Abstract
Introduction
This study compared the effects of downhill or horizontal treadmill running on the magnetic resonance imaging (MRI) transverse relaxation time constant (T2) in mdx mice.
Methods
Mice underwent either downhill (n=11 mdx, n=6 controls) or horizontal running (n=9, mdx only) on a treadmill. MRI was conducted prior to exercise, immediately afterwards (~20 min), 24, and 48 hours following exercise.
Results
A higher percentage of pixels with elevated T2 in the lower hindlimb muscles was observed in the mdx mice compared to controls both pre-exercise (p < 0.001) and at each time point following downhill running (p < 0.05), but not with horizontal running. The medial compartment muscles appeared to be the most susceptible to increased T2.
Discussion
Downhill running provides a stimulus for inducing acute changes in muscle T2 in mdx mice. MRI is a non-invasive approach for examining acute muscle damage and recovery in multiple muscle groups simultaneously.
Keywords: muscle damage, muscular dystrophy, magnetic resonance imaging, mdx mice, muscle T2
INTRODUCTION
The muscular dystrophies are a collection of degenerative neuromuscular diseases. Duchenne muscular dystrophy (DMD) is the most common and devastating form of muscular dystrophy, which affects one in every 3,500 male births.1 DMD is a hereditary disease caused by a mutation on the X chromosome and is characterized by the absence of functional dystrophin. Dystrophin is a 427kD cytoskeletal protein, which anchors F-actin to laminin in the extracellular matrix through the dystrophin-associated glycoprotein complex.2,3 When dystrophin is missing or nonfunctional, the entire complex is compromised, leading to increased vulnerability to muscle damage during contractions.4 Exercise-induced muscle damage occurs following high-force, unaccustomed exercise, particularly following eccentric (i.e. lengthening) muscle contractions.5–7 Although the mechanisms are not fully understood, the initial mechanical damage is followed by an inflammatory response and subsequent reparative and regenerative process within the muscle.8,9 The muscles of the mdx mouse, a model of DMD in which the dystrophin protein is absent, are known to be more susceptible to muscle damage from eccentric loading than wild-type mice.10–12 Even with normal cage activity, the skeletal muscles of the mdx mouse exhibit signs of muscle damage, degeneration and necrosis as early as two to four weeks in the postnatal period, and these muscle abnormalities persist throughout the lifespan despite low activity levels.13
Models of eccentric loading commonly employed in animal models, such as direct electrical stimulation of an excised muscle under passive stretch11,12 or electrical stimulation of intact muscles under lengthening conditions14,15 can be used to produce marked damage to individual muscles. However, these models are invasive and require sacrifice of the animal to examine muscle structure; therefore, they cannot be used for longitudinal studies.16,17 In contrast, downhill treadmill running has been used as a physiologically relevant model of eccentric loading in mdx mice,16–19 other rodent models,20–22 and humans.23,8 Downhill running has several advantages over eccentric contractions produced from electrical stimulation of muscles. First, it incorporates a physiological load and voluntary muscle contractions, which may translate into more relevant findings for studies involving human subjects. Second, downhill running has been used to accelerate muscle weakness and fibrosis in the limb muscles of the mdx mouse to more closely mirror the muscle pathology seen in patients with DMD.24,25 Finally, this method can be used for repeated bouts of muscle damage in the same animal, allowing for longitudinal studies of muscle damage and repair.22
Downhill treadmill running has previously been shown to produce muscle damage in the gastrocnemius and soleus muscles, as well as the tibialis anterior and extensor digitorum longus (EDL) of mdx mice.17 A limitation of this previous work was that the change in muscle damage following downhill running was compared between mdx mice that underwent downhill running to a group of mdx mice that did not run. This comparison has inherent limitations, since mdx mice have damaged muscle fibers even under conditions of normal cage activity, and there is high variability in muscle degeneration between animals.25 Ideally, the response of dystrophic muscle to downhill running should be examined in the same animal both pre- and post-exercise. This can be achieved using a non-invasive approach such as magnetic resonance imaging (MRI). The transverse relaxation time constant (T2) is a quantitative measure of a basic biophysical property that leads to signal contrast on MRI. Changes in the T2 of skeletal muscle have been observed during both acute physiological responses in healthy muscle and under pathophysiological conditions. For example, acute and transient increases in muscle T2 lasting approximately one hour have been observed in activated muscles following a bout of exercise.26,27 Although the exact mechanisms which account for these changes have not been fully elucidated, T2 changes have been attributed to a redistribution of water molecules in the muscle cells.28 Furthermore, following bouts of eccentric exercise, a later phase of increased muscle T2 occurs following the initial increase, and it peaks approximately 2 to 7 days after the initial exercise bout.29,27 Chronic changes in muscle T2 have also been detected in resting muscles of people with incomplete spinal cord injury,30 stroke, 31 DMD,32 and dermatomyositis,33 which are all conditions associated with muscle damage and inflammation.
Changes in muscle T2 have previously been used to monitor muscle damage and repair in animal models of cast immobilization,34 downhill running,22 tissue strain35 and muscular dystrophy.36–38 The results of these studies demonstrate that this fundamental MR property can be used to visualize muscle damage. Since MRI is non-invasive, it can be applied repeatedly in the same subject for longitudinal examination of muscle damage. MRI also has the added advantage of providing information on multiple muscles simultaneously; therefore differential responses to downhill running can be compared among different muscles of the same animal.
The objectives of this study were: 1) to compare the response of muscle T2 over a time course of 48 hours following a single bout of downhill running in mdx mice to wild-type (control) mice, 2) to compare the effects of horizontal versus downhill running on muscle damage in mdx mice, and 3) to compare the effects of downhill running among the lower hindlimb muscles of mdx mice. Muscle T2 and the percent of pixels with elevated T2 obtained from MRI were used as the main outcomes of interest. Histological confirmation of damaged muscle fibers was performed using Evans Blue Dye.
