levels of variability are tolerated (Table 3), with some genes harboring changes that lead to numerous different amino acid variants (e.g., MUTYH, XRCC1, and LIG1) and others tolerating almost none (e.g., PCNA and UNG). Mutations in the structure-specific 5'-flap endonuclease, FEN1, which operates as an important component of long-patch BER, as well as in DNA replication [81], have recently been connected to autoimmunity, chronic inflammation and cancer [82], yet the protein will not be discussed further herein.
Functional Consequences of Sequence Variants in BER
DNA Glycosylases
While many sequence variants have been identified and numerous molecular epidemiological studies have been carried out to assess the potential association of the high frequency DNA repair single nucleotide polymorphisms (SNPs) with a specific disease, particularly cancer (see for instance [83, 84]), comparatively few biochemical or cellular investigations have been performed to delineate the functional consequences of nucleotide changes that occur within the coding or regulatory regions of DNA repair genes. In the case of the BER DNA glycosylase OGG1, studies have by and large consistently indicated that the polymorphic variant Ser326Cys (observed at a frequency of ~32%) results in a protein that exhibits a slightly defective ability to process substrate base lesions in vitro or complement repair-deficient bacteria or human cells [85–87]. OGG1 is the major repair protein for removing not only 8-oxo-dG base damages from DNA when paired with cytosine, but has robust activity on 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FaPy) and 8-oxoadenine lesions as well [88]. More recent biochemical experiments have indicated that the extent to which the Ser326Cys polymorphism negatively impacts OGG1 function is influenced by the base opposite 8-oxo-dG in DNA, and that the OGG1-Cys326 variant has an aberrant DNA binding conformation involving dimerization (perhaps via a disulfide linkage) that may contribute to its inability to coordinate with other proteins of the BER pathway [89]. There is also evidence that oxidation of Cys326 alters the repair competence of the variant protein, and consequently, the overall capacity of the cell to cope with oxidative stress [90, 91]. In addition, it has been suggested that the Ser to Cys substitution adversely affects the dynamic intracellular localization of OGG1, possibly by altering the phosphorylation status of the protein [92]. These data collectively indicate that the OGG1-Cys326 variant is hampered in its ability to initiate BER in comparison to the wild-type protein. Although the relationship of the variant to cancer risk remains unclear, there appears to be a consensus building for an association with at least lung cancer susceptibility [93, 94]. Another polymorphic variant (observed at ~3%), Arg229Gln, was found to be thermolabile both in cells and in vitro, although the protein exhibited normal 8-oxo-dG excision activity in controlled biochemical assays [95].
Several possible tumor-associated variants have been described in OGG1, including Gly12Glu, Arg46Gln, Arg131Gln, Arg154His, Arg169Gln, Ser232Thr, and Ile321Thr [85, 96–99]. Of these, Arg46Gln (identified in 1 of 52 lung cancer cell lines), Arg131Gln (found in 1 of 40 human tumors), and Arg154His (detected in 1 of 9 gastric cancer cell lines) have been reported to exhibit impaired DNA glycosylase function, while the substitutions at codons 12, 169 and 232 had no effect on repair activity. Additional OGG1 variants (Ser31Pro and Thr398Ser) have been observed in the chronic inflammatory disease, primary sclerosing cholangitis (PSC), but neither amino acid change appears to alter the enzymatic activity of the protein [100]. Mutations in OGG1 have also been found in 4 of 14 Alzheimer disease patients analyzed, including a deletion that results in a C-terminal truncation (found in 2 of 14 AD samples) and the missense mutations Ala53Thr (found in 1 of 14 AD samples) and Ala288Val (also observed in the normal population at a frequency of ~1%) [101]. The truncated protein lacked 8-oxo-dG glycosylase activity, while the two amino acid variants exhibited around 50% of wild-type activity in vitro, suggesting a possible role in disease pathology.
Sequencing efforts have identified nucleotide changes that modify the amino acid composition of E. coli endonuclease 8-like protein (NEIL1). NEIL1 is a DNA glycosylase that removes oxidative base lesions from DNA and there is some suggestion based on a mouse knockout model that the protein plays an important role in the prevention of diseases associated with metabolic syndrome [102]. Four rare (≤1%) variants found within the normal population, Ser82Cys, Gly83Asp, Cys136Arg and Asp252Asn, have recently been characterized biochemically [103]. Cys136Arg appears to be deficient for both DNA glycosylase and AP site nicking activity, Gly83Asp exhibits reduced base excision activity and an altered nicking function, while the other two variants display essentially normal in vitro enzymatic properties. A more recent report observed Gly83Asp in two patients suffering from PSC with cholangiocarcinoma, and found that the variant, while defective in double-stranded DNA glycosylase activity, was able to excise substrate bases from single-stranded DNA with wild-type efficiency [100]. A Glu181Lys NEIL1 mutant was also observed in a patient with PSC and the protein was insoluble upon expression in bacteria, perhaps suggesting mis-folding of the gene product. In addition, three amino acid variants [Lys242Arg, Gly245Arg (found in 1 of 20 blood samples from Chinese patients) and Arg334Gly (identified in 1 of 71 Japanese patients)] and two deletion mutants (Pro2del and Glu28del) have been reported in gastric cancer, with only the truncated proteins exhibiting reduced repair capacity [104]. Finally, two rare NEIL1 variants (frequencies of < 1%), Pro208Ser and Arg339Gln, were observed in patients with multiple colorectal adenomas, yet neither variant was functionally characterized, and the latter was also detected in a normal control [99].
There are several reported amino acid variants, both population-based and potentially disease-associated (Table 3), for the other BER DNA glycosylases – MUTYH, UNG, NEIL2, NEIL3, the E. coli endonuclease 3-like protein (NTH1/NTHL1), the methylpurine DNA glycosylase (MPG), the G/T mismatch specific thymine DNA glycosylase (TDG), and the single-strand selective monofunctional uracil DNA glycosylase (SMUG1). Specifically, although rare [105], a single sequence variant has been observed in a case of sporadic glioblastoma (Gly143Arg) [106] and familial colorectal cancer (Arg88Cys) in UNG [107]. Missense mutations have also been detected in a single heterozygous colorectal cancer patient in NEIL2 (Pro123Thr, Arg164Thr), NEIL3 (Asp132Val), and TDG (Arg66Gly); unique amino acid variants were not found in NTHL1, NEIL1, MPG, or SMUG1 in familial colorectal cancer samples [99, 107]. The relationship of any of these disease-associated variants to disease pathology is unknown. To our knowledge, none or very few of the variants in many of the DNA glycosylases listed in Table 3 have been functionally evaluated, either biochemically or in cell biology experiments.
AP Endonuclease
An early report described missense mutations in APE1, the major human AP endonuclease, in 6 of 11 patients with sporadic amyotrophic lateral sclerosis (ALS, better known as Lou Gehrig disease) or familial ALS (FALS), including Leu104Arg, Glu126Asp, Asp148Glu, Asp283Gly and Gly306Ala [108]. However, other than the Asp148Glu substitution and a Gln51His variant, both of which are now recognized as polymorphic variants (observed at a frequency of ~46% and ~3%, respectively), no nonsynonymous missense mutations were observed in two larger independent screening efforts of either 84 or 153 ALS and FALS patients [109, 110]. These data suggest that the original list of APE1 mutants were experimental artifacts.
Additional resequencing of genomic DNA from 128 unrelated, presumably healthy individuals found not only the common Asp148Glu and Gln51His variants, but also a Gly241Arg substitution [111]. Biochemical characterization of many of the reported APE1 variants at that time revealed that Asp148Glu, Gly241Arg, and Gly306Ala displayed essentially normal endonuclease and AP-DNA binding activities, whereas Leu104Arg, Glu126Asp, and Arg237Ala (found in the NCBI database) exhibited ~40–60% reductions in AP site incision capacity. Although not specifically characterized, the Asp283Gly variant was noted to be similar in nature to an Asp283Ala mutant previously shown to exhibit ≤10% repair capacity. While some genotype association studies have suggested a role for the Asp148Glu substitution in cancer risk, such as of the breast [112], a more recent investigation [113], genome wide association studies (GWAS), [114–117], and meta-analysis [84], have not found an association with APE1 and cancer susceptibility, consistent with the Asp148Glu variant exhibiting normal in vitro nuclease capacity [111, 118].
