Abstract
Chronic widespread pain, such as observed in irritable bowel (IBS) and fibromyalgia (FMS) syndrome, are markedly affected by stress. While such forms of stress-induced hyperalgesia are generally considered manifestations of ‘central sensitization’, recent studies in patients with IBS and FMS suggest an additional, peripheral contribution. To examine the effect of stress on muscle nociceptor function, we evaluated activity in nociceptors innervating the gastrocnemius muscle in an animal model of chronic widespread pain, water avoidance stress, in the rat. This stressor, which produces mechanical hyperalgesia in skeletal muscle produced a significant decrease (~34%) in mechanical threshold of muscle nociceptors and a marked, ~2-fold increase in the number of action potentials produced by a prolonged (60 s) fixed intensity suprathreshold 10 g stimulus. Stress also induced an increase in conduction velocity from 1.25 m/s to 2.09 m/s, and increased variability in neuronal activity. Given that these changes, each of at least moderate magnitude, would be expected to enhance nociceptor activity, it is likely that, taken together, they contribute to the enhanced nociception observed in this model of stress-induced chronic widespread pain.
Keywords: Stress, Skeletal muscle, Hyperalgesia, Peripheral neuropathy, Nociceptors, Conduction velocity
Clinical conditions characterized by chronic widespread pain, such as irritable bowel syndrome (IBS), temporomandibular disorder (TMD) and fibromyalgia syndrome (FMS) are well recognized to be exacerbated by stressful life events13,19,32,35,36,41,51,71. The mechanisms by which stress negatively impacts these patients have generally been considered to be at the level of the central nervous system, via changes in descending modulatory nociceptive controls 52,57,67. The balance between descending inhibitory and facilitatory controls on spinal nociceptive circuits is believed to be affected by stress 5,21,25,40,58,72. The magnitude, duration and nature of the stress (e.g. continuous or intermittent/unpredictable or not) are determining factors, with recent evidence indicating that chronic stress facilitates pain transmission in animal models 24,25 and in patients with chronic pain 27.
Recent experiments in patients with IBS and FMS have provided evidence for a further contribution by peripheral mechanisms. For example, application of local anesthetics, in the gastrointestinal tract in patients with IBS 54,68 or to somatic tissues in patients with FMS 62, produces a widespread improvement in symptoms. Furthermore, in rodent models of IBS, a contribution of a peripheral mechanism has been suggested based on observations that chronic visceral hypersensitivity in adult rats, produced by colon irritation in neonates, is correlated with greater spontaneous firing, enhanced response to mechanical stimulation and lowered mechanical threshold in visceral afferents 38 and that intracolonic lidocaine decreases somatic as well as visceral nociceptive response in the trinitrobenzene sulfonic acid (TNBS) model of IBS 74. To provide more direct evidence that sensitization of nociceptive afferents is a component of chronic widespread musculoskeletal pain syndromes we evaluated muscle nociceptor function in a model of stress-induced muscle hyperalgesia in the rat. Given the marked co-morbidity between IBS and FMS, we elected to use water-avoidance stress, which has been shown to produce visceral hyperalgesia 6,7,23,73, and more recently to produce mechanical hyperalgesia in the gastrocnemius and masseter muscles, as well as an anxiety phenotype on the elevated plus maze 20.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague Dawley rats (250–350 g, Charles River, Hollister, CA, USA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h, room temperature 21–23°C) with food and water available ad libitum. Animal care and use conformed to NIH guidelines and experimental protocols were approved by the University of California San Francisco Committee on Animal Research.
Water avoidance stress
We used the water-avoidance model for irritable bowel syndrome 6, which we have recently shown also produces mechanical hyperalgesia in skeletal muscle 20. Rats were placed on a 10 cm high acrylic platform (8 × 8 cm) in the center of a clear plastic tank (45 cm length × 25 cm width × 25 cm height) filled with room temperature tap water to a depth of 9 cm, for 1 h/day, for 10 consecutive days. The water-avoidance protocol produces a psychological stress as indicated by the large increase in ACTH and glucocorticoids within 30 min of the start of stress exposure 6. Single fiber electrophysiology was performed 1 day after the last stress exposure.
Single fiber recording
The in vivo single fiber electrophysiology technique employed was similar to that used previously in recordings from cutaneous afferents 11. Rats were anesthetized with sodium pentobarbital (initially 50 mg/kg, i.p., with additional doses given throughout the experiment to maintain areflexia), their trachea cannulated, and heart rate monitored. Anesthetized animals were positioned on their right side and an incision made on the dorsal skin of the left leg, between the mid-thigh and calf, and the biceps femoris muscle partially removed to expose the sciatic nerve and gastrocnemius muscle. The edges of the incised skin were fixed to a metal loop to provide a pool that was filled with warm mineral oil, bathing the sciatic nerve and gastrocnemius muscle.
