Abstract
With intensified treatment leading to longer survival, complications of therapy for brain tumours are more frequently observed. Regarding radiation therapy, progressive and irreversible white matter disease with cognitive decline is most feared. We report on four patients with reversible clinical and radiological features occurring years after radiation for brain tumours, suggestive for the so called SMART syndrome (stroke-like migraine attacks after radiation therapy). All four patients (males, age 36–60 years) had been treated with focal brain radiation for a primary brain tumour or with whole-brain radiation therapy for brain metastases. Ranging from 2 to 10 years following radiation therapy patients presented with headache and focal neurological deficits, suggestive for tumour recurrence. Two patients also presented with focal seizures. MRI demonstrated typical cortical swelling and contrast enhancement, primarily in the parieto-occipital region. On follow-up both clinical and MRI features improved spontaneously. Three patients eventually proved to have tumour recurrence. The clinical and radiological picture of these patients is compatible with the SMART syndrome, a rare complication of radiation therapy which is probably under recognized in brain tumour patients. The pathophysiology of the SMART syndrome is poorly understood but bears similarities with the posterior reversible encephalopathy syndrome (PRES). These four cases underline that the SMART syndrome should be considered in patients formerly treated with radiation therapy for brain tumours, who present with new neurologic deficits. Before the diagnosis of SMART syndrome can be established other causes, such as local tumour recurrence, leptomeningeal disease or ischemic disease should be ruled out.
Keywords: Reversible late effects, Radiation therapy, Brain tumours
Introduction
With improved treatments for brain tumours leading to longer survival, late complications of therapy are more frequently observed. The development of new neurological symptoms following brain tumour treatment is primarily suggestive for tumour recurrence, but when this has been ruled out, adverse treatment effects as a cause of the symptoms should be considered. Amongst late complications of radiation therapy, progressive leukoencephalopathy with cognitive decline and focal radiation necrosis mimicking recurrent tumour growth are the most worrisome [1].
We report on four patients with reversible clinical and radiological features occurring years after radiation for brain tumours, suggestive for the so-called SMART syndrome: stroke-like migraine attacks after radiation therapy [2–4].
Case reports
Patient 1
In 1999 at age 32 years, this man had progressive headache and diplopia due to an obstructive hydrocephalus caused by a pineal tumour. Following resection a pineoloma with malignant histological features was diagnosed. MRI of the craniospinal axis and CSF examination demonstrated no other tumour localizations. He was subsequently treated with craniospinal irradiation (36 Gy in 20 fractions with a boost of 18 Gy to the original tumour site). Following a minor head trauma 4 years after RT he developed increasing headache over the following days and a left hemianopia. No tumour recurrence was observed on MRI, nor in the cerebrospinal fluid (CSF) (Table 1). Additional imaging ruled out ischemic stroke, vertebral artery dissection and venous sinus thrombosis. However, on MRI, gyral thickening of the right parieto-occipital cortex with contrast enhancement was seen (Fig. 1a, b). During hospitalisation he developed several generalized seizures with persistent post-ictal weakness of the left arm and confusion, lasting for several weeks. He was treated with valproate and levetiracetam. EEG showed slowing over the right hemisphere but no epileptic activity. The observed MRI abnormalities recovered over the weeks thereafter, with concomitant clinical improvement (Fig. 1c, d).
Table 1.