Somatic mutations have also been found in APE1 in 3 of 20 endometrial tumors, one resulting in a premature stop codon and two changing the amino acid composition of the protein (Pro112Leu and Arg237Cys) [119]. Preliminary in vitro data indicate that the Arg237Cys mutant behaves similarly to Arg237Ala, including the altered protein stability described previously (unpublished observation and [111]). The other APE1 variants (Table 3) have not been characterized functionally, and none of the variants have been examined in more inclusive cell-based assays to date. It might also be interesting to explore the consequences of amino acid substitutions in the N-terminus of APE1 (e.g.., Gln51His and Ile64Val) on its so-called REF-1 activity, a distinct function of the protein involved in regulating the DNA binding efficiency of transcriptional activators such as AP-1, p53, and NF-κB [5, 120]. Finally, a recent study employing bioinformatic evaluation of the expressed sequence tag (EST) database identified a total of 80 missense mutations in APE1 [121], although most of these require follow-up validation and are presumably sequencing artifacts.
Strand Break Processing Proteins
Besides the tumor-associated variants of DNA POLβ described earlier, a number of unique amino acid changes have been identified in resequencing efforts of the population (Table 3). To our knowledge, none of these variants have been functionally characterized. Similarly, there are no published reports describing the functional impact of the population-based amino acid substitutions in LIG1 or LIG3, or in the BER genes that have been causally linked to disease, i.e., MUTYH, APTX, TDP1 and PNKP. It's worth pointing out that in one individual – who displayed symptoms of immunodeficiency, stunted growth and cellular DNA-damaging agent sensitivity – two missense mutations (Glu566Lys and Arg771Trp) have been found in the different alleles of LIG1 that strongly reduce the ability of the enzyme to form a critical, covalent adenylate reaction intermediate [122]. LIG1, however, not only functions in specific pathways of BER, but is also the major DNA ligase in proliferating mammalian cells for replication, as well as having roles in other DNA repair responses [7].
XRCC1 is a non-enzymatic nuclear protein that operates as a support scaffold, facilitating primarily protein-protein interactions, to coordinate BER and single-strand break repair responses [123, 124]. Quite a number of amino acid variants have been identified in XRCC1 (Table 3), with some of these occurring at sufficiently high frequency that they have been the focus of many epidemiology association studies (see below). There have been a number of experimental efforts to characterize the three main human polymorphic variants: Arg194Trp, Arg280His, and Arg399Gln. The first such analysis found that Arg399Gln was able to fully rescue the alkylating agent methyl methanesulfonate (MMS) sensitivity and the DNA repair defect of the XRCC1-deficient Chinese hamster ovary cell line EM9 [125]. A subsequent study reported that Arg399Gln, unlike the Arg194Trp or Arg280His variants, was unable to correct the bleomycin-induced increase in micronuclei frequency of EM9 cells [126]. Yet another investigation found that Arg280His was able to only partially correct the MMS sensitivity and single-strand break repair defect of EM9 cells, while Arg194Trp showed wild-type complementation activity [127]. In still another study, Arg280His and Arg399Gln exhibited a weak or marginally impaired ability, respectively, to restore wild-type DNA repair capacity to EM9 cells challenged with a range of genotoxins [128]. A major weakness of this latter effort (unlike the other studies) is the fact that variant protein expression was not examined, and thus, the differences seen in complementation efficacy may simply reflect differing protein levels. Finally, examining a series of biochemical activities of several XRCC1 variants, including Arg194Trp, Arg280His and Arg399Gln, revealed that Arg280His displayed a mild defect in DNA binding capacity, while exhibiting a normal interaction with the protein partners POLβ, PARP1, LIG3α, and PCNA [129]. The other polymorphic variants, as well as the rare variants P161L and Y576S, exhibited essentially wild-type interactions with the proteins above, as well as with DNA. Seemingly consistent with the DNA binding defect seen for Arg280His in vitro, this protein, unlike the other variants, also displayed a slightly shorter retention time at site-specific, laser-induced DNA breaks in HeLa cells, perhaps underlying a repair deficiency.
Despite the numerous efforts to characterize the polymorphic variants of XRCC1, the picture remains cloudy as to which exhibit altered activity, although a majority of the evidence points to a defect in Arg280His and there is currently no evidence for Arg194Trp possessing impaired function. The disparity in experimental results may stem from the fact that the reported amino acid substitutions impart only a minor, and thus difficult to detect defect on protein activity, or differences in the technique or endpoint used to evaluate protein function. Whatever the case, this fact stresses the importance of applying multiple methods, perhaps a series that takes into account the different independent molecular responsibilities of the protein in question, to quantify functional capacity. Epidemiology studies or meta-analysis has found that the Arg194Trp variant may provide a protective advantage for certain cancers; the Arg280His substitution is not associated with colon cancer risk, yet may modify breast cancer risk differently in Caucasian and Asian populations; and Arg399Gln is associated with increased or decreased susceptibility depending on cancer type, smoking habits and potentially ethnicity [130–132]. However, again, like the functional assays, the epidemiology results for each of these variants are largely conflicting and require further clarification.
There are many reports of aberrant BER gene expression associated with various disease states, particularly specific cancer types [133]. Whether or not these changes in gene expression arise from nucleotide sequence alterations or from modifications in chromatin structure or epigenetics is unknown, but it remains possible that nucleotide changes in regulatory regions, such as untranslated regions or promoters, could alter transcription rates, transcript stability, or translation efficiency. In addition, a variety of lifestyle practices and environmental exposures have the potential to alter BER gene expression, presumably in a way that can either negatively or positively affect the ability of a cell to cope with DNA damage. To make this review manageable, we have elected not to touch upon the ongoing studies aimed at characterizing the functional consequences of non-coding or silent nucleotide substitutions, or the role of BER gene expression patterns in disease risk. However, we did not intend to minimize the possible contributions of these factors to the genesis and perpetuation of disease.
Considerations for Today and Tomorrow
As seen with other DNA repair pathways [134], a quantifiable defect in BER, or one of its related subpathways, has been genetically linked to disease (Table 2). In addition, disruption of BER coordination or efficacy through the aberrant activity of a variant protein, such as seen with DNA POLβ, can potentially foster tumorigenesis. Nevertheless, in the case of BER, it is likely that severe deficiency, at least at specific steps of the pathway, will be incompatible with life. Thus, it is anticipated that more subtle defects in BER will be related to disease proneness or susceptibility, particularly in the face of a relevant exposure or during the cumulative life span of the individual [135, 136].
Employment of the Comet assay and other methods to measure BER capacity has indicated variability in repair efficiency. Moreover, epidemiology studies have suggested an association of reduced BER capacity with cancer risk. However, as noted earlier, many studies in this regard are conflicting. In addition, whether the assays will be predictive of cancer or other disease risk remains far from definitive because of a bias inherent in the case-control study design. The bias, known as “reverse causation”, posits that the assay may be measuring the consequence and not the cause of cancer; reverse causation bias has been thoughtfully discussed in previous articles and editorials [51, 52, 59, 137, 138]. In one nested case-control study that assessed the alkaline Comet assay (endogenous damage), the Host Cell Reactivation assay (using the mutagen benzo[a]pyrene diol epoxide), and the G2-phase mutagen sensitivity assay (using bleomycin) among samples collected before lung cancer diagnosis, only the G2-phase mutagen sensitivity assay was associated with lung cancer risk [139].