The sciatic nerve was cut proximally to prevent flexor reflexes during electrical stimulation of sensory neurons. Fine fascicles of axons were then dissected from the distal stump, and placed on a recording electrode. Single units were first detected by mechanical stimulation of the gastrocnemius muscle with a small blunt-tipped glass bar. Bipolar stimulating electrodes were then placed and held on the center of the receptive field of the muscle afferent, by a micromanipulator (MM-3, Narishige, Japan). Conduction velocity of each fiber was calculated by dividing the distance between the stimulating and recording electrodes by the latency of the electrically evoked action potential. All recorded muscle afferents had conduction velocities in the range of type III (12%) or type IV (88%) fibers. Mechanical threshold was determined with calibrated von Frey hairs (VFH Ainsworth, London, UK). The #10 (1.66g) VFH was used first to elicit spikes, and if a response was elicited, then the #8 (0.603 g) was used. If this also elicited spikes, then the #6 (0.219 g) was used; if there was no response to #6, then #7 (0.363 g) VFH was used. Threshold is defined as the lowest force that elicited at least 2 spikes within 1 s, in at least 50% of trials. Sustained (60 s) suprathreshold (10 g) mechanical stimulation was accomplished by use of a mechanical stimulator that consisted of a force-measuring transducer (Entran, Fairfield, NJ, USA) with a blunt plastic tip that was applied by a micromanipulator (BC-3 and BE-8, Narishige) on the center of the receptive field, for 60 s. Neural activity and timing of stimulus onset and termination were monitored and stored on a Windows OS computer with Micro 1401 interface (CED, Cambridge, UK) and analyzed off-line with Spike2 software (CED).
Interspike interval (ISI) analysis
ISI analysis, used to evaluate the temporal characteristics of the response of C-fiber nociceptors to sustained mechanical stimulation, was adopted from our study of nociceptor activity in the rat model of vincristine-induced painful neuropathy 63. The ISIs for the responses of each C-fiber were grouped into 100 ms bins between 0 and 499 ms; the few ISIs greater than or equal to 500 ms were not analyzed 63. This bin width also allows comparison of data with that from previous studies 2,18,46. The number of intervals occurring in each bin was expressed as the percentage of the total number of ISIs in the trial. This normalization procedure allowed the distribution of ISIs from several fibers to be averaged together.
Coefficient of variation analysis
Coefficient of variation of the ISIs does not give an accurate estimate of the variability of neuronal firing if the mean rate changes over time, a common occurrence. Therefore, we calculated the coefficient of variability (CV2) which compares the relative difference between adjacent ISIs 22. CV2 is defined as the square root of 2 multiplied by the S.D. of two ISIs divided by their mean 22:
Error! Bookmark not defined. , where ti is the latency for the ith action potential.
Thus, CV2 is a dimensionless number that is independent of absolute firing rate.
Statistical analyses
Group data are expressed as mean ± SEM of n distinct observations. Statistical comparisons were made by a Student’s t-test (for one or two independent populations) or by one-way ANOVA for comparing multiple treatments, using Prism statistical software. To take uneven variances into account, for comparisons between groups of unequal numbers, Welch’s correction for the Student’s t-test was used. To compare CV2 analyses, a one-way repeated-measures ANOVA was used; P < 0.05 was considered statistically significant.
RESULTS
Mechanical threshold
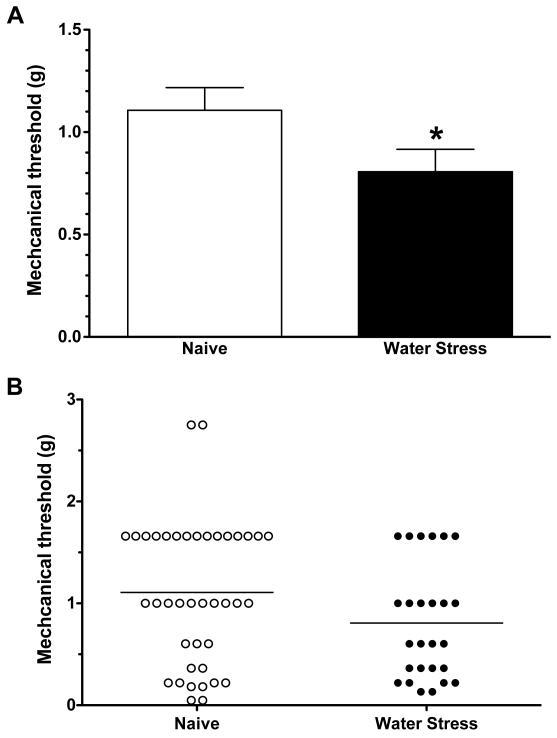
When tested by application of von Frey hairs to the peripheral receptive field in the gastrocnemius muscle, the mechanical threshold of muscle afferents in rats exposed to water avoidance stress (0.81 ± 0.11 g, n= 26) was significantly lower than the mechanical threshold of muscle afferents in naïve control animals (1.11 ± 0.11 g, n= 40, P<0.05, Student’s t-test; Fig. 1). Thus, water-avoidance stress produces ~34% decrease in mechanical threshold for activation in skeletal muscle nociceptors.
Figure 1.
