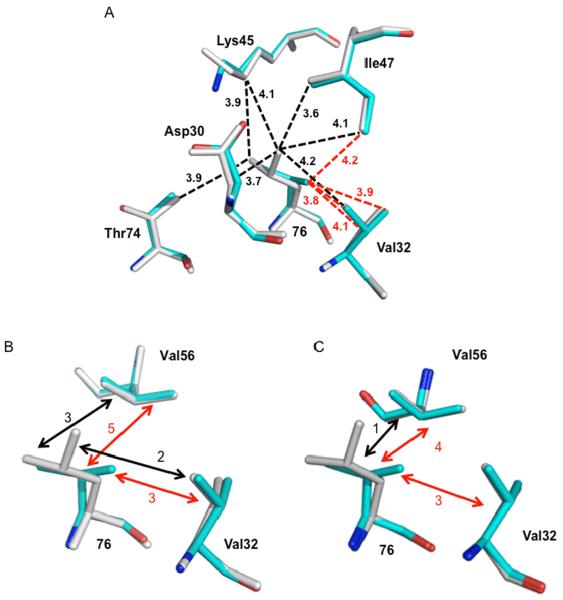
Figure 5.
Hydrophobic interactions of residue 76 in the crystal structures of PRL76V (cyan), and wild type PR (grey). A. Interactions of residue 76 in subunit A of PRL76V-DRV and PR-DRV. Leu76 forms van der Waals contacts (3.6–4.2 Å) with the side chains of Asp30, Val32 Lys45, Ile47, and Thr74 (black dashed lines), while Val76 in PRL76V only has hydrophobic contacts with Val32 and Ile47 (red dashed lines). Interatomic distances are indicated in Å. Neighboring residue Gln58 is not shown since it forms similar interactions with residue 76 in PR and PRL76V. Val56 is omitted for clarity. B. Interactions of residue 76 with Val32 and Val56 in PRL76V-DRV and PRDRV. C. Interactions of residue 76 with Val32 and Val56 in PRL76V-SQV and PR-SQV. In B and C the number of hydrophobic contacts between the side chains is indicated by black (with Leu76) and red (with Val76) dashed arrows.