As the connection between repair capacity and disease gains clarity and predictive usefulness, the requirement for high-throughput, epidemiologic-grade BER-type assays will become more prevalent. Such assays, irrespective of how DNA damage or repair is measured, should be carefully validated and must thoughtfully consider the assumptions under which the samples are collected. Quality control samples (taking into account age, race, ethnicity, dietary and lifestyle practices, etc.), selection of an appropriate surrogate cell type (when necessary), blinded testing of samples, and assessing laboratory drift over time are all important aspects of developing a rigorous set of assays going forward (see further discussion in [140–142]). Moreover, strategies to more clearly distinguish subtle DNA repair pathway deficiencies along the continuum of normal, such as the low-dose-rate assays [54–56], should continue to be explored and then tested in a study design where reverse causation bias would not affect the outcome. Novel approaches, such as the recently described automated method for measuring the formation and repair of DNA single-strand breaks in live cells, should also be investigated in appropriately designed population studies [143]. Work to assess the reproducibility, sensitivity, and specificity of existing or newly developed assays in prospective study designs or using stored samples collected before diagnosis will go a long way towards determining an assay's merit.
Because BER is involved in remediating some radiation-induced DNA damage, it will be important to follow results from GWAS that are specifically designed to detect gene-radiation interactions. The Women's Environmental Cancer and Radiation Epidemiology (WECARE) study has been designed to evaluate the effect of polymorphic variation and ionizing radiation dose on risk of contra-lateral breast cancer. The investigators used a counter-matched design to increase statistical power to detect radiation-gene interactions [144]. Previously the WECARE study has focused on candidate genes, such as ATM, but they are currently conducting a GWAS to assess whether radiation-related risk of contra-lateral breast cancer is modified by any polymorphic variant(s). Should study findings implicate BER-related genes, these would be worthwhile to pursue. Other GWAS projects that might be germane to BER are those that examine potential genetic relationships with cancers that arise from chronic oxidative stress, such as of the stomach, colon, prostate and breast. One could envision BER playing a potentially important role in the etiology of other diseases involving relevant gene-environment interactions as well [135].
It is evident from Table 3 that there exists significant genetic complexity in BER among individuals within the population. A challenge going forward will be determining one's BER capacity from their genetic composition. Towards this end, the plethora of missense mutations listed in Table 3 provides a rich resource of experimental targets for computational scientists, biochemists and cell biologists to determine functional capacity. Given the number of genetic variants observed, there would appear to be a need for more complete and robust high-throughput experimental techniques for assessing function, and ultimately pathway throughput, keeping in mind the limitation of the different possible approaches. Indeed, how one goes about measuring the functional consequence of a particular amino acid or promoter change, and how that is then translated to pathway repair capacity, is a complex issue that requires several levels of consideration [135]. One idea to circumnavigate the available functional data is to use a mathematical systems biology model that mimics the kinetics of BER to analyze the in silico effects of genetic variation on pathway throughput [145]. As more information becomes available on the intricacies of the BER network, more complex model systems can be developed that would allow for the accurate prediction of the impact of functional changes on pathway efficiency.
Seeing that the relationship of BER variants, repair capacity, and disease risk remains unclear, it is imperative that the reasons behind the disparity in the epidemiology association studies be better understood, such as the contribution of the methodological approach and the role of potential modifier genes. Until that time, the current data on BER polymorphisms and cancer should be interpreted with caution, and better designed prospective studies, with sufficient statistical power, are necessary going forward. The possible contribution of the sizeable number of rare BER variants to disease should also be considered, as collectively, these variants make up a significant proportion of the total number. In the end, having a more complete and accurate picture of one's repair capacity will have significant implications in predicting exposure risk and designing treatment strategies, particularly for cancer [146].
Acknowledgements
As the reports on the various topics covered herein are enormous, we apologize in advance if relevant articles were missed or not cited. This research was supported by the Intramural Research Programs of the National Institute on Aging, NIH and the Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Friedman JI, Stivers JT. Detection of damaged DNA bases by DNA glycosylase enzymes. Biochemistry. 2010;49:4957–4967. doi: 10.1021/bi100593a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wilson DM, III, Barsky D. The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutat. Res. 2001;485:283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- [4].Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair (Amst) 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [5].Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid. Redox. Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beard WA, Wilson SH. Structure and mechanism of DNA polymerase Beta. Chem. Rev. 2006;106:361–382. doi: 10.1021/cr0404904. [DOI] [PubMed] [Google Scholar]
- [7].Ellenberger T, Tomkinson AE. Eukaryotic DNA ligases: structural and functional insights. Annu. Rev. Biochem. 2008;77:313–338. doi: 10.1146/annurev.biochem.77.061306.123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fortini P, Dogliotti E. Base damage and single-strand break repair: mechanisms and functional significance of short- and long-patch repair subpathways. DNA Repair (Amst) 2007;6:398–409. doi: 10.1016/j.dnarep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- [9].Wilson DM., III Processing of nonconventional DNA strand break ends. Environ. Mol. Mutagen. 2007;48:772–782. doi: 10.1002/em.20346. [DOI] [PubMed] [Google Scholar]
- [10].Caldecott KW. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- [11].Robertson AB, Klungland A, Rognes T, Leiros I. DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell Mol. Life Sci. 2009;66:981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Al Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat. Genet. 2002;30:227–232. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- [13].Sampson JR, Jones N. MUTYH-associated polyposis. Best. Pract. Res. Clin. Gastroenterol. 2009;23:209–218. doi: 10.1016/j.bpg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- [14].Dunlop MG, Farrington SM. MUTYH-associated polyposis and colorectal cancer. Surg. Oncol. Clin. N. Am. 2009;18:599–610. doi: 10.1016/j.soc.2009.08.003. [DOI] [PubMed] [Google Scholar]