A. Mechanical threshold of nociceptors innervating the gastrocnemius muscle, from water-avoidance stress-exposed (n = 26) and control rats (n = 40). Muscle nociceptors in water-avoidance-exposed rats had significantly lower mechanical thresholds than nociceptors from naïve control rats (P<0.05, Welch’s correction for the Student’s t-test).
B. Scattergram of mechanical thresholds of individual muscle nociceptors from naïve control and water-avoidance stress-exposed rats.
Response to sustained stimulation
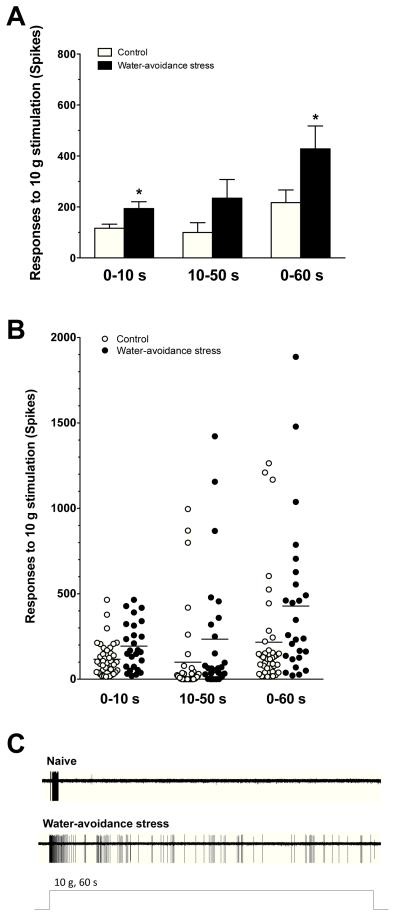
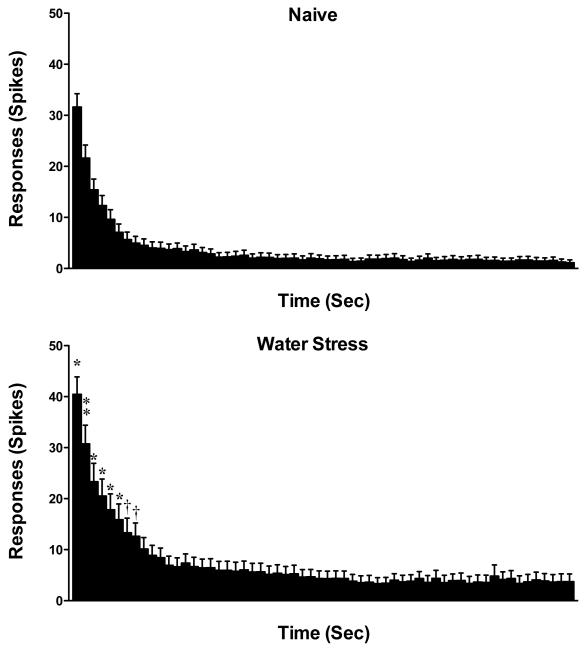
To examine excitability in muscle nociceptors from stressed rats, we evaluated their response to a sustained suprathreshold (10 g) von Frey stimulus. The response of muscle afferents to this sustained mechanical stimulation, in rats previously exposed to water avoidance stress (193.6 ± 27.0 action potentials/60 sec stimulus, n= 26), was significantly greater than in afferents from control animals (116.8 ± 15.7 action potentials/60 sec stimulus, n= 40, P = 0.005, Student’s t-test, Fig. 2); single-unit C-fiber recordings of action potentials evoked by a 10-g stimulus in naïve control and in water-avoidance stressed rats is shown in Fig. 2C. In rats exposed to stress, there was also an increase in firing frequency of muscle nociceptors for the first 8 s of the sustained 10 g stimulation (two-way repeated measures ANOVA, with Bonferroni post hoc test, P<0.05, control vs. stress exposed, Fig. 3). Thus, water-avoidance stress produces a marked increase in the response of muscle nociceptors to mechanical stimulation.
Figure 2.
The response of nociceptors to sustained (60 s) suprathreshold (10 g) von Frey hair mechanical stimuli in muscle nociceptors. Mean values (A) and individual fibers (B) are shown from naïve control and water-avoidance stress-exposed rats. The responses of the nociceptors from the water-avoidance group (n = 26) were significantly higher (for 0-10s and 0-60s) than those of control rats (n = 40, *P<0.05, Welch’s correction for the Student’s t-test). (C) Single-unit C-fiber recordings of action potentials evoked by a 10-g stimulus in naïve control and in water-avoidance stressed rats.
Figure 3.
The time course of the average responses of nociceptors during the 60 s suprathreshold stimulus, for naïve control (n = 40) and water-avoidance stressed (n = 26) rats; bin width 1 s. The number of action potentials in muscle afferents from stressed rats was significantly greater during the first 1 – 8 s stimulus period (two-way repeated measures ANOVA, with Bonferroni post hoc test; † P<0.05; * P < 0.01; and, □ P<0.001; naïve control vs. stress).
