Abstract
Background: The correlation between efficacy end points in randomized controlled trials (RCTs) of systemic therapy for non-Hodgkin's lymphoma (NHL) was investigated to identify an appropriate surrogate end point for overall survival (OS).
Methods: RCTs of previously untreated NHL published from 1990 to 2009 were identified. Associations between absolute differences in efficacy end points were determined using nonparametric Spearman's rank correlation coefficients (rs).
Results: Thirty-eight RCTs representing 85 treatment arms for aggressive NHL and 20 RCTs representing 42 arms for indolent NHL were included. For aggressive NHL, differences in 3-year progression-free survival (PFS)/event-free survival (EFS) were high correlated with differences in 5-year OS {rs of 0.90 [95% confidence interval (CI) 0.73–0.96]} and linear regression determined that a 10% improvement in 3-year EFS or PFS would predict for a 7% ± 1% improvement in 5-year OS. For indolent histology disease, differences in complete response were strongly correlated with differences in 3-year EFS [rs 0.86 (95% CI 0.35–0.97)], but there was no correlation between 3-year time-to-event end points and 5-year OS.
Conclusions: Improvements in 3-year EFS/PFS are highly correlated with improvements in 5-year OS in aggressive NHL and should be explored as a candidate surrogate end point. Definition of these relationships may inform future clinical trial design and interpretation of interim trial data.
Keywords: clinical trials, non-Hodgkin's lymphoma, surrogate end points
introduction
Selection of efficacy end points for randomized controlled trials (RCTs) of non-Hodgkin's lymphoma (NHL) depends largely on histology and treatment goals. In untreated aggressive histology lymphomas, primary treatment with chemotherapy is undertaken with curative intent, so the development of new treatments to increase the rate of overall survival (OS) remains an important goal for this patient population. In contrast, indolent histology lymphomas have a very long natural history and are generally incurable, and systemic treatment is generally directed at improving symptoms and prolonging progression-free survival (PFS).
Although OS is an unambiguous measure of efficacy in clinical trials, its use as a primary end point requires a long duration of follow-up and may prolong the process of identifying novel and potentially beneficial therapy. Surrogate end points for OS have been explored in breast [1, 2], lung [3], and rectal cancers [4] but only have been validated in colon cancer [5–7]. Nonetheless, there is emerging acceptance of such end points by the oncology community and by regulatory agencies [8–10].
While there is great interest in developing validated surrogate end points for OS, there is no consensus on the necessary validation process [5,11–14]. Prentice et al. proposed that the surrogate marker should correlate with the true end point and capture the net effect of the treatment on the true end point [11, 12]. More recently, Buyse et al. [13] stated that the surrogate should be predictive of the final end point using both trial- and individual-level data. Additionally, Begg and Leung [14] argued that significant differences observed for the candidate surrogate end point in trials should be concordant with results for the true end point.
Surrogate end points have yet to be explored in the trials of NHL. Time-to-event end points including event-free survival (EFS) or PFS permit earlier reporting of results, but their ability to predict OS is unknown. The purpose of this study is to describe reporting of primary and secondary end points in RCTs of NHL and to determine the correlations between response, intermediate time-to-event, and survival end points in the treatment of NHL with the goal of identifying a surrogate end point for OS.
methods
trial selection
RCTs were eligible for inclusion if they enrolled previously untreated aggressive NHL with at least 100 patients per arm or untreated indolent NHL with at least 75 patients per arm. Studies were excluded if they primarily investigated the effect of autologous stem-cell transplantation (ASCT), maintenance, or local therapies (i.e. surgery, radiation); exclusively enrolled T cell, mantle cell, HIV-associated Burkitt, primary central nervous system, or small-cell lymphocytic lymphomas (including chronic lymphocytic leukemia); and those reporting pooled data from multiple trials.