- [15].David SS, O'Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447:941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cheadle JP, Sampson JR. MUTYH-associated polyposis--from defect in base excision repair to clinical genetic testing. DNA Repair (Amst) 2007;6:274–279. doi: 10.1016/j.dnarep.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [17].Chmiel NH, Livingston AL, David SS. Insight into the functional consequences of inherited variants of the hMYH adenine glycosylase associated with colorectal cancer: complementation assays with hMYH variants and pre-steady-state kinetics of the corresponding mutated E.coli enzymes. J. Mol. Biol. 2003;327:431–443. doi: 10.1016/s0022-2836(03)00124-4. [DOI] [PubMed] [Google Scholar]
- [18].Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, Ochs HD, Fischer A, Durandy A. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat. Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- [19].Kavli B, Andersen S, Otterlei M, Liabakk NB, Imai K, Fischer A, Durandy A, Krokan HE, Slupphaug G. B cells from hyper-IgM patients carrying UNG mutations lack ability to remove uracil from ssDNA and have elevated genomic uracil. J. Exp. Med. 2005;201:2011–2021. doi: 10.1084/jem.20050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Minegishi Y, Lavoie A, Cunningham-Rundles C, Bedard PM, Hebert J, Cote L, Dan K, Sedlak D, Buckley RH, Fischer A, Durandy A, Conley ME. Mutations in activation-induced cytidine deaminase in patients with hyper IgM syndrome. Clin. Immunol. 2000;97:203–210. doi: 10.1006/clim.2000.4956. [DOI] [PubMed] [Google Scholar]
- [21].Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- [22].Sousa MM, Krokan HE, Slupphaug G. DNA-uracil and human pathology. Mol. Aspects Med. 2007;28:276–306. doi: 10.1016/j.mam.2007.04.006. [DOI] [PubMed] [Google Scholar]
- [23].Durandy A, Taubenheim N, Peron S, Fischer A. Pathophysiology of B-cell intrinsic immunoglobulin class switch recombination deficiencies. Adv. Immunol. 2007;94:275–306. doi: 10.1016/S0065-2776(06)94009-7. [DOI] [PubMed] [Google Scholar]
- [24].Kracker S, Gardes P, Durandy A. Inherited defects of immunoglobulin class switch recombination. Adv. Exp. Med. Biol. 2010;685:166–174. doi: 10.1007/978-1-4419-6448-9_15. [DOI] [PubMed] [Google Scholar]
- [25].Date H, Onodera O, Tanaka H, Iwabuchi K, Uekawa K, Igarashi S, Koike R, Hiroi T, Yuasa T, Awaya Y, Sakai T, Takahashi T, Nagatomo H, Sekijima Y, Kawachi I, Takiyama Y, Nishizawa M, Fukuhara N, Saito K, Sugano S, Tsuji S. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat. Genet. 2001;29:184–188. doi: 10.1038/ng1001-184. [DOI] [PubMed] [Google Scholar]
- [26].Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, Mendonca P, Costa M, Barros J, Yanagisawa T, Watanabe M, Ikeda Y, Aoki M, Nagata T, Coutinho P, Sequeiros J, Koenig M. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 2001;29:189–193. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- [27].Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–716. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- [28].Seidle HF, Bieganowski P, Brenner C. Disease-associated mutations inactivate AMP-lysine hydrolase activity of Aprataxin. J. Biol. Chem. 2005;280:20927–20931. doi: 10.1074/jbc.M502889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Takashima H, Boerkoel CF, John J, Saifi GM, Salih MA, Armstrong D, Mao Y, Quiocho FA, Roa BB, Nakagawa M, Stockton DW, Lupski JR. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- [30].Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF. Conversion of phosphoglycolate to phosphate termini on 3' overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J. Biol. Chem. 2002;277:27162–27168. doi: 10.1074/jbc.M204688200. [DOI] [PubMed] [Google Scholar]
- [31].Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286:552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- [32].Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ. SCAN1 mutant Tdp1 accumulates the enzyme--DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 2005;24:2224–2233. doi: 10.1038/sj.emboj.7600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shen J, Gilmore EC, Marshall CA, Haddadin M, Reynolds JJ, Eyaid W, Bodell A, Barry B, Gleason D, Allen K, Ganesh VS, Chang BS, Grix A, Hill RS, Topcu M, Caldecott KW, Barkovich AJ, Walsh CA. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat. Genet. 2010;42:245–249. doi: 10.1038/ng.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Karimi-Busheri F, Daly G, Robins P, Canas B, Pappin DJ, Sgouros J, Miller GG, Fakhrai H, Davis EM, Le Beau MM, Weinfeld M. Molecular characterization of a human DNA kinase. J. Biol. Chem. 1999;274:24187–24194. doi: 10.1074/jbc.274.34.24187. [DOI] [PubMed] [Google Scholar]
- [35].Jilani A, Ramotar D, Slack C, Ong C, Yang XM, Scherer SW, Lasko DD. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3'-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 1999;274:24176–24186. doi: 10.1074/jbc.274.34.24176. [DOI] [PubMed] [Google Scholar]
- [36].Wilson DM, III, Mattson MP. Neurodegeneration: nicked to death. Curr. Biol. 2007;17:R55–R58. doi: 10.1016/j.cub.2006.12.012. [DOI] [PubMed] [Google Scholar]
- [37].Loeb LA, Bielas JH, Beckman RA. Cancers exhibit a mutator phenotype: clinical implications. Cancer Res. 2008;68:3551–3557. doi: 10.1158/0008-5472.CAN-07-5835. [DOI] [PubMed] [Google Scholar]
- [38].Starcevic D, Dalal S, Sweasy JB. Is There a Link Between DNA Polymerase beta and Cancer? Cell Cycle. 2004;3 [PubMed] [Google Scholar]
- [39].Lang T, Maitra M, Starcevic D, Li SX, Sweasy JB. A DNA polymerase beta mutant from colon cancer cells induces mutations. Proc. Natl. Acad. Sci. U. S. A. 2004;101:6074–6079. doi: 10.1073/pnas.0308571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dalal S, Hile S, Eckert KA, Sun KW, Starcevic D, Sweasy JB. Prostate-Cancer-Associated I260M Variant of DNA Polymerase beta Is a Sequence-Specific Mutator. Biochemistry. 2005;44:15664–15673. doi: 10.1021/bi051179z. [DOI] [PubMed] [Google Scholar]
- [41].Sweasy JB, Lang T, Starcevic D, Sun KW, Lai CC, Dimaio D, Dalal S. Expression of DNA polymerase {beta} cancer-associated variants in mouse cells results in cellular transformation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14350–14355. doi: 10.1073/pnas.0505166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lang T, Dalal S, Chikova A, Dimaio D, Sweasy JB. The E295K DNA polymerase beta gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol. Cell Biol. 2007;27:5587–5596. doi: 10.1128/MCB.01883-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coquerelle T, Dosch J, Kaina B. Overexpression of N-methylpurine-DNA glycosylase in Chinese hamster ovary cells renders them more sensitive to the production of chromosomal aberrations by methylating agents--a case of imbalanced DNA repair. Mutat. Res. 1995;336:9–17. doi: 10.1016/0921-8777(94)00035-5. [DOI] [PubMed] [Google Scholar]