Nociceptor firing pattern
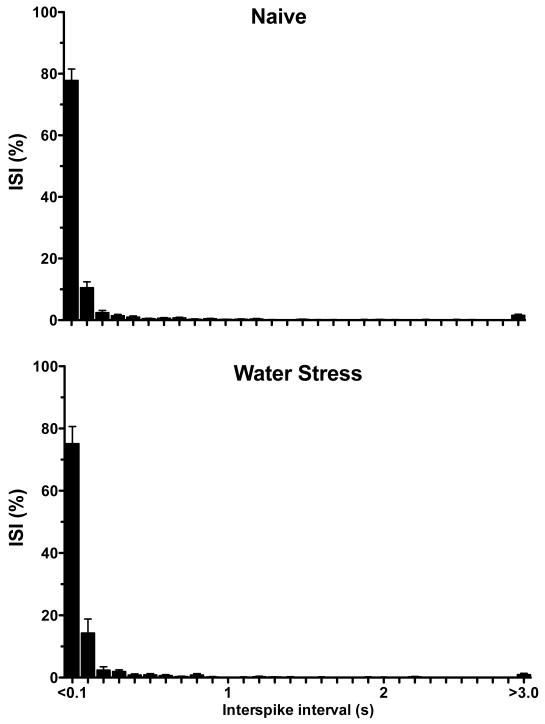
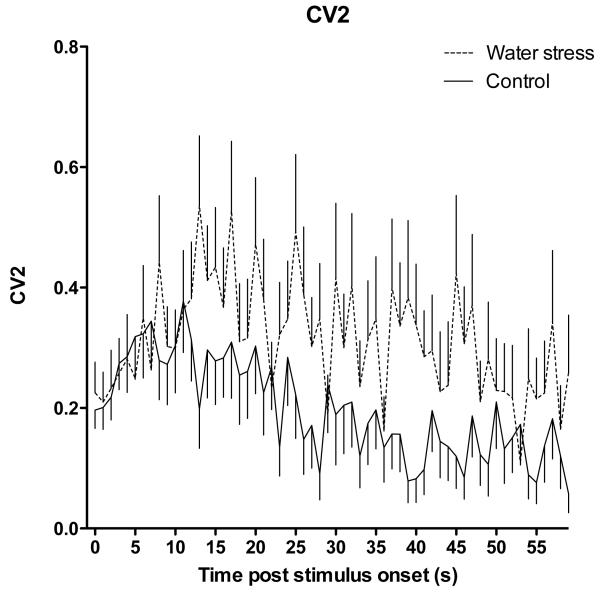
To examine the pattern of neural activity in nociceptors from stressed animals we generated inter-stimulus interval (ISI) histograms and performed coefficient of variation (CV2) analyses for muscle afferents recorded in stressed and control rats. We found no difference in the ISI histogram for muscle afferents from stressed and control rats (Fig. 4). However, we did find a significantly greater CV2 (indicating increased interspike interval variability) for muscle afferents from rats exposed to water-avoidance stress (P<0.05, two-way repeated measures ANOVA, Fig. 6). Thus, in addition to a significant decrease in mechanical nociceptive threshold and increase in response to sustained suprathreshold stimulation in muscle nociceptors from rats exposed to water-avoidance stress, there was also a difference in the firing patterns in these nociceptors.
Figure 4.
The inter-stimulus interval (ISI) distribution of muscle nociceptors in response to sustained (60 s) suprathreshold (10 g) von Frey hair mechanical stimuli, for water-avoidance stress-exposed (n = 26) and naïve control (n = 40) rats (bin width 1 s).
Figure 6.
The coefficient of variation (CV2) distribution of muscle nociceptors from water-avoidance stress-exposed (n = 26) and naïve control (n = 40) rats, in response to sustained (60 s) suprathreshold (10 g) von Frey hair mechanical stimulation. CV2 is significantly greater in neurons of rats exposed to water-avoidance stress, compared to naïve controls (two-way repeated measures ANOVA, P<0.05).