literature search
A systematic literature search was conducted to identify RCTs using Medline, EMBASE (Exerpta Medica Database), and the Cochrane Central Register of Controlled Trials databases from 1 January 1990 to 1 May 2009. Search strategy included MeSH headings and keywords such as ‘Lymphoma, Non-Hodgkin's’, ‘non-Hodgkin’ or ‘NHL’; ‘Antineoplastic Agents’, ‘Antineoplastic Combined Chemotherapy Protocols’ or ‘chemotherapy’; and ‘Randomized Controlled Clinical Trial’ or ‘Phase III Clinical Trial’. A manual search was also carried out for abstracts in the published proceedings of the annual meetings of the American Society of Clinical Oncology, American Society of Hematology, and the triennial International Conference on Malignant Lymphoma in Lugano from 2004 to 2009. Abstracts from all identified RCTs were manually reviewed for eligibility based on inclusion and exclusion criteria. RCTs were subgrouped for analysis by disease histology (i.e. aggressive or indolent).
data collection
For each eligible published RCT, data were extracted regarding study design, sample size, enrollment dates, experimental arms, and response rates (RRs). The standard chemotherapy arm and primary end point in each trial were determined by consensus of two investigators (LL and MC). Data on EFS, PFS, and OS were determined for all treatment arms using published data or survival curves. Reported time-to-event end points reflect the original terminology used by authors in the RCT. For our analysis, time-to-event end points were defined as PFS or EFS according to established (i.e. per-protocol) definitions in the International Working Group Revised Response Criteria for Lymphoma [15]. Results of each trial for PFS, EFS or OS were considered significant based on the per-protocol analysis with a P-value ≤ 0.05. RCTs were categorized as ‘positive’ if the specified primary end point was met. If a RCT was reported on multiple occasions, data were collated from all abstracts and the most recent data were used in the event of discrepancies.
statistical analysis
Descriptive statistics were used to summarize trial characteristics and end point selection. To evaluate changes in primary end point selection over time, studies were dichotomized into an earlier or later time period based on the year of study initiation and the frequency of time-to-event end points was examined using the Cochran–Armitage test for trend. In addition, trials were evaluated based on whether rituximab was included in at least one of the treatment arms, and differences in primary end point selection were determined using Fisher’s exact test. For each trial, the absolute differences in end points [complete response (CR), EFS, PFS, and OS] were calculated as the estimate in the experimental arm minus the estimate in the standard arm. For multiarm and factorial-design studies, only one randomly chosen experimental arm (or factorial group) was used to ensure that the absolute differences of the same end point were considered independent.
The nonparametric Spearman's rank correlation coefficient (rs) was used as a measure of correlation between the differences in (i) CR and intermediate time-to-event end points (3-year EFS/PFS or OS) and (ii) potential surrogate end points (CR, 3 year EFS/PFS) and 5-year OS. In this analysis, PFS and EFS were considered together as an intermediate time-to-event end point since the per-protocol definition of EFS always included progression and death as events; however, a separate analysis was also presented where possible. Correlation coefficients were compared using the normal approximation to the z-transformation of rs and its standard deviation. For strongly correlated end points, linear regression analysis was carried out to obtain slope, which served as a conversion factor between end points and determined the proportion of variability explained (R2). Furthermore, concordance of strongly correlated end points was assessed by determining the proportion of trials in which the set of end points led to the same conclusion based on statistical significance testing (P < 0.05).
results
A total of 58 RCTs conducted from 1978 to 2005 were identified: 38 in aggressive histology and 20 in indolent histology lymphomas (Table 1). The aggressive lymphoma RCTs included 85 treatment arms representing 16 103 patients and had a median follow-up of 55 months (range 20–108). The indolent lymphoma RCTs included 42 treatment arms and 5128 patients, with a median follow-up of 52 months (range 29–144).
Table 1.