MATERIALS & METHODS
Animals
Both C57BL/10ScSn-DMDmdx (mdx; n=25) and wild type, C57BL/10ScSn (controls, n=6) adult male mice (aged 5–15 months) were included in the study. Wild type mice and breeding pairs from the mdx colony were obtained from Jackson Laboratories (Bar Harbor, ME) and thereafter maintained in-house. Animals were housed in an AAALAC approved facility with a 12 hour light: dark cycle (72°F, 42% humidity) and free access to food and water. The University of Florida Institutional Animal Care and Use Committee approved the experimental protocol.
Experimental Protocol
MRI was conducted prior to treadmill running in both mdx and control mice to determine baseline muscle T2 values of the lower hindlimb muscles. The methods of the MRI protocol and T2 analysis are outlined below. Following the initial MRI, each mouse underwent one of two treadmill running protocols: a bout of downhill running (n=11 mdx, n=6 controls) or a bout of horizontal grade running (n=9 mdx only). Specifically, mice were run either on a downhill sloped (14 degree decline) or a horizontal (0% grade) motorized treadmill at a speed of 8 to 10m/min, for 45 minutes. To ensure the mice ran for the entire duration of the protocol, they were continually observed. If necessary, a short burst of compressed air or the use of a brush at the base of the tail was used to encourage running. From a total of 18 mdx mice screened, 11 were able to complete the downhill running protocol with some encouragement needed during the first five to ten minutes of running and near the end of the 45 minute period. All control mice (6 out of 6) were able to complete the downhill running protocol without encouragement. All mdx mice (9 out of 9) were able to complete the horizontal running protocol without external stimulation. MRI of the lower hindlimbs was conducted immediately post-exercise (approximately 20 minutes following the exercise bout), and at 24 and 48 hours post-exercise. In five of the mdx mice who ran downhill, a 10 day post-exercise follow-up MRI scan was also conducted. Lower hindlimb muscles were extracted in a subset of mdx (n=8) and control mice (n=5) at the end of the study for histological confirmation of muscle damage.
Magnetic Resonance Imaging
MRI was performed in a 4.7 T, horizontal bore magnet (Bruker Avance; ParaVision 3.02). The animal was anesthetized using an oxygen and isoflurane mixture (3% isoflurane) and maintained under 0.5–1% isoflurane for the duration of the MR procedure. The respiratory rate of the mouse was monitored for the duration of the scan (SA Instruments Inc., Stony Brook NY). The lower hindlimbs of the mouse were inserted up to the knee into a custom-built 2.0 cm internal diameter, solenoid 1H-coil (200 MHz). Multiple slice, diffusion controlled single spin-echo images were acquired with the following parameters: repetition time (TR) =2,000 ms, echo time (TE) = 14 ms and 40 ms, FOV 10–20 mm, slice thickness = 1 mm, acquisition matrix = 128 × 256 and two signal averages as previously described.34 Diffusion weighting was fixed at both TEs (diffusion weighting 3 mm2/s at both 14 and 40 ms). Hahn-spin echoes were implemented to avoid the contribution of stimulated echoes in the T2 measurement. Signal to noise ratios were 25:1 at TE = 14ms and 9:1 at TE = 40ms.
Muscle T2 Analysis from MRI
Muscle T2 values of the entire lower hindlimb (excluding bone) as well as of individual muscle compartments (anterior, posterior and medial; Figure 1) were computed from a T2 map, created from two echo times (TEs; 14 and 40ms) using in-house software as previously described.34,38 T2 relaxation time was calculated using single exponential decay with the following equation: T2 = (26 ms) / ln (SI14 / SI40), where SI14 and SI40 are the pixel intensities at 14 and 40 ms, respectively.39 The percent of muscle damage detected by MRI was defined as the percentage of pixels in a region of interest which had T2 values over two standard deviations above the mean muscle T2 found in control mice (> 29 ms) up to a maximum value of 100 ms.
Figure 1.
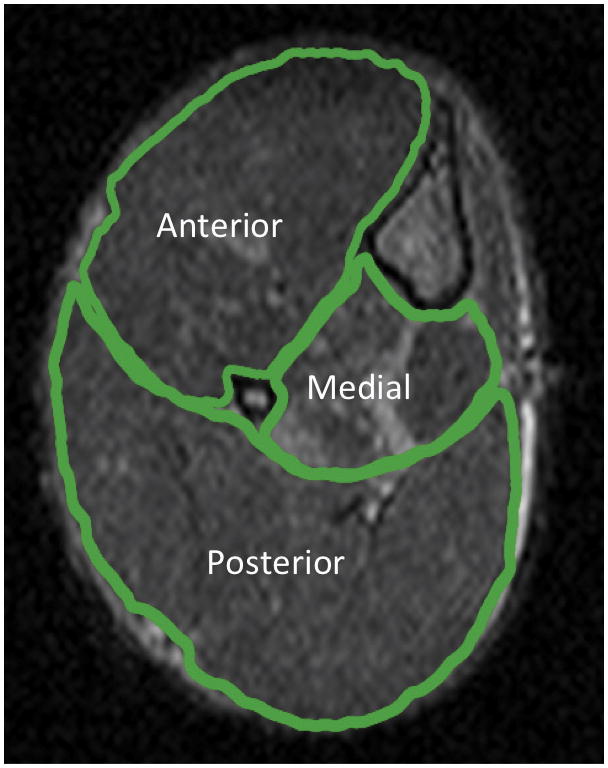
Trans-axial MR image of the right lower hindlimb showing regions of interest (ROIs) for muscle T2 analysis (TR = 2000ms, TE = 14ms). Muscles in each region include: tibialis anterior and extensor digitorum longus in the anterior compartment, flexor hallucis and flexor digitorum in the medial compartment and gastrocnemius, soleus and plantaris in the posterior compartment.