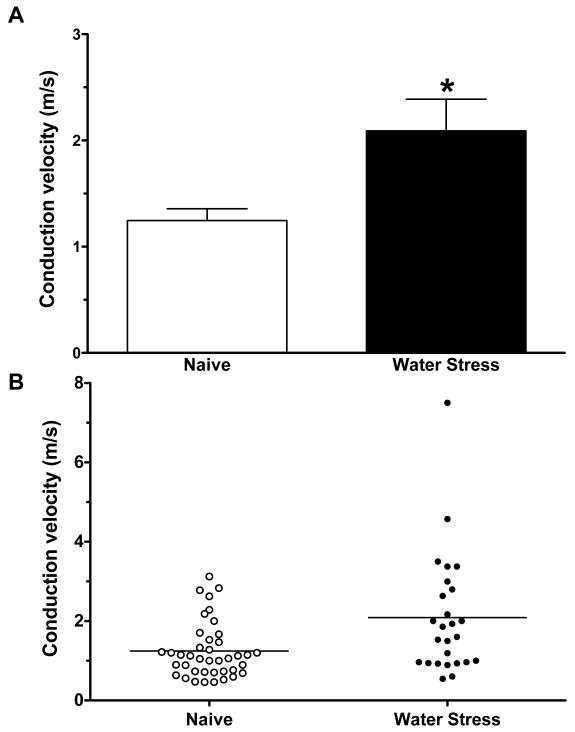
Conduction velocity
The conduction velocity of muscle afferents in rats exposed to water avoidance stress (2.09 ± 0.30 m/sec, n= 26) was markedly faster than that of afferents in control animals (1.25 ± 0.11 m/sec, n= 40 P<0.05, unpaired Student’s t test with Welch’s correction Fig. 5). When the distribution of conduction velocities in fibers of the two groups of animals (stress and control) was examined, a shift in the distribution of conduction velocities was observed. Thus, while not excluding a variation in percentage of a fiber type that was sampled, our data is most compatible with the suggestion that our findings are not explained by a larger percentage of type III afferents recorded in stressed rats (Fig. 5) (conduction velocities of type III and type IV fibers are 2.5 – 30 and <2.5 m/s, respectively 44).
Figure 5.
A. Mean conduction velocities from water-stressed rats (2.09 ± 0.3 n=26) were significantly greater than from naïve rats (1.25 ± 0.11 n=40, Welch’s correction for the Student’s t-test, p < 0.05).
B. Scattergram of conduction velocities of muscle nociceptors in naïve control and water-avoidance-treated rats.
DISCUSSION
Clinical conditions characterized by widespread pain (e.g., IBS, TMD and FMS) are amongst the most common forms of chronic pain 12,53,64, as well as amongst the most difficult to treat 34,42. The ability of stress to enhance pain has been well documented in a large number of chronic pain syndromes 32,35,36,51 and in animal models of these conditions 28,29,33,49,70. Indication of an involvement of CNS mechanisms in these pain syndromes arose, in part, from the observations of the major impact stress has on these clinical conditions 13,19,32,35,36,41,51,71, and a contribution of CNS mechanisms is further supported from experimental studies 43,61,69. More recently peripheral nociceptive effects of stress axis mediators (catecholamines and glucocorticoids) 30 have raised interest in a peripheral contribution, as well. Since these latter studies of peripheral mechanisms have used behavioral or reflex measures of nociception, how these peripheral mechanisms are reflected in activity of nociceptive afferents has not been directly studied. Of note, in a rat model of chronic visceral hypersensitivity, there is greater spontaneous activity, response to mechanical stimulation and lowered mechanical threshold in visceral primary afferent fibers 38, as well as a contribution of central sensitization 1, suggesting a contribution of peripheral as well as central mechanisms. In the present experiments we examined skeletal muscle nociceptor function in an animal model of chronic widespread pain, water-avoidance stress, which produces both musculoskeletal 20 and visceral 37 hyperalgesia in the rat.
The psychological stress produced by water-avoidance, which induces robust mechanical hyperalgesia in skeletal muscle, also induced multiple changes in the function of muscle nociceptors, including ~34% decrease in mechanical threshold, an almost doubling of the number of action potentials produced by a sustained suprathreshold stimulus, an increase in interspike interval variability and an increase in nociceptor conduction velocity. Given that most if not all of these changes would, alone, be expected to be associated with enhanced nociceptor activity, taken together, we suggest that these changes in skeletal muscle primary afferent nociceptor function produced by chronic stress make a substantial contribution to musculoskeletal mechanical hyperalgesia in the water-avoidance stressed rat. Of note, alterations in cutaneous mechanical thresholds are not always observed and as such, these changes in muscle mechanical threshold may well occur more commonly for muscle nociceptor afferents
In the setting of inflammatory pain, neuronal conduction velocity has generally been found to be unchanged 4,47 and for peripheral neuropathies, even those associated with pain, most commonly an unchanged or slowing conduction velocity has been observed 17,45,47. This paradox between expected and observed changes may be explained, at least in part, by the fact that in patients with peripheral neuropathies 48,60,65, and in animal models of neuropathic pain 3,8,9,26,45 conduction velocity is generally measured in myelinated, non-nociceptive, afferents. However, there are studies in which slowing conduction velocity of cutaneous C-fiber were observed in neuropathic models (e.g. dideoxycytidine 10) and clinical studies with neuropathic pain (e.g. erythromelalgia 50). Methodological differences may also play a role, due to differences in properties of cutaneous versus muscle C-fibers (e.g. cutaneous C-fiber conduction velocity slows markedly with repetitive stimulation 55,59). Of note, in this regard, we have recently observed, in a model of cancer chemotherapy-induced painful peripheral neuropathy, produced by paclitaxel (Taxol; 14), that while there was no change in conduction velocity in C-fibers innervating the hind paw there was an increase in conduction velocity of the same magnitude produced in the present study, in nociceptors innervating the gastrocnemius muscle 20. While the underlying mechanism(s) responsible for the increased conduction velocity in muscle afferents is unknown, such changes must be due to changes in an ionic conductance in the axon. Changes in channel kinetics might also explain the significantly higher CV2 in muscle afferents from stressed rats. Djhouri and Lawson observed that chronic inflammation produced by intradermal injection of complete Freunds’ Adjuvant increased C-fiber conduction velocity16, suggesting that direct exposure of the peripheral nerve to the effect of cytokines might have produced an enhancement of conduction velocity in nociceptors. Possibly related to this, Gold and colleagues have recently shown that persistent inflammation alters the density and distribution of voltage-activated ion channels in DRG neurons 39.