Clinical details of reported cases with the SMART syndrome
| Age (years) | Tumour | CSF studies | MRI radiation Toxicity | Interval (years) | Symptoms | Original tumour site | Radiation type and dose |
|---|---|---|---|---|---|---|---|
| Patient 1 male, 36 | Pinealoma | Protein 0.33 leukocytes 1 | None | 4 | Headache neurologic deficit seizures | Pineal region | Craniospinal axis 36 Gy (20 × 1.8) tumour booster 18 Gy (10 × 1.8) |
| Patient 2 male, 60 | Brain metastases from small-cell lung cancer | Protein 0.81 leukocytes 1 | Diffuse white matter changes | 10 | Headache neurologic deficit seizures | Both hemispheres | WBRT 30 Gy (10 × 3) |
| Patient 3 male, 42 | Oligodendroglioma | Protein 0.43 leukocytes 1 | Deep white matter disease | 2 and 5 | Headache neurologic deficit seizures | Left parietal lobe | Focal 60 Gy (30 × 2) |
| Patient 4 male, 39 | Astrocytoma | Protein 0.71 leukocytes 2 | None | 5 and 10 | Neurologic deficit seizures | Right temporal lobe | Focal 50 Gy (20 × 2.5) |
Protein level in g/l
Interval, years between irradiation and the first symptoms; Gy, radiation dose in Grays; X, number of radiation fractions; WBRT, whole-brain radiation therapy; Focal, focal radiation on the original tumour site
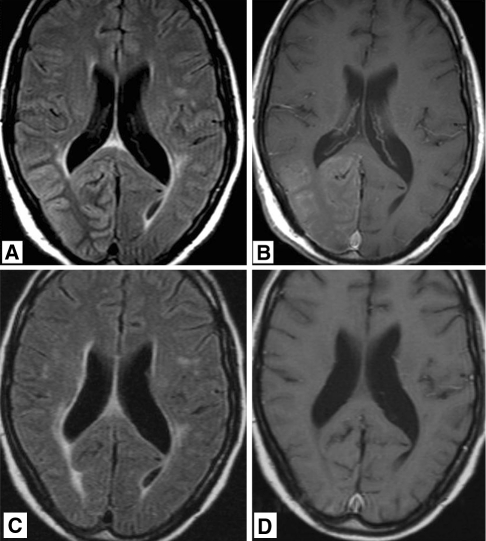
Fig. 1.
36-year-old man who was treated 4 years previously for pineal tumor with radiation therapy, now presenting with headache and left hemianopia. a T2 weighted, fluid attenuated inversion recovery (FLAIR) MR image showing diffuse gyral swelling and signal increase in the right occipital lobe. b T1 weighted MR image after gadolinium administration showing diffuse gyral and leptomeningeal enhancement. MR images obtained 9 month later showing non residual signal abnormalities on FLAIR (c) and no abnormal enhancement (d)
Two years later he developed a similar clinical episode for which he was admitted once more, this time without seizures. The same imaging abnormalities were observed which resolved once again over the following months without any treatment. Again, no tumour recurrence could be demonstrated.
However, in 2008, 8 years after the initial presentation he did develop spinal leptomeningeal metastases, for which he was treated with focal RT and systemic chemotherapy.
Patient 2
In 1997 at age 50 years, this man was treated for small cell lung cancer and two brain metastases with systemic chemotherapy and whole-brain radiation (30 Gy in 10 fractions). Ten years later he developed slowly progressive headache, and a few weeks later he woke up with an expressive dysphasia. During hospitalization he developed a right sided hemiparesis with right hemianopia and he suffered from frequent focal epileptic seizures of the right arm and leg, for which he received valproate and levetiracetam. MRI showed focal gyral thickening and enhancement of the left parieto-occipital lobe where metastases had not been observed previously (Fig. 2a–d). Apart from diffuse leukoencephalopathy, presumably due to the earlier brain radiotherapy, there were no signs of recurrent brain metastases. Repeated CSF analysis showed a slightly elevated protein level, but no leptomeningeal metastases (Table 1). There were no clues for extracranial tumour recurrence. During the hospital stay he had a gradual clinical improvement and was discharged fully recovered. A follow-up MRI 3 months after discharge also showed complete resolution of the abnormalities in the left parieto-occipital cortex (Fig. 2e, f).
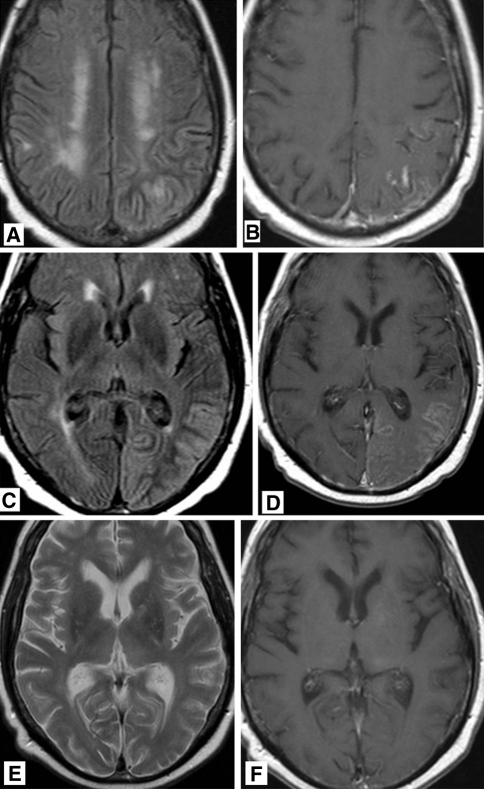
Fig. 2.