- [44].Glassner BJ, Rasmussen LJ, Najarian MT, Posnick LM, Samson LD. Generation of a strong mutator phenotype in yeast by imbalanced base excision repair. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9997–10002. doi: 10.1073/pnas.95.17.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Goula AV, Berquist BR, Wilson DM, III, Wheeler VC, Trottier Y, Merienne K. Stoichiometry of base excision repair proteins correlates with increased somatic CAG instability in striatum over cerebellum in Huntington's disease transgenic mice. PLoS. Genet. 2009;5:e1000749. doi: 10.1371/journal.pgen.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilson DM, III, Thompson LH. Life without DNA repair. Proc. Natl. Acad. Sci. U. S. A. 1997;94:12754–12757. doi: 10.1073/pnas.94.24.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hsu TC. Genetic instability in the human population: a working hypothesis. Hereditas. 1983;98:1–9. doi: 10.1111/j.1601-5223.1983.tb00574.x. [DOI] [PubMed] [Google Scholar]
- [48].Hsu TC, Johnston DA, Cherry LM, Ramkissoon D, Schantz SP, Jessup JM, Winn RJ, Shirley L, Furlong C. Sensitivity to genotoxic effects of bleomycin in humans: possible relationship to environmental carcinogenesis. Int. J. Cancer. 1989;43:403–409. doi: 10.1002/ijc.2910430310. [DOI] [PubMed] [Google Scholar]
- [49].Parshad R, Sanford KK, Jones GM. Chromatid damage after G2 phase xirradiation of cells from cancer-prone individuals implicates deficiency in DNA repair. Proc. Natl. Acad. Sci. U. S. A. 1983;80:5612–5616. doi: 10.1073/pnas.80.18.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Parshad R, Sanford KK, Jones GM. Chromosomal radiosensitivity during the G2 cell-cycle period of skin fibroblasts from individuals with familial cancer. Proc. Natl. Acad. Sci. U. S. A. 1985;82:5400–5403. doi: 10.1073/pnas.82.16.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J. Natl. Cancer Inst. 2000;92:874–897. doi: 10.1093/jnci/92.11.874. [DOI] [PubMed] [Google Scholar]
- [52].Wu X, Gu J, Spitz MR. Mutagen sensitivity: a genetic predisposition factor for cancer. Cancer Res. 2007;67:3493–3495. doi: 10.1158/0008-5472.CAN-06-4137. [DOI] [PubMed] [Google Scholar]
- [53].Li C, Wang LE, Wei Q. DNA repair phenotype and cancer susceptibility--a mini review. Int. J. Cancer. 2009;124:999–1007. doi: 10.1002/ijc.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kato TA, Wilson PF, Nagasawa H, Peng Y, Weil MM, Little JB, Bedford JS. Variations in radiosensitivity among individuals: a potential impact on risk assessment? Health Phys. 2009;97:470–480. doi: 10.1097/HP.0b013e3181b08eee. [DOI] [PubMed] [Google Scholar]
- [55].Wilson PF, Nagasawa H, Fitzek MM, Little JB, Bedford JS. G2-phase chromosomal radiosensitivity of primary fibroblasts from hereditary retinoblastoma family members and some apparently normal controls. Radiat. Res. 2010;173:62–70. doi: 10.1667/RR1943.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kato TA, Nagasawa H, Weil MM, Little JB, Bedford JS. Levels of gamma-H2AX Foci after low-dose-rate irradiation reveal a DNA DSB rejoining defect in cells from human ATM heterozygotes in two AT families and in another apparently normal individual. Radiat. Res. 2006;166:443–453. doi: 10.1667/RR3604.1. [DOI] [PubMed] [Google Scholar]
- [57].Kato TA, Wilson PF, Nagasawa H, Fitzek MM, Weil MM, Little JB, Bedford JS. A defect in DNA double strand break processing in cells from unaffected parents of retinoblastoma patients and other apparently normal humans. DNA Repair (Amst) 2007;6:818–829. doi: 10.1016/j.dnarep.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [58].Collins AR, Gaivao I. DNA base excision repair as a biomarker in molecular epidemiology studies. Mol. Aspects Med. 2007;28:307–322. doi: 10.1016/j.mam.2007.05.005. [DOI] [PubMed] [Google Scholar]
- [59].Decordier I, Loock KV, Kirsch-Volders M. Phenotyping for DNA repair capacity. Mutat. Res. 2010;705:107–129. doi: 10.1016/j.mrrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- [60].Collins AR. Investigating oxidative DNA damage and its repair using the comet assay. Mutat. Res. 2009;681:24–32. doi: 10.1016/j.mrrev.2007.10.002. [DOI] [PubMed] [Google Scholar]
- [61].Sigurdson AJ, Hauptmann M, Alexander BH, Doody MM, Thomas CB, Struewing JP, Jones IM. DNA damage among thyroid cancer and multiple cancer cases, controls, and long-lived individuals. Mutat. Res. 2005;586:173–188. doi: 10.1016/j.mrgentox.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [62].Schabath MB, Spitz MR, Grossman HB, Zhang K, Dinney CP, Zheng PJ, Wu X. Genetic instability in bladder cancer assessed by the comet assay. J. Natl. Cancer Inst. 2003;95:540–547. doi: 10.1093/jnci/95.7.540. [DOI] [PubMed] [Google Scholar]
- [63].Shao L, Lin J, Huang M, Ajani JA, Wu X. Predictors of esophageal cancer risk: assessment of susceptibility to DNA damage using comet assay. Genes Chromosomes. Cancer. 2005;44:415–422. doi: 10.1002/gcc.20254. [DOI] [PubMed] [Google Scholar]
- [64].Lin X, Wood CG, Shao L, Huang M, Yang H, Dinney CP, Wu X. Risk assessment of renal cell carcinoma using alkaline comet assay. Cancer. 2007;110:282–288. doi: 10.1002/cncr.22792. [DOI] [PubMed] [Google Scholar]
- [65].El-Zein RA, Monroy CM, Cortes A, Spitz MR, Greisinger A, Etzel CJ. Rapid method for determination of DNA repair capacity in human peripheral blood lymphocytes amongst smokers. BMC. Cancer. 2010;10:439. doi: 10.1186/1471-2407-10-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Paz-Elizur T, Elinger D, Leitner-Dagan Y, Blumenstein S, Krupsky M, Berrebi A, Schechtman E, Livneh Z. Development of an enzymatic DNA repair assay for molecular epidemiology studies: distribution of OGG activity in healthy individuals. DNA Repair (Amst) 2007;6:45–60. doi: 10.1016/j.dnarep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [67].Paz-Elizur T, Sevilya Z, Leitner-Dagan Y, Elinger D, Roisman LC, Livneh Z. DNA repair of oxidative DNA damage in human carcinogenesis: potential application for cancer risk assessment and prevention. Cancer Lett. 2008;266:60–72. doi: 10.1016/j.canlet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McNeill DR, Narayana A, Wong HK, Wilson DM., III Inhibition of Ape1 nuclease activity by lead, iron, and cadmium. Environ. Health Perspect. 2004;112:799–804. doi: 10.1289/ehp.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Wong HK, Kim D, Hogue BA, McNeill DR, Wilson DM., III DNA Damage Levels and Biochemical Repair Capacities Associated with XRCC1 Deficiency. Biochemistry. 2005;44:14335–14343. doi: 10.1021/bi051161o. [DOI] [PubMed] [Google Scholar]
- [70].Redaelli A, Magrassi R, Bonassi S, Abbondandolo A, Frosina G. AP endonuclease activity in humans: development of a simple assay and analysis of ten normal individuals. Teratog. Carcinog. Mutagen. 1998;18:17–26. [PubMed] [Google Scholar]
- [71].Rossi O, Carrozzino F, Frosina G. Age-independency of AP site incision capacity in women. Environ. Mol. Mutagen. 1999;34:256–259. [PubMed] [Google Scholar]