The mechanism by which stress produces such marked changes in nociceptor function are, at the moment, unknown. However, chronic stress is known to upregulate Na+ channels 66, and the sympathoadrenal stress hormone, epinephrine, enhances tetrodotoxin-resistant Na+ currents in dorsal root ganglion neurons 31. We have provided evidence that stress can induce neuroplasticity in peripheral sensitization mechanisms in skin and muscle in the rat 28-30,56 and even induce mechanical hyperalgesia 20. These effects of stress are due, at least in part, to neuroendocrine stress axis mediators, catecholamines and corticosteroids 29, acting on adrenergic and glucocorticoid receptors on sensory neurons 28. And, in a model of alcohol-induced painful peripheral neuropathy, we have shown that alcohol consumption fails to produce mechanical hyperalgesia in adrenal medullectomized rats 15.
CONCLUSION
This is the first demonstration of stress-induced changes in primary afferent nociceptor function in skeletal muscle, produced by analysis of nociceptor activity in a model of stress-induced musculoskeletal hyperalgesia in the rat. Stress produced a modest decrease in mechanical nociceptive threshold, an increase in the number of action potentials produced in response to a prolonged stimulus, and an increase in conduction velocity of muscle nociceptors. Given that these changes in nociceptor function would be expected to predispose to increased activity in muscle nociceptors and are at least moderate in magnitude, it is likely that they make a substantial contribution to the enhanced nociception observed in this model of chronic widespread pain.
Acknowledgments
We thank Dr. Robert Gear for help with CV2 calculations. This work was supported by NIH grant AR054635.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Sonnenborg FA, Andersen OK. Facilitation of the withdrawal reflex by repeated transcutaneous electrical stimulation: an experimental study on central integration in humans. Eur J Appl Physiol. 2000;81:165–173. doi: 10.1007/s004210050026. [DOI] [PubMed] [Google Scholar]
- 3.Authier N, Gillet JP, Fialip J, Eschalier A, Coudore F. Description of a short-term Taxol-induced nociceptive neuropathy in rats. Brain Res. 2000;887:239–249. doi: 10.1016/s0006-8993(00)02910-3. [DOI] [PubMed] [Google Scholar]
- 4.Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J. Neurosci. 1999;19:859–867. doi: 10.1523/JNEUROSCI.19-02-00859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackburn-Munro G, Blackburn-Munro RE. Chronic pain, chronic stress and depression: coincidence or consequence? J. Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 6.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 7.Bradesi S, Svensson CI, Steinauer J, Pothoulakis C, Yaksh TL, Mayer EA. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology. 2009;136:1339–1348. e1–2. doi: 10.1053/j.gastro.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, Glazner GW, Fernyhough P, Zochodne DW. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008;57:1664–1673. doi: 10.2337/db07-1737. [DOI] [PubMed] [Google Scholar]
- 9.Cermenati G, Giatti S, Cavaletti G, Bianchi R, Maschi O, Pesaresi M, Abbiati F, Volonterio A, Saez E, Caruso D, Melcangi RC, Mitro N. Activation of the liver X receptor increases neuroactive steroid levels and protects from diabetes-induced peripheral neuropathy. J. Neurosci. 2010;30:11896–11901. doi: 10.1523/JNEUROSCI.1898-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Levine JD. Mechanically-evoked C-fiber activity in painful alcohol and AIDS therapy neuropathy in the rat. Mol Pain. 2007;3 doi: 10.1186/1744-8069-3-5. Article No. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X, Tanner K, Levine JD. Mechanical sensitization of cutaneous C-fiber nociceptors by prostaglandin E2 in the rat. Neurosci. Lett. 1999;267:105–108. doi: 10.1016/s0304-3940(99)00345-6. [DOI] [PubMed] [Google Scholar]
- 12.Croft P, Rigby AS, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. J. Rheumatol. 1993;20:710–713. [PubMed] [Google Scholar]
- 13.Delvaux MM. Stress and visceral perception. Can J Gastroenterol. 1999;13(Suppl A):32A–36A. doi: 10.1155/1999/235916. [DOI] [PubMed] [Google Scholar]
- 14.Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase Cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/s0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 15.Dina OA, Khasar SG, Alessandri-Haber N, Green PG, Messing RO, Levine JD. Alcohol-induced stress in painful alcoholic neuropathy. Eur J Neurosci. 2008;27:83–92. doi: 10.1111/j.1460-9568.2007.05987.x. [DOI] [PubMed] [Google Scholar]
- 16.Djouhri L, Lawson SN. Increased conduction velocity of nociceptive primary afferent neurons during unilateral hindlimb inflammation in the anaesthetised guinea-pig. Neuroscience. 2001;102:669–679. doi: 10.1016/s0306-4522(00)00503-0. [DOI] [PubMed] [Google Scholar]