60-year-old man who was treated 10 years previously for brain metastases of bronchial carcinoma with chemo- and radiation therapy, now presenting with gradually progressive headache and acute dysphasis. a T2 weighted MR image showing diffuse gyral swelling and signal increase in the left parietal lobe. b T1 weighted MR image after gadolinium administration showing abnormal regional leptomeningeal enhancement. c T2 weighted FLAIR MR image showing diffuse gyral swelling and signal increase in the left occipital lobe. d T1 weighted MR image after gadolinium administration showing abnormal leptomeningeal enhancement. MR images after 4 months. e T2 weighted MR image no residual signal abnormalities. f T1 weighted MR image after gadolinium administration showing no abnormal leptomeningeal enhancement
Patient 3
In 1997 at the age of 42 years, this man suffered from an epileptic seizure caused by an anaplastic oligodendroglioma in the left hemisphere. The tumour was resected and he subsequently received focal brain radiation (60 Gy in 30 fractions). He was discharged from hospital on valproate. Two years later he was admitted to the hospital twice within a short time period with reversible neurological deficits: dysphasia, right hemianopia, headache and neglect of his right arm. Imaging showed no signs of recurrent tumour, but possibly a small infarction in the left occipital region and some diffuse white matter disease, possibly due to radiation. An EEG showed no epileptic activity. Diagnosis of reversible ischemic neurological deficit was hypothesized, possibly due to former radiotherapy.
Three years thereafter he was again taken into hospital with focal epileptic seizures of the right side of his face. He suffered from headache, progressive aphasia, right hemianopia and mild paresis of his right arm and leg. An MRI scan now showed diffuse cortical enhancement of the left occipital and temporal cortex (Fig. 3a, b). This enhancement disappeared on a subsequent MRI performed 8 days later (Fig. 3c, d). An EEG showed no epileptic activity. Clinically, he recovered substantially.
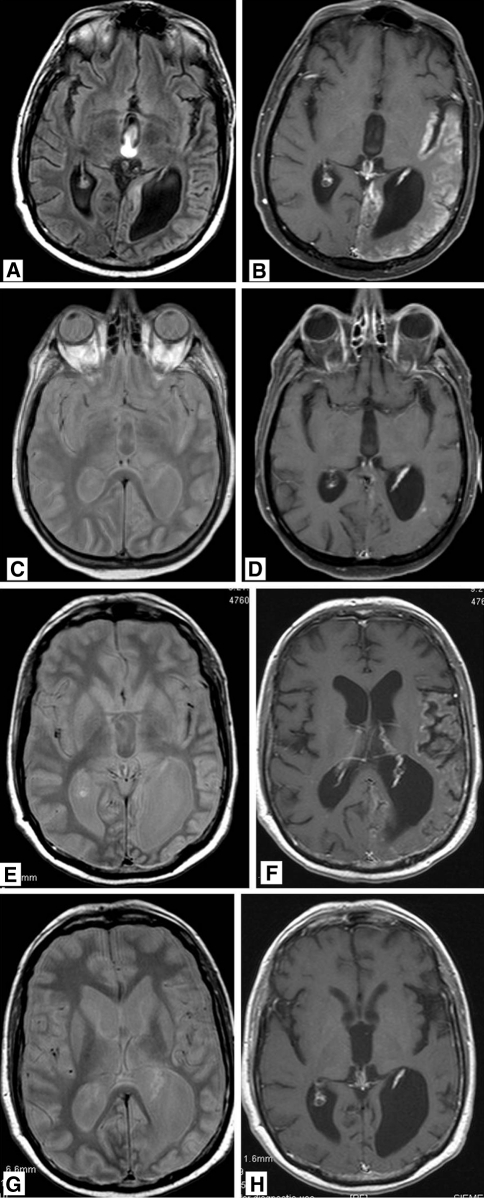
Fig. 3.