Histological confirmation of muscle damage
Histological confirmation of muscle damage was conducted in a subset of mdx and control mice, from each of the protocols: downhill running (n=4 mdx, n=5 control) and horizontal grade running (n=4 mdx only). Twenty-four hours following the exercise bout, mice were injected with Evans Blue Dye (EBD) (0.10 mL/10 g of body weight), via intraperitoneal injection.40 Forty-eight hours following exercise (i.e. 24 hours post-injection), the following muscles of the lower hindlimb were harvested: tibialis anterior (TA), extensor digitorum longus (EDL), flexor digitorum longus (FDL), soleus and gastrocnemius. Muscles were coated with OCT, frozen in isopentane cooled with liquid nitrogen and stored at −80°C. Each muscle was sectioned at the mid-belly region, at 10μm thickness and mounted on slides using a fluorescence-mounting medium with DAPI (Vectashield, Vector Labs). Slides were viewed using fluorescence microscopy, optimized for EBD (Texas Red filter). Images of the entire muscle cross-section were captured at 10x magnification using a light microscope (Leica Microsystems, Bannockburn IL) attached to a digital camera with slightly overlapping fields to obtain images from the entire muscle. The cross-section of the muscle was reconstructed in Adobe Photoshop from the digital images. EBD positive fibers were outlined using NIH Image J (available from http://rsbweb.nih.gov/ij/), and the total area occupied by EBD positive fibers was calculated as a percentage of the total muscle area.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS), version 11.0 (SPSS Inc., Chicago IL). The descriptive data are presented using means and standard error of the mean (SEM). To compare the differences in muscle T2 and percent muscle damage between mdx and control mice following a single bout of downhill running (study objective 1), independent samples t-tests were used to make comparisons at each time point between groups (α= 0.05, adjusted for multiple comparisons using a modified Bonferroni correction).41 Paired t-tests were used to compare differences in muscle T2 and percent damage from pre-exercise values to post-exercise time points (immediately post-exercise, 24 and 48 hours post-exercise), in each of the mdx and control groups (α= 0.05, adjusted for multiple comparisons). A comparison of the response of muscle T2 and percent damage in mdx mice between downhill running and horizontal running (study objective 2) was conducted using independent samples t-tests at each timepoint (p < 0.05). To examine study objective 3, the response of each muscle compartment to one bout of downhill treadmill running in mdx mice was compared using a two-way, repeated measures ANOVA and the Tukey post-hoc tests (main – factors timepoint and muscle, p < 0.05). Lastly, a Spearman rank correlation was used to examine the relationship between the percent of muscle cross-sectional area positive for EBD and the percent of pixels with elevated T2.
RESULTS
Downhill running in mdx compared to control mice
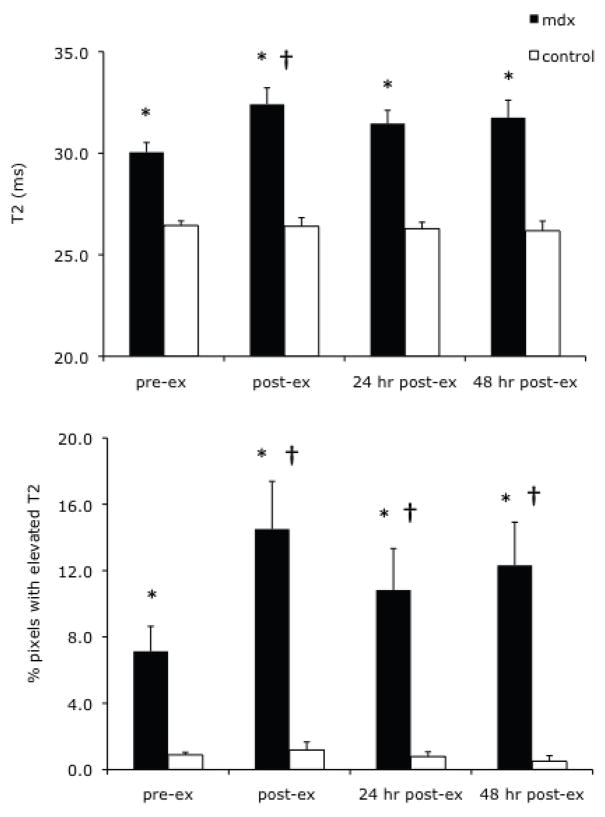
Muscle T2 and the percent of pixels with elevated T2 in the lower hindlimb muscles was compared between mdx and control mice following a single bout of downhill running over a period of 48 hours. As shown in Figure 2A, mdx mice had a higher muscle T2 compared with control mice both pre-exercise and at each timepoint following downhill running (p < 0.001). A twofold increase in the number of pixels with elevated T2 was detected immediately post-exercise in mdx mice (14.5 ± 2.9% of pixels with elevated T2 post-exercise compared to 7.1 ± 1.5%, pre-exercise) and remained elevated compared to baseline values at 24 and 48 hours post-exercise (p < 0.001; Figure 2B). Muscle T2 was higher immediately post-exercise in the mdx mice (p < 0.05, Figure 2A), but not at 24 or 48 hours. The percent of pixels with elevated T2 consistently returned to baseline values ten days after downhill running in mdx mice (Fig 3A). Immediately following downhill running and 24 and 48 hours later, the control mice did not show any difference in their muscle T2 or percent of elevated pixels compared to their pre-exercise values. An example of transaxial T2-weighted images from an mdx mouse following downhill running is shown in Figure 3B, with follow-up MRI taken ten days after the exercise bout. A distinct hyperintense region is observed on the T2-weighted images of the medial muscle compartment. Figure 3C shows a projection from a three-dimensional rendering of the medial compartment with elevated muscle T2 following downhill running.
Figure 2.
Panel A. Muscle T2 of the lower hindlimb muscles of mdx and control mice before and after a single bout of downhill running (mean ± SEM). The mdx mice had higher T2 values than controls at all time points (pre and post-exercise) (p < 0.001; denoted by *). The mdx mice showed a significant increase in muscle T2 immediately post-exercise (p < 0.05, denoted by †). No significant difference was observed in T2 values of control mice following a bout of downhill running. Panel B. Percentage of pixels with elevated T2 (% damaged muscle; mean ± SEM) in the lower hindlimb muscles of mdx and control mice before and after downhill running. The mdx mice had higher % damage at all time points (pre and post-exercise) than controls (p < 0.001; denoted by *). Also, mdx mice showed a significant increase in % damaged muscle immediately following the downhill running bout (post-ex), as well as 24 hr and 48 hrs later, compared to pre-exercise values (p < 0.05, denoted by †). The control mice did not show a significant change in % damaged muscle following downhill running.
Figure 3.