- 17.Elliott MB, Barr AE, Clark BD, Amin M, Amin S, Barbe MF. High force reaching task induces widespread inflammation, increased spinal cord neurochemicals and neuropathic pain. Neuroscience. 2009;158:922–931. doi: 10.1016/j.neuroscience.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franck J, Brodin E, Fried G. Differential release of endogenous 5-hydroxytryptamine, substance P, and neurokinin A from rat ventral spinal cord in response to electrical stimulation. J. Neurochem. 1993;61:704–711. doi: 10.1111/j.1471-4159.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- 19.Giske L, Bautz-Holter E, Sandvik L, Roe C. Relationship between pain and neuropathic symptoms in chronic musculoskeletal pain. Pain Med. 2009;10:910–917. doi: 10.1111/j.1526-4637.2009.00622.x. [DOI] [PubMed] [Google Scholar]
- 20.Green PG, Alvarez P, Gear RW, Mendoza D, Levine JD. Further validation of a model of fibromyalgia syndrome in the rat. J Pain. 2011 doi: 10.1016/j.jpain.2011.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holt GR, Softky WR, Koch C, Douglas RJ. Comparison of discharge variability in vitro and in vivo in cat visual cortex neurons. J. Neurophysiol. 1996;75:1806–1814. doi: 10.1152/jn.1996.75.5.1806. [DOI] [PubMed] [Google Scholar]
- 23.Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imbe H, Murakami S, Okamoto K, Iwai-Liao Y, Senba E. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Imbe H, Okamoto K, Donishi T, Senba E, Kimura A. Involvement of descending facilitation from the rostral ventromedial medulla in the enhancement of formalin-evoked nocifensive behavior following repeated forced swim stress. Brain Res. 2010;1329:103–112. doi: 10.1016/j.brainres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Jolivalt CG, Mizisin LM, Nelson A, Cunha JM, Ramos KM, Bonke D, Calcutt NA. B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur. J. Pharmacol. 2009;612:41–47. doi: 10.1016/j.ejphar.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Karp JF, Shega JW, Morone NE, Weiner DK. Advances in understanding the mechanisms and management of persistent pain in older adults. Br J Anaesth. 2008;101:111–120. doi: 10.1093/bja/aen090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J. Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khasar SG, Dina OA, Green PG, Levine JD. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 31.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korszun A, Papadopoulos E, Demitrack M, Engleberg C, Crofford L. The relationship between temporomandibular disorders and stress-associated syndromes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:416–420. doi: 10.1016/s1079-2104(98)90366-3. [DOI] [PubMed] [Google Scholar]
- 33.Larauche M, Gourcerol G, Million M, Adelson DW, Tache Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13:343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson K. Treatment options and patient perspectives in the management of fibromyalgia: future trends. Neuropsychiatr Dis Treat. 2008;4:1059–1071. doi: 10.2147/ndt.s3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am. J. Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 36.Lew HL, Otis JD, Tun C, Kerns RD, Clark ME, Cifu DX. Prevalence of chronic pain, posttraumatic stress disorder, and persistent postconcussive symptoms in OIF/OEF veterans: polytrauma clinical triad. J Rehabil Res Dev. 2009;46:697–702. doi: 10.1682/jrrd.2009.01.0006. [DOI] [PubMed] [Google Scholar]
- 37.Liebregts T, Adam B, Bertel A, Lackner C, Neumann J, Talley NJ, Gerken G, Holtmann G. Psychological stress and the severity of post-inflammatory visceral hyperalgesia. Eur J Pain. 2007;11:216–222. doi: 10.1016/j.ejpain.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 39.Lu SG, Zhang XL, Luo ZD, Gold MS. Persistent inflammation alters the density and distribution of voltage-activated calcium channels in subpopulations of rat cutaneous DRG neurons. Pain. 2010;151:633–643. doi: 10.1016/j.pain.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin AL, Halket E, Asmundson GJ, Flora DB, Katz J. Posttraumatic stress symptoms and the diathesis-stress model of chronic pain and disability in patients undergoing major surgery. Clin J Pain. 2010;26:518–527. doi: 10.1097/AJP.0b013e3181e15b98. [DOI] [PubMed] [Google Scholar]
- 42.McBeth J, Macfarlane GJ, Hunt IM, Silman AJ. Risk factors for persistent chronic widespread pain: a community-based study. Rheumatology (Oxford) 2001;40:95–101. doi: 10.1093/rheumatology/40.1.95. [DOI] [PubMed] [Google Scholar]
- 43.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26:465–473. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mense S, Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J. Physiol. 1983;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer L, Patte-Mensah C, Taleb O, Mensah-Nyagan AG. Cellular and functional evidence for a protective action of neurosteroids against vincristine chemotherapy-induced painful neuropathy. Cell Mol Life Sci. 2010;67:3017–3034. doi: 10.1007/s00018-010-0372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller BA, Woolf CJ. Glutamate-mediated slow synaptic currents in neonatal rat deep dorsal horn neurons in vitro. J. Neurophysiol. 1996;76:1465–1476. doi: 10.1152/jn.1996.76.3.1465. [DOI] [PubMed] [Google Scholar]