47-year-old man who was treated 5 years previously for a left parietal oligodendrolioma with chemo- and radiation therapy, now presenting with right hemianopia and mild paresis of his right arm and leg. T2 weighted FLAIR MR images a showing gyral swelling and signal increase in the temporal lobe. b After gadolinium administration, diffuse gyral enhancement is seen in the occipital, temporal and insular lobe. Proton density (c) and T1 post gadolinium (d) MR imaging 8 days later showing normalization of signal intensity in the temporal lobe and marked reduction in abnormal gyral enhancement. After 3 years, the patient presented again with right sided paresis. MR imaging again shows signal increase in the temporal lobe on PD weighted images (e) while on T1 post gadolinium imaging (f) abnormal gyral enhancement is again visible. Like after the previous episode, the MR abnormalities disappeared (g, h)
Three years later he was again admitted to hospital because of a new episode of worsening aphasia and paresis of his right arm and leg.
MR imaging, similar to 3 years earlier, demonstrated diffuse cortical enhancement of the left occipital and temporal cortex (Fig. 3e, f). Although CSF examination showed some atypical cells, he again partially recovered spontaneously and almost completely, making leptomeningeal seeding unlikely (Table 1). A follow-up MRI scan performed 1 month later again showed complete disappearance of the contrast enhancement (Fig. 3g, h), and he recovered afterwards in a rehabilitation centre. On longer follow-up, he was treated successfully with temozolomide chemotherapy for local tumour recurrence.
Patient 4
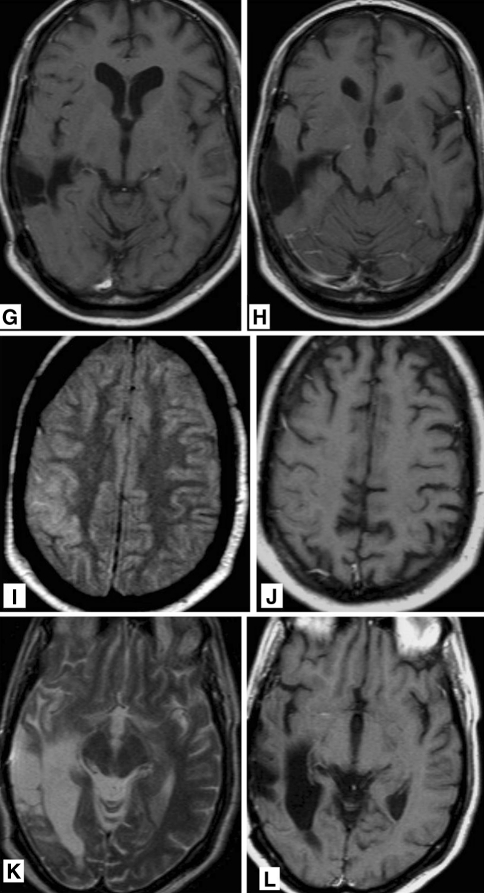
In 1999 at the age of 34 years, this man suffered from an epileptic seizure caused by a right temporal anaplastic astrocytoma. The tumour was resected and he subsequently had focal brain radiation (50 Gy in 20 fractions). In the following 5 years he had infrequent focal seizures of the left arm but no tumour recurrence was observed on repeated MRI. Five years after initial treatment, however, he developed a progressive clumsy left hand, left-sided facial paresis and dysarthria in combination with an increase of focal seizures. An EEG showed nearly continuous epileptic discharges diffusely over the right hemisphere. He was treated with intravenous clonazepam and valproate. The MRI demonstrated no tumour recurrence but a gyriform enhancement and swelling of the right parieto-temporal cortex (Fig. 4a–f).
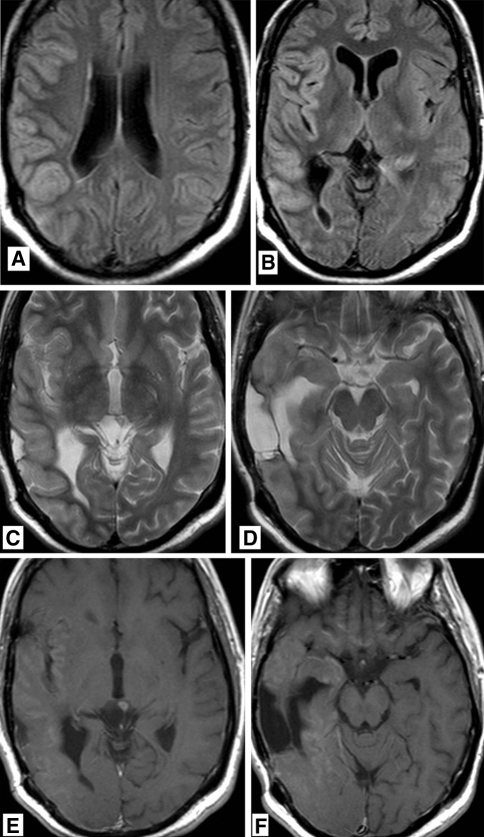
Fig. 4.