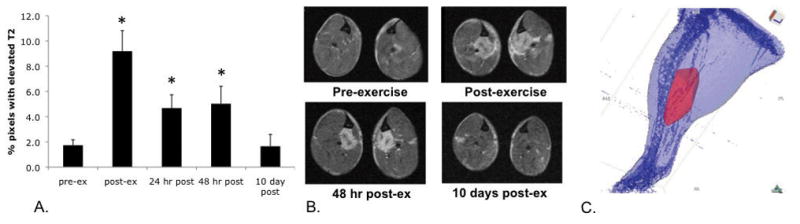
Panel A. Percent of pixels with elevated muscle T2 recovered to baseline values ten days after downhill running (* denotes significant difference from baseline, p < 0.05). Panel B. Transaxial, T2-weighted images from the lower hindlimb of an mdx mouse before and after a single bout of downhill running for 45 min (−14 degrees, 8–10 m/min). Note specific area of hyperintensity in the medial region, between the tibia and fibula, which is recovered after 10 days. Panel C. Three-dimensional rendering of a single mouse hindlimb showing region of muscle damage in the medial compartment.
Downhill versus horizontal running in mdx mice
The response of muscle T2 and percent of muscle damage was compared in two independent groups of mdx mice that underwent either a bout of downhill or horizontal treadmill running. As shown in Figure 4, muscle T2 only increased in the group of mdx mice immediately following downhill running whereas no change in T2 was observed in those that underwent horizontal treadmill running. The percent of pixels with elevated T2 showed an increase in the horizontal running mice immediately post-exercise (5.0 ±1.0% at baseline versus 6.8 ±1.5% immediately post-exercise, p < 0.05), but only remained elevated in the downhill running group at 24 and 48 hours following the exercise bout.
Figure 4.
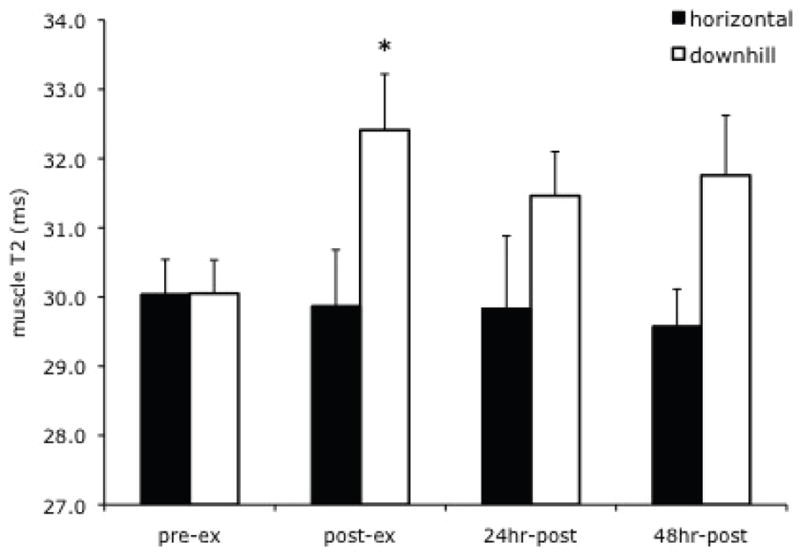
Muscle T2 values in mdx mice following a single bout of downhill running compared to horizontal running. Muscle T2 showed a significant increase immediately following downhill running (compared to pre-exercise, p < 0.05, denoted by *) but not following horizontal grade running.
Comparison between muscle compartments in mdx mice following downhill running
As mdx mice showed an increase in muscle damage of their lower hindlimb muscles following downhill running, a further examination of response in the three muscle compartments (anterior, medial and posterior) was conducted. As shown in Figure 5, the medial muscle compartment had a significantly higher percentage of pixels with elevated T2 at baseline compared to the anterior and posterior muscle groups. Furthermore, the medial compartment showed a significant increase in the percent of elevated pixels at each time point following exercise compared to pre-exercise values (p < 0.05). However, a significant change was not observed in the anterior and posterior muscle compartments.
Figure 5.
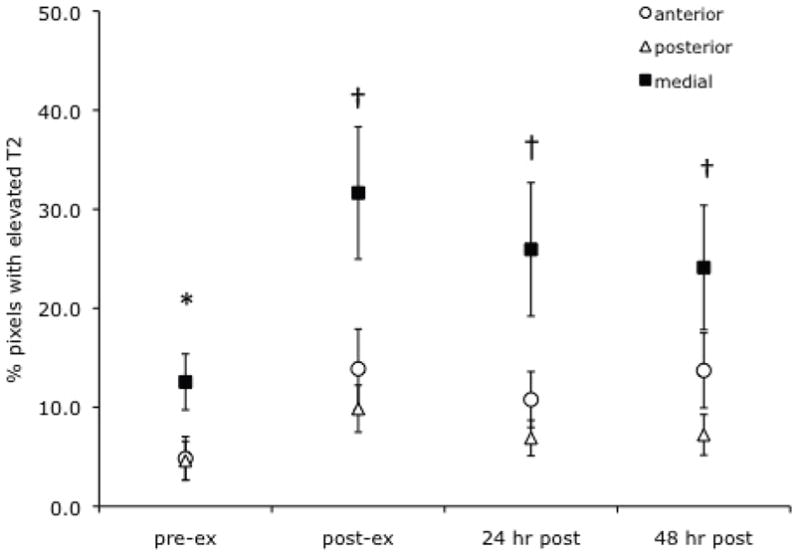
Change in % muscle damage in individual muscle compartments of mdx mice following a bout of downhill running. The medial muscle compartment (flexor hallucis/digitorum longus muscles) had a higher % of damaged muscle at baseline than the anterior and posterior muscle compartments (p < 0.05; denoted by *) and also showed significant increases in % damage from pre-exercise values, immediately post-exercise and at 24 and 48 hours (p < 0.05, denoted by †).