- 47.Nakatsuka T, Park JS, Kumamoto E, Tamaki T, Yoshimura M. Plastic changes in sensory inputs to rat substantia gelatinosa neurons following peripheral inflammation. Pain. 1999;82:39–47. doi: 10.1016/S0304-3959(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 48.Nardone A, Schieppati M. Group II spindle fibres and afferent control of stance. Clues from diabetic neuropathy. Clin Neurophysiol. 2004;115:779–789. doi: 10.1016/j.clinph.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur J Pain. 2010;14:236–244. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Orstavik K, Jorum E. Microneurographic findings of relevance to pain in patients with erythromelalgia and patients with diabetic neuropathy. Neurosci. Lett. 2010;470:180–184. doi: 10.1016/j.neulet.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 51.Paras ML, Murad MH, Chen LP, Goranson EN, Sattler AL, Colbenson KM, Elamin MB, Seime RJ, Prokop LJ, Zirakzadeh A. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- 52.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 53.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5:317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Price DD, Craggs JG, Zhou Q, Verne GN, Perlstein WM, Robinson ME. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. Neuroimage. 2009;47:995–1001. doi: 10.1016/j.neuroimage.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raymond SA, Thalhammer JG, Popitz-Bergez F, Strichartz GR. Changes in axonal impulse conduction correlate with sensory modality in primary afferent fibers in the rat. Brain Res. 1990;526:318–321. doi: 10.1016/0006-8993(90)91239-d. [DOI] [PubMed] [Google Scholar]
- 56.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- 58.Rivat C, Becker C, Blugeot A, Zeau B, Mauborgne A, Pohl M, Benoliel JJ. Chronic stress induces transient spinal neuroinflammation, triggering sensory hypersensitivity and long-lasting anxiety-induced hyperalgesia. Pain. 2010;150:358–368. doi: 10.1016/j.pain.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 59.Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J. Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shefner JM, Buchthal F, Krarup C. Slowly conducting myelinated fibers in peripheral neuropathy. Muscle Nerve. 1991;14:534–542. doi: 10.1002/mus.880140608. [DOI] [PubMed] [Google Scholar]
- 61.Staud R. Evidence of involvement of central neural mechanisms in generating fibromyalgia pain. Curr Rheumatol Rep. 2002;4:299–305. doi: 10.1007/s11926-002-0038-5. [DOI] [PubMed] [Google Scholar]
- 62.Staud R, Nagel S, Robinson ME, Price DD. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145:96–104. doi: 10.1016/j.pain.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanner KD, Reichling DB, Gear RW, Paul SM, Levine JD. Altered temporal pattern of evoked afferent activity in a rat model of vincristine-induced painful peripheral neuropathy. Neuroscience. 2003;118:809–817. doi: 10.1016/s0306-4522(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 64.Toda K, Harada T. Prevalence, classification, and etiology of pain in Parkinson’s disease: association between Parkinson’s disease and fibromyalgia or chronic widespread pain. Tohoku J Exp Med. 2010;222:1–5. doi: 10.1620/tjem.222.1. [DOI] [PubMed] [Google Scholar]
- 65.Truini A, Padua L, Biasiotta A, Caliandro P, Pazzaglia C, Galeotti F, Inghilleri M, Cruccu G. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain. 2009;145:105–109. doi: 10.1016/j.pain.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 66.Van Zundert J, Harney D, Joosten EA, Durieux ME, Patijn J, Prins MH, Van Kleef M. The role of the dorsal root ganglion in cervical radicular pain: diagnosis, pathophysiology, and rationale for treatment. Reg Anesth Pain Med. 2006;31:152–167. doi: 10.1016/j.rapm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 68.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 69.Whiting W. Quality Assurance Network Newsletter. Hooked on quality. Nurs Stand. 1991;6(suppl):1–2. [PubMed] [Google Scholar]
- 70.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304.e3. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62:420–424. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 72.Yilmaz P, Diers M, Diener S, Rance M, Wessa M, Flor H. Brain correlates of stress-induced analgesia. Pain. 2010;151:522–529. doi: 10.1016/j.pain.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 73.Yu YB, Yang J, Zuo XL, Gao LJ, Wang P, Li YQ. Transient receptor potential vanilloid-1 (TRPV1) and ankyrin-1 (TRPA1) participate in visceral hyperalgesia in chronic water avoidance stress rat model. Neurochem Res. 2010;35:797–803. doi: 10.1007/s11064-010-0137-z. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intracolonic lidocaine. Pain. 2008;139:218–224. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]