38-year-old man who was treated 5 years previously for a right temporal anaplastic astrocytoma with chemo- and radiation therapy, now presenting with increasing epileptic activity and gradually progressive left sided hemiparesis and dysarthria. T2 weighted FLAIR MR images (a, b) showing gyral swelling and signal increase in the temporal lobe. T2 weighted MR image (c, d) showing gyral swelling and signal increase in the temporal lobe. A post-operative parenchymal defect is seen in the area of the previously treated astrocytoma. T1 weighted MR images after gadolinium administration (e, f) abnormal gyral and leptomeningeal enhancement in the temporal lobe and in the basal frontal areas. No focal enhancement in post-operative parenchymal defect. MR images 3 months later. g, h T1 weighted MR image after gadolinium administration showing persistent gyral abnormalities but disappearance of abnormal enhancement. MR images 6 months after treatment for recurrent anaplastic astrocytoma using chemotherapy. i T2 weighted FLAIR MR image and j T1 weighted MR image after gadolinium administration showing persistent gyral abnormalities but disappearance of abnormal enhancement. MR images 6 months after treatment for recurrent anaplastic astrocytoma using chemotherapy. k T2 weighted MR image and l T1 weighted MR image after gadolinium administration resolution of T2 abnormalities and disappearance of abnormal enhancement in the deep temporal area
CSF examination was, besides a slightly elevated protein level, completely normal (Table 1). Over the following weeks the focal seizures disappeared and the left sided weakness improved. The patient was discharged from hospital on valproate and nearly complete resolution of the clinical picture. The abnormalities on MRI did partially resolve (Fig. 4g, h). Three months later, a new MRI was suggestive for recurrent tumour in the right temporal lobe, showing several nodular enhancements. A biopsy confirmed recurrent high-grade glioma, for which he was treated with temozolomide. Following chemotherapy treatment, the abnormal gyral and leptomeningeal enhancement temporarily disappeared, but recurred for which PCV chemotherapy was started. Again, a complete response ensued. Ten years after the initial diagnosis of anaplastic astrocytoma he developed left hemianopia and a clumsy left hand, but had no seizures. On MRI a right occipital contrast enhancing tumour was found, suggestive for recurrent tumour. Resection was planned, but pre-operative imaging showed the lesion to be smaller and an open biopsy did not confirm active tumour growth. Postoperatively he suffered from very frequent focal seizures of the left side of his face and a clumsy left hand, which did not subside until he was put into a propofol induced coma. He completely recovered within several weeks. Follow-up MRI demonstrated the occipital contrast-enhancing to have disappeared completely (Fig. 4i–l). The patient died a year later from tumour recurrence, 11 years after the initial diagnosis.
Discussion
All four patients had (partly) reversible neurological symptoms as well as reversible imaging findings several years after radiation therapy for their brain tumours. The combination of headaches in three of the four patients, as well as development of new neurological deficit and seizures in all four patients was clinically suggestive for tumour recurrence. However, neither local tumour recurrence, nor leptomeningeal disease were found to be the cause of the clinical deterioration.
The radiological differential diagnosis of diffuse gyral signal increase and leptomeningeal enhancement as observed in these cases also includes vascular disorders such as ischemia, venous sinus thrombosis and dural arteriovenous fistula, and infection [6, 7]. Using CSF examination and additional radiological techniques such as diffusion and perfusion weighted MRI and digital subtraction angiography, these potentially treatable causes could be ruled out. Also post-ictal imaging changes (swelling and enhancement) and posterior reversible encephalopathy syndrome (PRES) as observed in patients with hypertensive encephalopathy or following the use of immunosuppressive agents should be considered [8, 9].