Characteristics of included phase III trials
| Characteristic | Aggressive histology (N = 38), n (%) | Indolent histology (N = 20), n (%) |
| Sample size | ||
| Median | 382 | 244 |
| Range | 177–1222 | 131–428 |
| Time period of study | ||
| Before 1990 | 16 (42) | 7 (35) |
| 1991–2005 | 22 (58) | 13 (65) |
| Accrual duration (years) | ||
| Median | 4 | 5 |
| Range | 1–10 | 2–9 |
| Follow-up duration (months) | ||
| Median | 55 | 53 |
| Range | 20–108 | 29–144 |
| Design | ||
| Two-arm | 25 (66) | 12 (60) |
| Three-arm | 1 (3) | 2 (10) |
| Four-arm | 1 (3) | 0 |
| Two-arm, Two-stage | 5 (13) | 6 (30) |
| 2 × 2 factorial | 6 (16) | 0 |
| Number of comparisons per trial | ||
| 1 | 33 (87) | 18 (90) |
| 2 | 2 (5) | 2 (10) |
| 3 | 3 (8) | 0 |
| Frequency of reported end pointa | ||
| OS | 36 (94) | 19 (95) |
| EFS | 10 (26) | 6 (30) |
| PFS | 12 (32) | 6 (30) |
| DFS/RFS | 15 (39) | 4 (20) |
| FFS | 8 (21) | 2 (10) |
| TTF | 4 (10) | 10 (50) |
| TTP | 2 (5) | 4 (20) |
| RR | 37 (97) | 15 (75) |
| Outcomes | ||
| Positive | 12 (32) | 11 (55) |
| Negative | 26 (68) | 9 (45) |
Includes primary and secondary end points, with percentages presented as a ratio of total number of randomized clinical trials.
OS, overall survival; EFS, event-free survival; PFS, progression-free survival; DFS, disease-free survival; RFS, relapse-free survival; FFS, failure-free survival; TTF, time-to-failure; TTP, time-to-progression; RR, response rate.
end point selection and reporting
Regardless of lymphoma histology, almost all trials reported OS (94% for aggressive, 95% for indolent), most trials included RR (97% for aggressive, 75% for indolent), but only approximately one-third of the trials reported at least one other time-to-event end point (Table 1).
Seven different primary end points were reported, reflecting heterogeneity in reporting terminology (Table 2). OS and RR were unambiguously defined as demonstrated by their consistent frequency of use as primary end points regardless of reported or per-protocol definitions for both histologies of lymphoma (Table 2). Discrepancies between reported and per-protocol end point definitions arose from the use of ‘event’ or ‘failure’, which affected time-to-event end points, such as PFS, EFS, time-to-failure (TTF), failure-free survival (FFS), and disease-free survival. For example if failure was defined as progression or death, FFS would be classified as PFS by the per-protocol definition, whereas inclusion nonprogression events suggested that this term was being used synonymously with EFS.
Table 2.
Frequency and reporting of primary end points in lymphoma randomized clinical trialsa
| Primary end point | Aggressive (N = 38) |
Indolent (N = 20) |
||
| Reported, n (%) | Per-protocol, n (%) | Reported, n (%) | Per-protocol, n (%) | |
| OS | 21 (55) | 21 (55) | 2 (10) | 2 (10) |
| EFS | 8 (21) | 12 (32) | 2 (10) | 5 (25) |
| PFS | 0 | 0 | 3 (15) | 5 (25) |
| DFS | 0 | 1 (3) | 0 | 0 |
| FFS | 4 (10) | 0 | 0 | 0 |
| TTF | 1 (3) | 0 | 5 (25) | 0 |
| RR | 2 (5) | 2 (5) | 5 (25) | 5 (25) |
| CR | 2 (5) | 2 (5) | 3 (15) | 3 (15) |
Primary end point based at time of study initiation. ‘Reported’ refers to the original terminology used by study authors, whereas ‘per-protocol’ refers to classification of the end point according to International Working Group guidelines based on its definition within the protocol [15].
OS, overall survival; EFS, Event-Free Survival; PFS, Progression-Free Survival; DFS, Disease-Free Survival; FFS, Failure-Free Survival; TTF, Time-to-Failure; RR, Response Rate; CR, Complete Response.