Histological Confirmation of Muscle Damage using Evans Blue Dye
Individual muscles from the lower hindlimb of mdx and control mice were extracted following the 48 hour MRI scan and examined for EBD uptake, as an indicator of muscle damage. The percent of the total muscle cross-sectional area positive for EBD fibers was compared to the percent of pixels with elevated T2 from the corresponding muscle compartment (i.e. tibialis anterior and extensor digitorum longus muscles were compared to the anterior muscle compartment; gastrocnemius and soleus muscles to the posterior muscle compartment and flexor digitorum muscle to the medial muscle compartment). Bivariate correlational analysis demonstrated a strong relationship between the percent of muscle cross-sectional area that was EBD positive and the percent of pixels with elevated T2 (r = 0.79, p < 0.001; see Figure 6).
Figure 6.
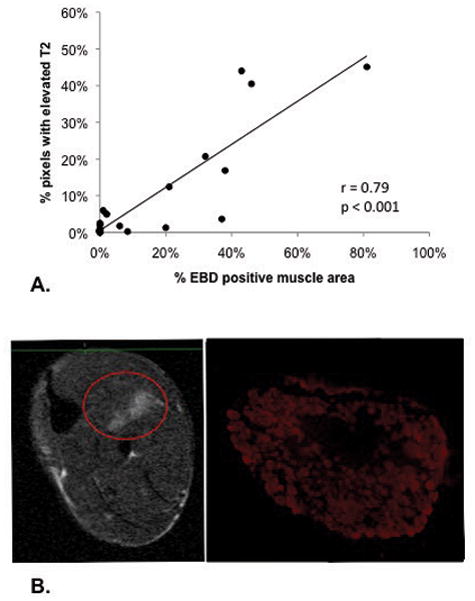
Panel A. Correlation between % of pixels with elevated T2 and % of area showing damaged fibers using Evans Blue Dye (r = 0.79, p < 0.001, n = 23). Panel B. Trans-axial MRI of the lower hindlimb and corresponding histological section of the flexor digitorum longus muscle from the medial compartment showing damaged muscle fibers, positive for Evans Blue Dye.
DISCUSSION
The findings of our study demonstrate that increases in muscle T2 can be visualized using MRI following downhill running in mdx mice. Furthermore, T2 elevation was evident in mdx following downhill running but not control mice after downhill running or mdx mice after horizontal (flat) treadmill running, who did not experience muscle damage. A relationship between histological evidence of muscle damage (Evans Blue Dye positive fibers) and T2 elevation was found. Furthermore, MRI allowed for individual muscle compartments to be examined simultaneously in the same animal. The medial muscle compartment had greater changes in muscle T2 following downhill running in mdx mice and therefore may be more prone to muscle damage than the anterior and posterior muscle compartments.
The effects of two standardized, exercise-based perturbations resulted in different responses in mdx mice. Downhill treadmill running consistently caused muscle T2 elevation above baseline in mdx mice (measured as the percent of pixels with elevated T2), whereas horizontal running resulted in no change in muscle T2 in mdx mice. These physiologically relevant protocols may prove useful in pre-clinical testing of the effects of therapeutic interventions, which are aimed at improving the integrity of dystrophic muscle.42,43 Activity-based outcomes such as treadmill running have been identified as relevant endpoints for translational research as they parallel the outcome measures used in clinical trials. The addition of MRI assessment following a known exercise perturbation would allow for the direct visualization of tissue damage and inflammation.25
Muscle T2 was higher in the mdx mice compared to controls at baseline, and is likely due to regions of muscle degeneration and damage in the dystrophic mice.44,45,38 Elevated muscle T2 has been observed in the pelvic and thigh muscles, particularly the gluteus maximus, of boys with DMD.32 However, the high T2 values in the muscles of boys with DMD were attributed to fatty infiltration, which does not occur in young mdx mice.46 In other animal models of DMD that are characterized by large amounts of fatty tissue replacement similar to the human condition (e.g. golden retriever dog model), changes in muscle T2 have been used to visualize both the corrective43 and the inflammatory42 aspects of therapeutic intervention.
In our study, we found that mdx mice showed an additional increase in the percent of pixels with elevated T2 from baseline values following a bout of downhill running, which remained elevated up to 48 hours. On the other hand, following the same exercise protocol in control mice, the percent of elevated pixels did not show an immediate (20 min post) or prolonged increase. An acute elevation of muscle T2 observed immediately after exercise has been attributed to changes in intra- and extramyocellular water content47,48 and the redistribution of water molecules within muscle49,28 which typically dissipates within one hour post-exercise.29 T2 elevation that lasts longer than 24 hours post-exercise is most likely due to the inflammatory response combined with increased membrane permeability, resulting from the initial muscle damage.34,22 In our study, the control mice did not show an acute increase in muscle T2, which may indicate that their level of exercise was not sufficiently intense to cause a prolonged shift in water compartmentalization.50 This is consistent with previous findings of Kobyashi et al.37 who reported that mdx mice have an accumulation of muscle water content in their hindlimbs following mild treadmill exercise, which is not seen in wild-type mice. Vilquin et al.18 also showed that wild type mice that ran downhill for 5 minutes did not experience muscle damage as measured by an increase in plasma creatine kinase (CK), whereas mdx mice did. However using a more intense protocol, Lynch et al.21 reported histological signs of muscle damage in the soleus and EDL muscles in wild type mice (60 min at 16% decline). Similarly, Carter et al.51 reported a large increase in plasma CK in wild type mice that ran downhill until exhaustion. Therefore, the amount of muscle damage that occurs in controls is likely dependent on the intensity of the running protocol, and this was not achieved with our protocol.
We found that the percent of pixels with elevated T2 48 hours after downhill running in mdx mice was associated with a histological measurement of increased uptake of Evans Blue Dye, which is indicative of increased permeability of the sarcolemma from membrane damage.40 Muscle T2 elevation occurring 48 hours following exercise is likely due to multiple causes, including fiber damage, inflammatory responses, and subsequent edema. Marqueste et al.22 reported that increased plasma markers of muscle damage (creatine kinase and lactate dehydrogenase) were correlated with increased muscle T2, 3 to 17 days after downhill running in rabbits. However the authors also noted an increase in muscle edema, which could also contribute to elevated T2.22 The process of muscle damage is associated with an inflammatory response as well as edema within the muscle,22 both of which can independently result in increased T2.52,33 Future studies using other imaging methods, such as those that enable multiexponential analysis,48 along with histological analysis of inflammation and edema may further elucidate the mechanisms accounting for increases in muscle T2.