The episodic nature of the deficits with spontaneous resolution over weeks in combination with unilateral enhancement and thickening of the posterior cortex on MRI might suggest focal epileptic activity with long-lasting post-ictal deficit as the cause of the clinical symptoms. Yaffe and Ferreiro described eight patients who had all been treated with systemic chemotherapy with comparable reversible MRI changes following seizures. The majority of the lesions involved grey and white matter, predominantly in the posterior vascular boundary zones [10]. This phenomena was attributed to damage to the blood–brain barrier by systemic chemotherapy in combination with seizures. In our patients, however, damage to the parieto-occipital blood brain barrier was presumably due to radiation therapy. Irradiation affects primarily the vasculature or the trigeminovascular system and engender a reversible disturbance of homeostasis. Most previously presented cases [2–4] had been treated with posterior fossa irradiation, and it is presumed that the posterior lobes are specific vulnerable for radiation damage. Regarding epileptic seizures as the underlying mechanism, in two of our patients the seizures occurred either before the development of MRI abnormalities or after the MRI abnormalities had subsided. Also, repeated EEG in three of four patients did not show epileptic activity during the symptoms. Additionally, post-ictal MRI abnormalities in patients without previous radiation therapy or chemotherapy are not confined to the parieto-occipital region [8]. We therefore argue that the MRI findings as observed in our patients cannot be explained by post-ictal enhancement alone. Reversible white matter enhancement, but not cortical enhancement has been described in patients with migraine [11, 12]. The underlying mechanisms of these transient imaging abnormalities in migraine include meningeal and parenchymal hyperperfusion, edema or inflammatory plasma protein extravasation after disruption of the blood brain barrier. Besides, the prolonged duration of the symptoms in our patients, who had no history of migraine headaches, are unlikely due to migraine.
Otherwise, the clinical and radiological picture of these patients is compatible with the SMART syndrome. SMART syndrome involves transient, reversible neurological dysfunction which may include migrainous headache, at times preceded by aura, prolonged hemispheric neurological impairment and sometimes seizure activity [2, 3]. Neuroimaging studies of patients with SMART syndrome typically show focal gyral thickening of the affected cortex and gyriform contrast enhancement. Before SMART was defined as a distinct syndrome, similar patients with sort-like reversible MRI changes and clinical symptoms after radiation were reported [5, 13–15].
The pathophysiology of the SMART syndrome is poorly understood. A specific vulnerability of the parieto-occipital cortex for radiation or chemotherapy, similar to that observed in PRES, may explain why the imaging findings are preferentially observed in this region [1]. Patient 2 had been treated with whole-brain radiation and developed parieto-occipital abnormalities in the left hemisphere, not related to the original site of the brain metastases, which supports this concept. The clinical presentation of PRES has similarities with SMART, including headaches, neurological deficits and, more frequently than in SMART, seizures. The pathophysiology of PRES is combined vasoconstriction and vasodilation and has the same patterns as seen in vasculopathy [9]. This results in blood brain barrier disruption leading to symmetric hemispheric edema and contrast enhancement on MRI [9]. Since radiation may preferentially damage endothelial cells, the SMART syndrome might be a reversible radiation vasculopathy comparable with PRES. An alternative hypothesis is that post-radiation neuronal dysfunction is the underlying mechanism, such as in migraine or epilepsy, with impairment of the trigeminovascular system or a lowered threshold for cortical spreading depression [21].The parieto-occipital cortical damage might even be the cause of epileptic seizures rather than being the result of seizures [13].
Development of the SMART syndrome has been related to a radiation dose of at least 50 Gy, as described in most of the previous cases [3–5]. In our cases, a dose exceeding 50 Gy had only be applied to patients 1 and 3. In the other two patients, however, the daily fraction doses were relatively high (3 Gy in patient 2 and 2.5 Gy in patient 4, respectively), which may have attributed to the radiation toxicity.
Late effects of radiotherapy have mainly been blamed on vascular endothelial injury and demyelination, and include diffuse white matter changes or focal necrosis with mass effect [16–18]. In contrast with the SMART syndrome these late effects of radiation therapy are gradual in onset and may have a progressive nature [19, 20].
If these reversible, stroke-like migraine attacks are related to remote radiation therapy, this relationship may be under recognized so far because many patients did not live long enough to experience these late treatment effects.