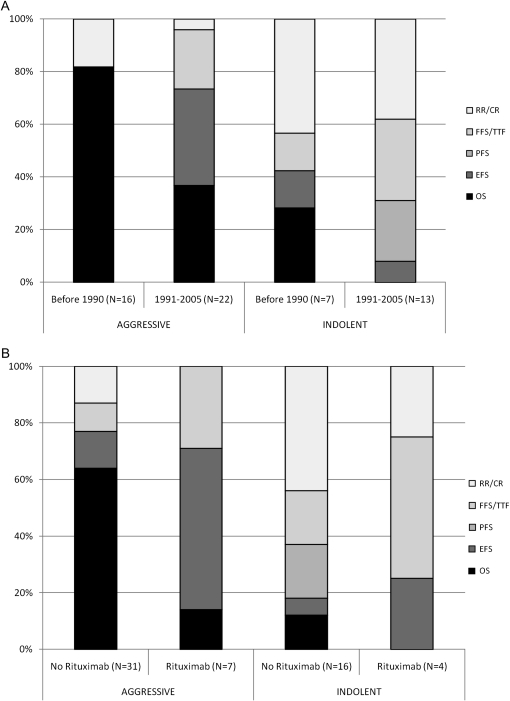
For aggressive lymphoma RCTs, the most commonly reported primary end point was OS followed by EFS. For indolent lymphoma RCTs, choice of primary end point was more heterogeneous, but use of either TTF or RR was most common (Table 2). Trend in the selection of primary end points was evaluated by comparing RCTs initiated before 1990 to those initiated following 1991 (Figure 1A) and by comparing RCTs based on the presence of rituximab in at least one treatment arm (Figure 1B). In the more recent time period, use of OS decreased in both indolent (28% versus 0%, P = 0.042) and aggressive NHL trials (81% versus 36%, P = 0.006). In the latter histologic subgroup, use of EFS became more common (0% versus 36%, P = 0.007). In aggressive NHL, RCTs evaluating rituximab were significantly less likely to use OS as the primary end point than RCTs without rituximab (14% versus 64%, P = 0.013), but there was no such difference noted for indolent histology NHL.
Figure 1.
Trends in selection of primary end points according to (A) time period and (B) presence or absence of rituximab in at least one treatment arm of the trial. RR/CR, response rate or complete response; FFS, failure-free survival/TTF, time-to-failure; PFS, progression-free survival; EFS, event-free survival; OS, overall survival.
correlation between response and intermediate time-to-event end points
For aggressive NHL, differences in CR rates strongly correlated with differences in 3-year EFS/PFS with an rs of 0.70 [95% confidence interval (CI) 0.42–0.86] (Table 3). The rs between differences in CR rate and differences in 3-year EFS and 3-year PFS were 0.88 (95% CI 0.57–0.97) and 0.63 (95% CI 0.21–0.84), respectively. There was a moderate correlation between differences in CR rate and differences in 3-year OS with a rs of 0.58 (95% CI 0.29–0.77).
Table 3.
Correlation between CR, time-to-event, and OS end points
| Aggressive |
Indolent |
|||||
| Nonparametric Spearman rank coefficient | 95% CI | P-value | Nonparametric Spearman rank coefficient | 95% CI | P-value | |
| CR and 3-year time-to-event and OS end points | ||||||
| CR and 3-year EFS | 0.88 | 0.57–0.97 | 0.0003 | 0.86 | 0.35 to 0.97 | 0.0059 |
| CR and 3-year PFS | 0.63 | 0.21–0.84 | 0.005 | 0.41 | −0.52 to 0.88 | 0.35 |
| CR and 3-year PFS/EFS | 0.70 | 0.42–0.86 | <0.0001 | 0.77 | 0.41–0.92 | 0.0007 |
| CR and 3-year OS | 0.58 | 0.29–0.77 | 0.004 | 0.41 | −0.1 to 0.74 | 0.098 |
| Potential surrogate end points and 5-year OS | ||||||
| CR and 5-year OS | 0.50 | 0.23–0.74 | 0.01 | 0.21 | −0.34 to 0.5 | 0.44 |
| 3-year EFS or PFS and 5-year OS | 0.90 | 0.73–0.96 | <0.0001 | 0.26 | −0.38 to 0.72 | 0.41 |
CI, confidence interval; CR, complete response; EFS, Event-Free Survival; PFS, Progression-Free Survival; OS, overall survival.