In comparison to downhill running, mdx mice that ran on a horizontal grade treadmill only demonstrated an acute increase in the percentage of pixels with an elevated T2, which then returned to baseline levels by 24 hours post-exercise. This indicates that although horizontal running caused significant muscle activation in mdx mice, it did not result in muscle damage to the same extent as downhill running. This result is consistent with the well established finding that downhill running, an eccentrically-biased activity,53 is more damaging than horizontal running, which is predominately concentric. The comparison between horizontal and downhill running in mdx mice is an interesting model with which to compare the effects of dystrophin replacement or other therapeutic interventions, as it provides an option for an exercised “control” that does not cause muscle damage in untreated mdx mice.
Previous studies on downhill running in mice and rats have shown that the tibialis anterior muscle is damaged to a lesser extent than the gastrocnemius and soleus muscles,17,22 which was attributed to the plantarflexor muscles undergoing a longer period of eccentric work than the dorsiflexors during downhill running.53 We found no difference between the anterior and posterior muscle compartments in T2 elevation following downhill running in mdx mice. However muscles of the medial compartment of the lower hindlimb showed a higher muscle T2 at baseline than both the anterior or posterior compartment muscles. There was also an increase in muscle T2 in the medial compartment following downhill running, which was not observed in the other two muscle compartments. These findings imply that the medial compartment muscles may be more susceptible to contraction-induced muscle damage both during regular cage activity (i.e. baseline) and during exercise. The medial compartment muscles consist of the long toe flexors located between the tibia and fibula (flexor digitorum longus and flexor hallucis longus), tibialis posterior and the peroneals. We have not identified other studies that have examined the response of these muscles to downhill running in rodents. It is plausible that one or more of these muscles undergoes greater strain or eccentric activity during downhill running than the others, resulting in greater muscle damage and T2 elevation. Furthermore, these muscles are primarily composed of fast-twitch fibers in rodents54 which also increases their susceptibility to muscle damage. The functional role of these muscles during downhill running and their susceptibility to muscle damage in mdx mice warrants further investigation.
We utilized two measures from T2-weighted imaging to examine changes in signal intensity after exercise: mean T2 relaxation time (ms) from a region of interest and the percentage of pixels that exceeded a pre-defined threshold. We found that the threshold method used in this study (% of pixels between 30 and 100 ms) was more sensitive for detecting differences in T2 between mdx and control mice after downhill running than the mean T2 of the ROI. Threshold methods for pixel intensity from MRI have previously been used to examine dystrophic muscle38 and muscle activation following exercise.55 This method differs from the calculation of the mean T2 from a specified region, as it identifies the proportion of pixels that exceed a given threshold. In our study the threshold was set two standard deviations above the mean T2 of uninjured control muscle, therefore it was used to identify muscle which had a particularly high signal intensity on T2-weighted imaging.
About 60% of mdx mice in our study were able to complete the downhill running protocol with encouragement, and 100% of control mice were able to run downhill and did not require any external encouragement. Previous reports have indicated that mdx mice have a greater fatigue response to treadmill or free wheel running than wild type mice.56,37. Vilquin et al.18 reported that mdx mice aged 9 to14 months were unable to run for more than 5 minutes without external stimulation on a more intense downhill running protocol than used in our study (−16 degree decline at 10m/min). They also found that although there were a few “natural runners” in their group (5 out of 15), two of the mice died one hour post-exercise. We did not have any fatalities from running in our study, although the protocol was much longer (45 minutes) and the mice were of a similar age. We also found that all of the mdx mice were able to complete the horizontal running protocol, even though downhill running is less energy consuming than horizontal or uphill activity. Therefore, this difference may not have only to do with muscle fatigue but also a protective response to muscle damage. The tolerance of mdx mice to downhill running has important implications when planning studies to examine the response to intervention. Depending on the age of the animal and the intensity of the protocol a proportion of mice may not be able to complete the protocol, therefore a “drop-out” rate must be established and accounted for in sample size estimations.
The limitations of this study must also be considered when interpreting the results. First, we did not include a group of control mice in the horizontal running protocol to compare with the mdx group. However, since there was no prolonged T2 elevation observed in the mdx mice following horizontal running, the comparison to controls may not have provided any additional information regarding susceptibility to muscle damage. Second, the T2 measurements were made using single exponential analysis from two echo times, which has previously been employed by our group34,52 and others.55 Although the experiment may have benefitted from multi-exponential analysis in order to further differentiate muscle damage from inflammation, it is challenging to obtain images at multiple TEs during in-vivo experiments without the contribution of stimulated echoes. Therefore this approach was beyond the scope of this study. Finally, the correlation between Evans Blue Dye positive fibers and percent of pixels with elevated T2 is limited by the fact that perfect registration between the histological cross-section and trans-axial MR image was not obtained, although the mid-belly of the muscle was sampled for both measures. However, we observed macroscopically that bundles of damaged muscle fibers, positive for EBD, ran continuously along the entire length of a muscle and were therefore captured on multiple cross-sections. This has also been shown using longitudinal muscle sections of EBD positive fibers in mdx mice by Straub et al.3 Similarly, areas of elevated muscle T2 were observed in the same spatial location on multiple trans-axial images of the muscle. Although the cross-sections were not exactly registered, the correlation between histology and MRI findings of muscle damage based on using the mid-belly region of the muscle seems acceptable based on the distribution of damaged fibers along the entire length of a muscle.
Conclusion
The protocol for downhill running used in this study provides a stimulus to induce muscle damage in the lower hindlimbs of mdx mice, which can be noninvasively visualized using T2-weighted MRI. Using MRI to examine T2 changes following exercise allows for repeated and longitudinal measurements to be made from multiple muscles in a single animal. The ability to examine several muscles simultaneously is particularly important, since not all muscles respond in the same way to downhill running. Therefore MRI may be used to complement histological studies by determining which muscles experience the most damage and should be examined further. An important aspect of the downhill running protocol used in this study was its ability to differentiate mdx mice who experience muscle T2 changes from downhill run wild type mice and mdx mice run on a horizontal grade treadmill, who do not experience elevated T2 after running. In addition, this protocol allowed for the examination of acute muscle damage following a known and timed event. This can be often be difficult in dystrophic mice due to the background presence of asynchronous bouts muscle damage and recovery.