- [72].Rossi O, Carrozzino F, Cappelli E, Carli F, Frosina G. Analysis of repair of abasic sites in early onset breast cancer patients. Int. J. Cancer. 2000;85:21–26. doi: 10.1002/(sici)1097-0215(20000101)85:1<21::aid-ijc4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [73].Mazzei F, Guarrera S, Allione A, Simonelli V, Narciso L, Barone F, Minoprio A, Ricceri F, Funaro A, D'Errico M, Vogel U, Matullo G, Dogliotti E. 8-Oxoguanine DNA-glycosylase repair activity and expression: A comparison between cryopreserved isolated lymphocytes and EBV-derived lymphoblastoid cell lines. Mutat. Res. 2010 doi: 10.1016/j.mrgentox.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [74].Briegert M, Kaina B. Human monocytes, but not dendritic cells derived from them, are defective in base excision repair and hypersensitive to methylating agents. Cancer Res. 2007;67:26–31. doi: 10.1158/0008-5472.CAN-06-3712. [DOI] [PubMed] [Google Scholar]
- [75].Paz-Elizur T, Krupsky M, Blumenstein S, Elinger D, Schechtman E, Livneh Z. DNA repair activity for oxidative damage and risk of lung cancer. J. Natl. Cancer Inst. 2003;95:1312–1319. doi: 10.1093/jnci/djg033. [DOI] [PubMed] [Google Scholar]
- [76].Auckley DH, Crowell RE, Heaphy ER, Stidley CA, Lechner JF, Gilliland FD, Belinsky SA. Reduced DNA-dependent protein kinase activity is associated with lung cancer. Carcinogenesis. 2001;22:723–727. doi: 10.1093/carcin/22.5.723. [DOI] [PubMed] [Google Scholar]
- [77].Cabelof DC, Ikeno Y, Nyska A, Busuttil RA, Anyangwe N, Vijg J, Matherly LH, Tucker JD, Wilson SH, Richardson A, Heydari AR. Haploinsufficiency in DNA polymerase beta increases cancer risk with age and alters mortality rate. Cancer Res. 2006;66:7460–7465. doi: 10.1158/0008-5472.CAN-06-1177. [DOI] [PubMed] [Google Scholar]
- [78].Shen MR, Jones IM, Mohrenweiser H. Nonconservative amino acid substitution variants exist at polymorphic frequency in DNA repair genes in healthy humans. Cancer Res. 1998;58:604–608. [PubMed] [Google Scholar]
- [79].Mohrenweiser HW, Xi T, Vazquez-Matias J, Jones IM. Identification of 127 amino acid substitution variants in screening 37 DNA repair genes in humans. Cancer Epidemiol. Biomarkers Prev. 2002;11:1054–1064. [PubMed] [Google Scholar]
- [80].Mohrenweiser HW. Genetic variation and exposure related risk estimation: will toxicology enter a new era? DNA repair and cancer as a paradigm. Toxicol. Pathol. 2004;32(Suppl 1):136–145. doi: 10.1080/01926230490424671. [DOI] [PubMed] [Google Scholar]
- [81].Liu Y, Kao HI, Bambara RA. Flap endonuclease 1: a central component of DNA metabolism. Annu. Rev. Biochem. 2004;73:589–615. doi: 10.1146/annurev.biochem.73.012803.092453. [DOI] [PubMed] [Google Scholar]
- [82].Zheng L, Dai H, Zhou M, Li M, Singh P, Qiu J, Tsark W, Huang Q, Kernstine K, Zhang X, Lin D, Shen B. Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat. Med. 2007;13:812–819. doi: 10.1038/nm1599. [DOI] [PubMed] [Google Scholar]
- [83].Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol. Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- [84].Hung RJ, Hall J, Brennan P, Boffetta P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am. J. Epidemiol. 2005;162:925–942. doi: 10.1093/aje/kwi318. [DOI] [PubMed] [Google Scholar]
- [85].Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J. Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene. 1998;16:3219–3225. doi: 10.1038/sj.onc.1201872. [DOI] [PubMed] [Google Scholar]
- [86].Dherin C, Radicella JP, Dizdaroglu M, Boiteux S. Excision of oxidatively damaged DNA bases by the human alpha-hOgg1 protein and the polymorphic alpha-hOgg1(Ser326Cys) protein which is frequently found in human populations. Nucleic Acids Res. 1999;27:4001–4007. doi: 10.1093/nar/27.20.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yamane A, Kohno T, Ito K, Sunaga N, Aoki K, Yoshimura K, Murakami H, Nojima Y, Yokota J. Differential ability of polymorphic OGG1 proteins to suppress mutagenesis induced by 8-hydroxyguanine in human cell in vivo. Carcinogenesis. 2004;25:1689–1694. doi: 10.1093/carcin/bgh166. [DOI] [PubMed] [Google Scholar]
- [88].Klungland A, Bjelland S. Oxidative damage to purines in DNA: role of mammalian Ogg1. DNA Repair (Amst) 2007;6:481–488. doi: 10.1016/j.dnarep.2006.10.012. [DOI] [PubMed] [Google Scholar]
- [89].Hill JW, Evans MK. Dimerization and opposite base-dependent catalytic impairment of polymorphic S326C OGG1 glycosylase. Nucleic Acids Res. 2006;34:1620–1632. doi: 10.1093/nar/gkl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Smart DJ, Chipman JK, Hodges NJ. Activity of OGG1 variants in the repair of pro-oxidant-induced 8-oxo-2'-deoxyguanosine. DNA Repair (Amst) 2006;5:1337–1345. doi: 10.1016/j.dnarep.2006.06.001. [DOI] [PubMed] [Google Scholar]
- [91].Bravard A, Vacher M, Moritz E, Vaslin L, Hall J, Epe B, Radicella JP. Oxidation status of human OGG1-S326C polymorphic variant determines cellular DNA repair capacity. Cancer Res. 2009;69:3642–3649. doi: 10.1158/0008-5472.CAN-08-3943. [DOI] [PubMed] [Google Scholar]
- [92].Luna L, Rolseth V, Hildrestrand GA, Otterlei M, Dantzer F, Bjoras M, Seeberg E. Dynamic relocalization of hOGG1 during the cell cycle is disrupted in cells harbouring the hOGG1-Cys326 polymorphic variant. Nucleic Acids Res. 2005;33:1813–1824. doi: 10.1093/nar/gki325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Weiss JM, Goode EL, Ladiges WC, Ulrich CM. Polymorphic variation in hOGG1 and risk of cancer: a review of the functional and epidemiologic literature. Mol. Carcinog. 2005;42:127–141. doi: 10.1002/mc.20067. [DOI] [PubMed] [Google Scholar]
- [94].Li H, Hao X, Zhang W, Wei Q, Chen K. The hOGG1 Ser326Cys polymorphism and lung cancer risk: a meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2008;17:1739–1745. doi: 10.1158/1055-9965.EPI-08-0001. [DOI] [PubMed] [Google Scholar]
- [95].Hill JW, Evans MK. A novel R229Q OGG1 polymorphism results in a thermolabile enzyme that sensitizes KG-1 leukemia cells to DNA damaging agents. Cancer Detect. Prev. 2007;31:237–243. doi: 10.1016/j.cdp.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chevillard S, Radicella JP, Levalois C, Lebeau J, Poupon MF, Oudard S, Dutrillaux B, Boiteux S. Mutations in OGG1, a gene involved in the repair of oxidative DNA damage, are found in human lung and kidney tumours. Oncogene. 1998;16:3083–3086. doi: 10.1038/sj.onc.1202096. [DOI] [PubMed] [Google Scholar]
- [97].Audebert M, Chevillard S, Levalois C, Gyapay G, Vieillefond A, Klijanienko J, Vielh P, El Naggar AK, Oudard S, Boiteux S, Radicella JP. Alterations of the DNA repair gene OGG1 in human clear cell carcinomas of the kidney. Cancer Res. 2000;60:4740–4744. [PubMed] [Google Scholar]
- [98].Audebert M, Radicella JP, Dizdaroglu M. Effect of single mutations in the OGG1 gene found in human tumors on the substrate specificity of the Ogg1 protein. Nucleic Acids Res. 2000;28:2672–2678. doi: 10.1093/nar/28.14.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dallosso AR, Dolwani S, Jones N, Jones S, Colley J, Maynard J, Idziaszczyk S, Humphreys V, Arnold J, Donaldson A, Eccles D, Ellis A, Evans DG, Frayling IM, Hes FJ, Houlston RS, Maher ER, Nielsen M, Parry S, Tyler E, Moskvina V, Cheadle JP, Sampson JR. Inherited predisposition to colorectal adenomas caused by multiple rare alleles of MUTYH but not OGG1, NUDT1, NTH1 or NEIL 1, 2 or 3. Gut. 2008;57:1252–1255. doi: 10.1136/gut.2007.145748. [DOI] [PubMed] [Google Scholar]