Acknowledgments
Conflict of interest
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Kim JH, Brown SL. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 2.Lachance DH, Black DF (2005) SMART: strokelike migraine attacks after radiation therapy. Neurology (Suppl 1) A220
- 3.Pruitt A, Dalmau J, Detre J. Episodic neurologic dysfunction with migraine and reversible imaging findings after radiation. Neurology. 2006;67:676–678. doi: 10.1212/01.wnl.0000228862.76269.62. [DOI] [PubMed] [Google Scholar]
- 4.Black DF, Bartleson JD, Bell ML, Lachance DH. Smart: stroke-like migraine attacks after radiation therapy. Cephalagia. 2006;26:1137–1142. doi: 10.1111/j.1468-2982.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartleson JD, Krecke KN. Reversible, stroke like migraine attacks in patients with previous radiation therapy. Neuro-oncology. 2003;5:121–127. doi: 10.1215/S1522-8517-02-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon BJ, Han MH. MR imaging findings of intracranial dural arteriovenous fistulas; relations with venous drainage patterns. Am J Neuroradiol. 2005;26:2500–2507. [PMC free article] [PubMed] [Google Scholar]
- 7.Smirniotopoulos JG, Murphey FM. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007;27:525–551. doi: 10.1148/rg.272065155. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Chung J. Transient MR signal changes in patients with generalized toniclonic seizure or status epilepticus: periictal diffusion-weigthed imaging. Am J Neuroradiol. 2001;22:1149–1160. [PMC free article] [PubMed] [Google Scholar]
- 9.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. Am J Neuroradiol. 2007;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Ferriero D. Reversible MRI abnormalities following seizures. Neurology. 1995;45:104–108. doi: 10.1212/wnl.45.1.104. [DOI] [PubMed] [Google Scholar]
- 11.Arnold G, Reuter U. Migraine with aura shows gadolinium enhancement which is reversed following prophylactic treatment. Cephalagia. 1998;18:644–646. doi: 10.1046/j.1468-2982.1998.1809644.x. [DOI] [PubMed] [Google Scholar]
- 12.Maytal J, Libman RB. Basilar artery migraine and reversible imaging abnormalities. Am J Neuroradiol. 1998;19:1116–1119. [PMC free article] [PubMed] [Google Scholar]
- 13.Friedenberg S, Dodick DW. Migraine-associated seizure: a case of reversible MRI abnormalities and persistent non dominant hemisphere syndrome. Headache. 2000;40:487–490. doi: 10.1046/j.1526-4610.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- 14.Murthy SN, Cohen ME. Pseudomigraine with prolonged aphasia in a child with cranial irradiation for medulloblastoma. J Child Neurol. 2002;17:134–138. doi: 10.1177/088307380201700209. [DOI] [PubMed] [Google Scholar]
- 15.Shuper A, Packer RJ. Complicatde migraine-like episodes in children following cranial irradiation and chemotherapy. Neurology. 1995;45:1837–1840. doi: 10.1212/wnl.45.10.1837. [DOI] [PubMed] [Google Scholar]
- 16.Hopewell JW, van der Kogel AJ. Pathophysiological mechanisms leading to the development of the late radiation-induced damage to the central nervous system. Front Radiat Ther Oncol. 1999;33:265–275. doi: 10.1159/000061239. [DOI] [PubMed] [Google Scholar]
- 17.Tsuruda JS, Kortman KER. Radiation effects on white matter: MRI evaluation. Am J Neuroradiol. 1987;149:165–171. doi: 10.2214/ajr.149.1.165. [DOI] [PubMed] [Google Scholar]
- 18.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:TROTCN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Cohen BH, Packer RJ (1992) Adverse neurologic effects of chemotherapy and radiation therapy. In: Berg BO (ed) Neurologic aspects of pediatrics. Butterworth-Heinemann, Stoneham, pp 567–580
- 20.Surma-aho O, Niemela M. Adverse long-term effects of brain radiotherapy in adult low-grade glioma patients. Neurology. 2001;56:1285–1290. doi: 10.1212/wnl.56.10.1285. [DOI] [PubMed] [Google Scholar]
- 21.Farid K, Meissner WG. Normal cerebrovascular reactivity in strokleike migraine attacks after radiation therapy syndrome. Clin Nucl Med. 2010;35:583–585. doi: 10.1097/RLU.0b013e3181e4db6f. [DOI] [PubMed] [Google Scholar]