Acknowledgments
Muscular Dystrophy Association, National High Field Magnet Lab (NHFML), National Institutes of Health (R01HL78670, U54AR052646). The authors also thank Wendy Wang and Fan Ye for assistance with muscle histology.
ABBREVIATIONS
- CK
creatine kinase
- DMD
Duchenne muscular dystrophy
- EBD
Evans Blue Dye
- EDL
extensor digitorum longus
- FDL
flexor digitorum longus
- MRI
magnetic resonance imaging
- T2
muscle transverse relaxation time
- TA
tibialis anterior
- TE
echo time
Footnotes
The data have been presented in part at the Canadian Society for Exercise Physiology Meeting (Nov 2009, Vancouver BC) and the Combined Sections Meeting of the American Physical Therapy Association (Feb 2010, San Diego CA)
References
- 1.Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–1122. [PubMed] [Google Scholar]
- 2.Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Straub V, Campbell KP. Muscular dystrophies and the dystrophin-glycoprotein complex. Curr Opin Neurol. 1997;10:168–175. doi: 10.1097/00019052-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81:S52–69. doi: 10.1097/00002060-200211001-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lieber RL, Friden J. Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil. 2002;81:S70–79. doi: 10.1097/00002060-200211001-00008. [DOI] [PubMed] [Google Scholar]
- 7.Proske U, Allen TJ. Damage to skeletal muscle from eccentric exercise. Exerc Sport Sci Rev. 2005;33:98–104. doi: 10.1097/00003677-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Peake JM, Suzuki K, Wilson G, Hordern M, Nosaka K, Mackinnon L, Coombes JS. Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Med Sci Sports Exerc. 2005;37:737–745. doi: 10.1249/01.mss.0000161804.05399.3b. [DOI] [PubMed] [Google Scholar]
- 9.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol. 2005;288:R345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 10.Consolino CM, Brooks SV. Susceptibility to sarcomere injury induced by single stretches of maximally activated muscles of mdx mice. J Appl Physiol. 2004;96:633–638. doi: 10.1152/japplphysiol.00587.2003. [DOI] [PubMed] [Google Scholar]
- 11.Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol. 2000;279:C1290–1294. doi: 10.1152/ajpcell.2000.279.4.C1290. [DOI] [PubMed] [Google Scholar]
- 12.Petrof B, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord. 2009;19:241–249. doi: 10.1016/j.nmd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol. 2004;286:C230–238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovering RM, Hakim M, Moorman CT, 3rd, De Deyne PG. The contribution of contractile pre-activation to loss of function after a single lengthening contraction. J Biomech. 2005;38:1501–1507. doi: 10.1016/j.jbiomech.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brussee V, Merly F, Tardif F, Tremblay JP. Normal myoblast implantation in MDX mice prevents muscle damage by exercise. Biochemical Biophys Res Commun. 1998;250:321–327. doi: 10.1006/bbrc.1998.9276. [DOI] [PubMed] [Google Scholar]
- 17.Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 18.Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve. 1998;21:567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104:347–353. doi: 10.1152/japplphysiol.00128.2007. [DOI] [PubMed] [Google Scholar]
- 21.Lynch GS, Fary CJ, Williams DA. Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium. 1997;22:373–383. doi: 10.1016/s0143-4160(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 22.Marqueste T, Giannesini B, Fur YL, Cozzone PJ, Bendahan D. Comparative MRI analysis of T2 changes associated with single and repeated bouts of downhill running leading to eccentric-induced muscle damage. J Appl Physiol. 2008;105:299–307. doi: 10.1152/japplphysiol.00738.2007. [DOI] [PubMed] [Google Scholar]
- 23.Kingsley MI, Kilduff LP, McEneny J, Dietzig RE, Benton D. Phosphatidylserine supplementation and recovery following downhill running. Med Sci Sports Exerc. 2006;38:1617–1625. doi: 10.1249/01.mss.0000229459.11452.a0. [DOI] [PubMed] [Google Scholar]
- 24.Granchelli JA, Pollina C, Hudecki MS. Pre-clinical screening of drugs using the mdx mouse. Neuromuscul Disord. 2000;10:235–239. doi: 10.1016/s0960-8966(99)00126-1. [DOI] [PubMed] [Google Scholar]
- 25.Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, Van Der Meulen JH, Cha HJ, Pistilli EE, Partridge TA, Hoffman EP, Nagaraju K. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39:591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bickel CS, Slade JM, Dudley GA. Long-term spinal cord injury increases susceptibility to isometric contraction-induced muscle injury. Eur J Appl Physiol. 2004;91:308–313. doi: 10.1007/s00421-003-0973-5. [DOI] [PubMed] [Google Scholar]
- 27.Larsen RG, Ringgaard S, Overgaard K. Localization and quantification of muscle damage by magnetic resonance imaging following step exercise in young women. Scand J Med Sci Sports. 2007;17:76–83. doi: 10.1111/j.1600-0838.2006.00525.x. [DOI] [PubMed] [Google Scholar]
- 28.Meyer RA, Prior BM. Functional magnetic resonance imaging of muscle. Exerc Sport Sci Rev. 2000;28:89–92. [PubMed] [Google Scholar]
- 29.Foley JM, Jayaraman RC, Prior BM, Pivarnik JM, Meyer RA. MR measurements of muscle damage and adaptation after eccentric exercise. J Appl Physiol. 1999;87:2311–2318. doi: 10.1152/jappl.1999.87.6.2311. [DOI] [PubMed] [Google Scholar]