- [100].Forsbring M, Vik ES, Dalhus B, Karlsen TH, Bergquist A, Schrumpf E, Bjoras M, Boberg KM, Alseth I. Catalytically impaired hMYH and NEIL1 mutant proteins identified in patients with primary sclerosing cholangitis and cholangiocarcinoma. Carcinogenesis. 2009;30:1147–1154. doi: 10.1093/carcin/bgp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mao G, Pan X, Zhu BB, Zhang Y, Yuan F, Huang J, Lovell MA, Lee MP, Markesbery WR, Li GM, Gu L. Identification and characterization of OGG1 mutations in patients with Alzheimer's disease. Nucleic Acids Res. 2007;35:2759–2766. doi: 10.1093/nar/gkm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Vartanian V, Lowell B, Minko IG, Wood TG, Ceci JD, George S, Ballinger SW, Corless CL, McCullough AK, Lloyd RS. The metabolic syndrome resulting from a knockout of the NEIL1 DNA glycosylase. Proc. Natl. Acad. Sci. U. S. A. 2006;103:1864–1869. doi: 10.1073/pnas.0507444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Roy LM, Jaruga P, Wood TG, McCullough AK, Dizdaroglu M, Lloyd RS. Human polymorphic variants of the NEIL1 DNA glycosylase. J. Biol. Chem. 2007;282:15790–15798. doi: 10.1074/jbc.M610626200. [DOI] [PubMed] [Google Scholar]
- [104].Shinmura K, Tao H, Goto M, Igarashi H, Taniguchi T, Maekawa M, Takezaki T, Sugimura H. Inactivating mutations of the human base excision repair gene NEIL1 in gastric cancer. Carcinogenesis. 2004;25:2311–2317. doi: 10.1093/carcin/bgh267. [DOI] [PubMed] [Google Scholar]
- [105].Kvaloy K, Nilsen H, Steinsbekk KS, Nedal A, Monterotti B, Akbari M, Krokan HE. Sequence variation in the human uracil-DNA glycosylase (UNG) gene. Mutat. Res. 2001;461:325–338. doi: 10.1016/s0921-8777(00)00063-x. [DOI] [PubMed] [Google Scholar]
- [106].Moon YW, Park WS, Vortmeyer AO, Weil RJ, Lee YS, Winters TA, Zhuang Z, Fuller BG. Mutation of the uracil DNA glycosylase gene detected in glioblastoma. Mutat. Res. 1998;421:191–196. doi: 10.1016/s0027-5107(98)00165-1. [DOI] [PubMed] [Google Scholar]
- [107].Broderick P, Bagratuni T, Vijayakrishnan J, Lubbe S, Chandler I, Houlston RS. Evaluation of NTHL1, NEIL1, NEIL2, MPG, TDG, UNG and SMUG1 genes in familial colorectal cancer predisposition. BMC. Cancer. 2006;6:243. doi: 10.1186/1471-2407-6-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Olkowski ZL. Mutant AP endonuclease in patients with amyotrophic lateral sclerosis. Neuroreport. 1998;9:239–242. doi: 10.1097/00001756-199801260-00012. [DOI] [PubMed] [Google Scholar]
- [109].Hayward C, Colville S, Swingler RJ, Brock DJ. Molecular genetic analysis of the APEX nuclease gene in amyotrophic lateral sclerosis. Neurology. 1999;52:1899–1901. doi: 10.1212/wnl.52.9.1899. [DOI] [PubMed] [Google Scholar]
- [110].Tomkins J, Dempster S, Banner SJ, Cookson MR, Shaw PJ. Screening of AP endonuclease as a candidate gene for amyotrophic lateral sclerosis (ALS) Neuroreport. 2000;11:1695–1697. doi: 10.1097/00001756-200006050-00020. [DOI] [PubMed] [Google Scholar]
- [111].Hadi MZ, Coleman MA, Fidelis K, Mohrenweiser HW, Wilson DM., III Functional characterization of Ape1 variants identified in the human population. Nucleic Acids Res. 2000;28:3871–3879. doi: 10.1093/nar/28.20.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hu JJ, Smith TR, Miller MS, Lohman K, Case LD. Genetic regulation of ionizing radiation sensitivity and breast cancer risk. Environ. Mol. Mutagen. 2002;39:208–215. doi: 10.1002/em.10058. [DOI] [PubMed] [Google Scholar]
- [113].Zhang Y, Newcomb PA, Egan KM, Titus-Ernstoff L, Chanock S, Welch R, Brinton LA, Lissowska J, Bardin-Mikolajczak A, Peplonska B, Szeszenia-Dabrowska N, Zatonski W, Garcia-Closas M. Genetic polymorphisms in base-excision repair pathway genes and risk of breast cancer. Cancer Epidemiol. Biomarkers Prev. 2006;15:353–358. doi: 10.1158/1055-9965.EPI-05-0653. [DOI] [PubMed] [Google Scholar]
- [114].Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey CS, Bowman R, Meyer KB, Haiman CA, Kolonel LK, Henderson BE, Le ML, Brennan P, Sangrajrang S, Gaborieau V, Odefrey F, Shen CY, Wu PE, Wang HC, Eccles D, Evans DG, Peto J, Fletcher O, Johnson N, Seal S, Stratton MR, Rahman N, Chenevix-Trench G, Bojesen SE, Nordestgaard BG, Axelsson CK, Garcia-Closas M, Brinton L, Chanock S, Lissowska J, Peplonska B, Nevanlinna H, Fagerholm R, Eerola H, Kang D, Yoo KY, Noh DY, Ahn SH, Hunter DJ, Hankinson SE, Cox DG, Hall P, Wedren S, Liu J, Low YL, Bogdanova N, Schurmann P, Dork T, Tollenaar RA, Jacobi CE, Devilee P, Klijn JG, Sigurdson AJ, Doody MM, Alexander BH, Zhang J, Cox A, Brock IW, MacPherson G, Reed MW, Couch FJ, Goode EL, Olson JE, Meijers-Heijboer H, van den OA, Uitterlinden A, Rivadeneira F, Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Hopper JL, McCredie M, Southey M, Giles GG, Schroen C, Justenhoven C, Brauch H, Hamann U, Ko YD, Spurdle AB, Beesley J, Chen X, Mannermaa A, Kosma VM, Kataja V, Hartikainen J, Day NE, Cox DR, Ponder BA. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stacey SN, Manolescu A, Sulem P, Thorlacius S, Gudjonsson SA, Jonsson GF, Jakobsdottir M, Bergthorsson JT, Gudmundsson J, Aben KK, Strobbe LJ, Swinkels DW, van Engelenburg KC, Henderson BE, Kolonel LN, Le ML, Millastre E, Andres R, Saez B, Lambea J, Godino J, Polo E, Tres A, Picelli S, Rantala J, Margolin S, Jonsson T, Sigurdsson H, Jonsdottir T, Hrafnkelsson J, Johannsson J, Sveinsson T, Myrdal G, Grimsson HN, Sveinsdottir SG, Alexiusdottir K, Saemundsdottir J, Sigurdsson A, Kostic J, Gudmundsson L, Kristjansson K, Masson G, Fackenthal JD, Adebamowo C, Ogundiran T, Olopade OI, Haiman CA, Lindblom A, Mayordomo JI, Kiemeney LA, Gulcher JR, Rafnar T, Thorsteinsdottir U, Johannsson OT, Kong A, Stefansson K. Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat. Genet. 2008;40:703–706. doi: 10.1038/ng.131. [DOI] [PubMed] [Google Scholar]
- [116].Thomas G, Jacobs KB, Kraft P, Yeager M, Wacholder S, Cox DG, Hankinson SE, Hutchinson A, Wang Z, Yu K, Chatterjee N, Garcia-Closas M, Gonzalez-Bosquet J, Prokunina-Olsson L, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver R, Prentice R, Jackson R, Kooperberg C, Chlebowski R, Lissowska J, Peplonska B, Brinton LA, Sigurdson A, Doody M, Bhatti P, Alexander BH, Buring J, Lee IM, Vatten LJ, Hveem K, Kumle M, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF, Jr., Hoover RN, Chanock SJ, Hunter DJ. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat. Genet. 2009;41:579–584. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, Seal S, Ghoussaini M, Hines S, Healey CS, Hughes D, Warren-Perry M, Tapper W, Eccles D, Evans DG, Hooning M, Schutte M, van den OA, Houlston R, Ross G, Langford C, Pharoah PD, Stratton MR, Dunning AM, Rahman N, Easton DF. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat. Genet. 2010;42:504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Daviet S, Couve-Privat S, Gros L, Shinozuka K, Ide H, Saparbaev M, Ishchenko AA. Major oxidative products of cytosine are substrates for the nucleotide incision repair pathway. DNA Repair (Amst) 2007;6:8–18. doi: 10.1016/j.dnarep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- [119].Pieretti M, Khattar NH, Smith SA. Common polymorphisms and somatic mutations in human base excision repair genes in ovarian and endometrial cancers. Mutat. Res. 2001;432:53–59. doi: 10.1016/s1383-5726(00)00002-9. [DOI] [PubMed] [Google Scholar]
- [120].Luo M, He H, Kelley MR, Georgiadis MM. Redox regulation of DNA repair: implications for human health and cancer therapeutic development. Antioxid. Redox. Signal. 2010;12:1247–1269. doi: 10.1089/ars.2009.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yu ET, Hadi MZ. Bioinformatic processing to identify single nucleotide polymorphism that potentially affect Ape1 function. Mutat. Res. 2010 doi: 10.1016/j.mrgentox.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [122].Barnes DE, Tomkinson AE, Lehmann AR, Webster AD, Lindahl T. Mutations in the DNA ligase I gene of an individual with immunodeficiencies and cellular hypersensitivity to DNA-damaging agents. Cell. 1992;69:495–503. doi: 10.1016/0092-8674(92)90450-q. [DOI] [PubMed] [Google Scholar]
- [123].Thompson LH, West MG. XRCC1 keeps DNA from getting stranded. Mutat. Res. 2000;459:1–18. doi: 10.1016/s0921-8777(99)00058-0. [DOI] [PubMed] [Google Scholar]
- [124].Caldecott KW. XRCC1 and DNA strand break repair. DNA Repair (Amst) 2003;2:955–969. doi: 10.1016/s1568-7864(03)00118-6. [DOI] [PubMed] [Google Scholar]
- [125].Taylor RM, Thistlethwaite A, Caldecott KW. Central role for the XRCC1 BRCT I domain in mammalian DNA single-strand break repair. Mol. Cell Biol. 2002;22:2556–2563. doi: 10.1128/MCB.22.8.2556-2563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Qu T, Morii E, Oboki K, Lu Y, Morimoto K. Micronuclei in EM9 cells expressing polymorphic forms of human XRCC1. Cancer Lett. 2005;221:91–95. doi: 10.1016/j.canlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- [127].Takanami T, Nakamura J, Kubota Y, Horiuchi S. The Arg280His polymorphism in X-ray repair cross-complementing gene 1 impairs DNA repair ability. Mutat. Res. 2005;582:135–145. doi: 10.1016/j.mrgentox.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [128].Pachkowski BF, Winkel S, Kubota Y, Swenberg JA, Millikan RC, Nakamura J. XRCC1 genotype and breast cancer: functional studies and epidemiologic data show interactions between XRCC1 codon 280 His and smoking. Cancer Res. 2006;66:2860–2868. doi: 10.1158/0008-5472.CAN-05-3388. [DOI] [PubMed] [Google Scholar]
- [129].Berquist BR, Singh DK, Fan J, Kim D, Gillenwater E, Kulkarni A, Bohr VA, Ackerman EJ, Tomkinson AE, Wilson DM., III Functional capacity of XRCC1 protein variants identified in DNA repair-deficient Chinese hamster ovary cell lines and the human population. Nucleic Acids Res. 2010;38:5023–5035. doi: 10.1093/nar/gkq193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Li J, Humphreys K, Heikkinen T, Aittomaki K, Blomqvist C, Pharoah PD, Dunning AM, Ahmed S, Hooning MJ, Martens JW, van den Ouweland AM, Alfredsson L, Palotie A, Peltonen-Palotie L, Irwanto A, Low HQ, Teoh GH, Thalamuthu A, Easton DF, Nevanlinna H, Liu J, Czene K, Hall P. A combined analysis of genome-wide association studies in breast cancer. Breast Cancer Res. Treat. 2010 doi: 10.1007/s10549-010-1172-9. [DOI] [PubMed] [Google Scholar]
- [131].Jiang J, Zhang X, Yang H, Wang W. Polymorphisms of DNA repair genes: ADPRT, XRCC1, and XPD and cancer risk in genetic epidemiology. Methods Mol. Biol. 2009;471:305–333. doi: 10.1007/978-1-59745-416-2_16. [DOI] [PubMed] [Google Scholar]
- [132].Allan JM, Engelward BP, Dreslin AJ, Wyatt MD, Tomasz M, Samson LD. Mammalian 3-methyladenine DNA glycosylase protects against the toxicity and clastogenicity of certain chemotherapeutic DNA cross-linking agents. Cancer Res. 1998;58:3965–3973. [PubMed] [Google Scholar]
- [133].Tudek B. Base excision repair modulation as a risk factor for human cancers. Mol. Aspects Med. 2007;28:258–275. doi: 10.1016/j.mam.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [134].Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Mohrenweiser HW, Wilson DM, III, Jones IM. Challenges and complexities in estimating both the functional impact and the disease risk associated with the extensive genetic variation in human DNA repair genes. Mutat. Res. 2003;526:93–125. doi: 10.1016/s0027-5107(03)00049-6. [DOI] [PubMed] [Google Scholar]
- [136].Wilson DM, III, Bohr VA. The mechanics of base excision repair, and its relationship to aging and disease. DNA Repair (Amst) 2007;6:544–559. doi: 10.1016/j.dnarep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- [137].Caporaso N. The molecular epidemiology of oxidative damage to DNA and cancer. J. Natl. Cancer Inst. 2003;95:1263–1265. doi: 10.1093/jnci/djg065. [DOI] [PubMed] [Google Scholar]
- [138].Bhatti P, Sigurdson AJ, Thomas CB, Iwan A, Alexander BH, Kampa D, Bowen L, Doody MM, Jones IM. No evidence for differences in DNA damage assessed before and after a cancer diagnosis. Cancer Epidemiol. Biomarkers Prev. 2008;17:990–994. doi: 10.1158/1055-9965.EPI-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sigurdson AJ, Jones IM, Wei Q, Wu X, Spitz MR, Stram D, Gross MD, Huang WY, Wang LE, Gu J, Thomas CB, Reding D, Hayes RB, Caporaso NE. Prospective Analysis of DNA Damage and Repair Markers of Lung Cancer Risk From the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Carcinogenesis. 2010 doi: 10.1093/carcin/bgq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Trzeciak AR, Barnes J, Ejiogu N, Foster K, Brant LJ, Zonderman AB, Evans MK. Age, sex, and race influence single-strand break repair capacity in a human population. Free Radic. Biol. Med. 2008;45:1631–1641. doi: 10.1016/j.freeradbiomed.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gaivao I, Piasek A, Brevik A, Shaposhnikov S, Collins AR. Comet assay-based methods for measuring DNA repair in vitro; estimates of inter- and intra-individual variation. Cell Biol. Toxicol. 2009;25:45–52. doi: 10.1007/s10565-007-9047-5. [DOI] [PubMed] [Google Scholar]
- [142].Caple F, Williams EA, Spiers A, Tyson J, Burtle B, Daly AK, Mathers JC, Hesketh JE. Inter-individual variation in DNA damage and base excision repair in young, healthy non-smokers: effects of dietary supplementation and genotype. Br. J. Nutr. 2010;103:1585–1593. doi: 10.1017/S0007114509993540. [DOI] [PubMed] [Google Scholar]
- [143].Moreno-Villanueva M, Pfeiffer R, Sindlinger T, Leake A, Muller M, Kirkwood TB, Burkle A. A modified and automated version of the 'Fluorimetric Detection of Alkaline DNA Unwinding' method to quantify formation and repair of DNA strand breaks. BMC. Biotechnol. 2009;9:39. doi: 10.1186/1472-6750-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Bernstein JL, Haile RW, Stovall M, Boice JD, Jr., Shore RE, Langholz B, Thomas DC, Bernstein L, Lynch CF, Olsen JH, Malone KE, Mellemkjaer L, Borresen-Dale AL, Rosenstein BS, Teraoka SN, Diep AT, Smith SA, Capanu M, Reiner AS, Liang X, Gatti RA, Concannon P. Radiation exposure, the ATM Gene, and contralateral breast cancer in the women's environmental cancer and radiation epidemiology study. J. Natl. Cancer Inst. 2010;102:475–483. doi: 10.1093/jnci/djq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Sokhansanj BA, Wilson DM., III Estimating the effect of human base excision repair protein variants on the repair of oxidative DNA base damage. Cancer Epidemiol. Biomarkers Prev. 2006;15:1000–1008. doi: 10.1158/1055-9965.EPI-05-0817. [DOI] [PubMed] [Google Scholar]
- [146].Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- [147].Shinmura K, Yamaguchi S, Saitoh T, Kohno T, Yokota J. Somatic mutations and single nucleotide polymorphisms of base excision repair genes involved in the repair of 8-hydroxyguanine in damaged DNA. Cancer Lett. 2001;166:65–69. doi: 10.1016/s0304-3835(01)00435-9. [DOI] [PubMed] [Google Scholar]