- 30.Shah PK, Gregory CM, Stevens JE, Pathare NC, Jayaraman A, Behrman AL, Walter GA, Vandenborne K. Non-invasive assessment of lower extremity muscle composition after incomplete spinal cord injury. Spinal Cord. 2008;46:565–570. doi: 10.1038/sc.2008.10. [DOI] [PubMed] [Google Scholar]
- 31.Ploutz-Snyder LL, Clark BC, Logan L, Turk M. Evaluation of spastic muscle in stroke survivors using magnetic resonance imaging and resistance to passive motion. Arch Phys Med Rehabil. 2006;87:1636–1642. doi: 10.1016/j.apmr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Kim HK, Laor T, Horn PS, Racadio JM, Wong B, Dardzinski BJ. T2 mapping in Duchenne muscular dystrophy: distribution of disease activity and correlation with clinical assessments. Radiology. 2010;255:899–908. doi: 10.1148/radiol.10091547. [DOI] [PubMed] [Google Scholar]
- 33.Maillard SM, Jones R, Owens C, Pilkington C, Woo P, Wedderburn LR, Murray KJ. Quantitative assessment of MRI T2 relaxation time of thigh muscles in juvenile dermatomyositis. Rheumatology (Oxford) 2004;43:603–608. doi: 10.1093/rheumatology/keh130. [DOI] [PubMed] [Google Scholar]
- 34.Frimel TN, Walter GA, Gibbs JD, Gaidosh GS, Vandenborne K. Noninvasive monitoring of muscle damage during reloading following limb disuse. Muscle Nerve. 2005;32:605–612. doi: 10.1002/mus.20398. [DOI] [PubMed] [Google Scholar]
- 35.Ceelen KK, Stekelenburg A, Mulders JL, Strijkers GJ, Baaijens FP, Nicolay K, Oomens CW. Validation of a numerical model of skeletal muscle compression with MR tagging: a contribution to pressure ulcer research. J Biomech Eng. 2008;130:061015. doi: 10.1115/1.2987877. [DOI] [PubMed] [Google Scholar]
- 36.Dunn JF, Zaim-Wadghiri Y. Quantitative magnetic resonance imaging of the mdx mouse model of Duchenne muscular dystrophy. Muscle Nerve. 1999;22:1367–1371. doi: 10.1002/(sici)1097-4598(199910)22:10<1367::aid-mus5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter G, Cordier L, Bloy D, Sweeney HL. Noninvasive monitoring of gene correction in dystrophic muscle. Magn Reson Med. 2005;54:1369–1376. doi: 10.1002/mrm.20721. [DOI] [PubMed] [Google Scholar]
- 39.Prior BM, Ploutz-Snyder LL, Cooper TG, Meyer RA. Fiber type and metabolic dependence of T2 increases in stimulated rat muscles. J Appl Physiol. 2001;90:615–623. doi: 10.1152/jappl.2001.90.2.615. [DOI] [PubMed] [Google Scholar]
- 40.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. Journal Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 42.Kornegay JN, Li J, Bogan JR, Bogan DJ, Chen C, Zheng H, Wang B, Qiao C, Howard JF, Jr, Xiao X. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S, Hoffman E. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIntosh L, Granberg KE, Briere KM, Anderson JE. Nuclear magnetic resonance spectroscopy study of muscle growth, mdx dystrophy and glucocorticoid treatments: correlation with repair. NMR Biomed. 1998;11:1–10. doi: 10.1002/(sici)1099-1492(199802)11:1<1::aid-nbm493>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 45.Pacak CA, Walter GA, Gaidosh G, Bryant N, Lewis MA, Germain S, Mah CS, Campbell KP, Byrne BJ. Long-term skeletal muscle protection after gene transfer in a mouse model of LGMD-2D. Mol Ther. 2007;15:1775–1781. doi: 10.1038/sj.mt.6300246. [DOI] [PubMed] [Google Scholar]
- 46.Grounds MD, Radley HG, Lynch GS, Nagaraju K, De Luca A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol Dis. 2008;31:1–19. doi: 10.1016/j.nbd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleckenstein JL, Canby RC, Parkey RW, Peshock RM. Acute effects of exercise on MR imaging of skeletal muscle in normal volunteers. AJR Am J Roentgenol. 1988;151:231–237. doi: 10.2214/ajr.151.2.231. [DOI] [PubMed] [Google Scholar]
- 48.Le Rumeur E, De Certaines J, Toulouse P, Rochcongar P. Water phases in rat striated muscles as determined by T2 proton NMR relaxation times. Magn Reson Imaging. 1987;5:267–272. doi: 10.1016/0730-725x(87)90003-8. [DOI] [PubMed] [Google Scholar]
- 49.Conley MS, Foley JM, Ploutz-Snyder LL, Meyer RA, Dudley GA. Effect of acute head-down tilt on skeletal muscle cross-sectional area and proton transverse relaxation time. J Appl Physiol. 1996;81:1572–1577. doi: 10.1152/jappl.1996.81.4.1572. [DOI] [PubMed] [Google Scholar]
- 50.Kinugasa R, Kawakami Y, Fukunaga T. Quantitative assessment of skeletal muscle activation using muscle functional MRI. Magn Reson Imaging. 2006;24:639–644. doi: 10.1016/j.mri.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Carter GT, Kikuchi N, Abresch RT, Walsh SA, Horasek SJ, Fowler WM., Jr Effects of exhaustive concentric and eccentric exercise on murine skeletal muscle. Arch Phys Med Rehabil. 1994;75:555–559. [PubMed] [Google Scholar]
- 52.Liu M, Bose P, Walter GA, Anderson DK, Thompson FJ, Vandenborne K. Changes in muscle T2 relaxation properties following spinal cord injury and locomotor training. Eur J Appl Physiol. 2006;97:355–361. doi: 10.1007/s00421-006-0199-4. [DOI] [PubMed] [Google Scholar]
- 53.Eston RG, Mickleborough J, Baltzopoulos V. Eccentric activation and muscle damage: biomechanical and physiological considerations during downhill running. Br J Sports Med. 1995;29:89–94. doi: 10.1136/bjsm.29.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 55.Prior BM, Foley JM, Jayaraman RC, Meyer RA. Pixel T2 distribution in functional magnetic resonance images of muscle. J Appl Physiol. 1999;87:2107–2114. doi: 10.1152/jappl.1999.87.6.2107. [DOI] [PubMed] [Google Scholar]
- 56.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]