For indolent NHL, differences in CR rate also strongly correlated with differences in 3-year EFS/PFS with a rs of 0.77 (95% CI 0.41–0.92). While differences in CR rate correlated strongly with differences in 3-year EFS when considered alone with a rs of 0.86 (95% CI 0.35–0.97), there was no correlation between differences in CR rate and differences in 3-year PFS or 3-year OS.
correlation between potential surrogate and OS end points
There was no relationship between difference in CR and difference in 5-year OS in either aggressive or indolent NHL (Table 3). However, in aggressive NHL, differences in 3-year PFS/EFS were highly correlated with differences in 5-year OS with a rs of 0.90 (95% CI 0.73–0.96), and similarly strong correlations were noted when differences in 3-year PFS and 3-year EFS were separately correlated with 5-year OS. In contrast, there was no correlation between differences in these intermediate time-to-event end points and differences in 5-year OS in indolent NHL (Table 3).
In an exploratory analysis, we determined the correlation between 3-year PFS or EFS with 5-year OS within individual arms of the randomized trials (supplemental Data available at Annals of Oncology online). Similarly, these two end points were strongly correlated in aggressive NHL with a rs = 0.85 (95% CI 0.71–0.92, P < 0.001) (supplemental Figure S1, available at Annals of Oncology online) but only moderately correlated in indolent NHL with a rs = 0.56 (95% CI 0.2–0.78, P = 0.004) (supplemental Figure S2, available at Annals of Oncology online).
linear regression analysis
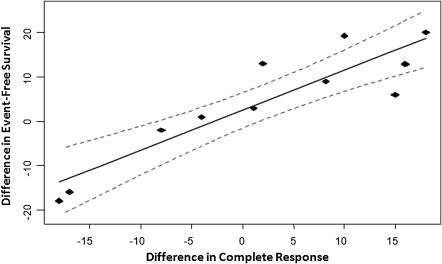
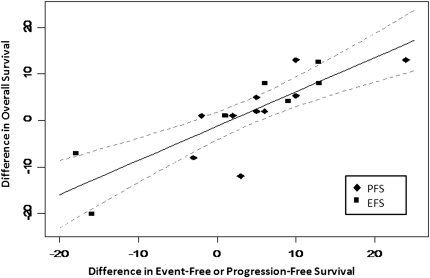
For strongly correlated end points, linear regression was carried out through the origin. In aggressive NHL, the regression of differences in CR and 3-year EFS yielded a slope of 0.9 ± 0.1 [±1 standard error (SE) of the estimate] with a R2 of 0.78 (Figure 2). The regression of differences in 3-year EFS/PFS and 5-year OS yielded a slope of 0.7 ± 0.1 (±1 SE of the estimate) with a R2 of 0.66 for aggressive histology NHL (Figure 3). In indolent NHL, the regression of differences in CR and 3-year EFS yielded a slope of 0.9 with a large SE (0.3) due to the smaller number of trials.
Figure 2.
Correlation between differences in complete response rates and differences in 3-year event-free survival in aggressive non-Hodgkin's lymphoma. Solid line represents the linear regression with 95% confidence intervals indicated by the dashed lines.
Figure 3.
Correlation between differences in 3-year event or progression-free survival and 5-year overall survival in aggressive non-Hodgkin's lymphoma. Solid line represents the linear regression with 95% confidence intervals indicated by the dashed line.
These findings suggest that in aggressive NHL, a 10% improvement in CR is estimated to correspond with a 9% ± 1% improvement in 3-year EFS and that a 10% improvement in 3-year EFS or PFS would predict for a 7% ± 1% improvement in 5-year OS. In indolent NHL, a 10% improvement in CR is estimated to predict a 9% ± 3% benefit in 3-year EFS.
concordance of trial results for correlated end points
For aggressive NHL, 26 trials had paired results where differences in 3-year EFS/PFS and 5-year OS between treatment arms were assessed for statistical significance. Concordant results were present in 23 trials (15 had no difference between arms for either end points and 8 had significant differences for both end points, Table 4). The three discordant trials all showed a significant difference in the 3-year time-to-event end point, but no difference in 5-year OS.
Table 4.
Concordance of randomized clinical trial outcomes
| Treatmenta | N | Primary end point (per-protocol) | Significant difference in CR | Significant difference in EFS/PFS | Significant difference in OS | Superior arm |
| Aggressive histology lymphoma | ||||||
| Dose-escalated CHOEP-21 versus CHOEP-21 [33] | 389 | EFS | No | No | No | No difference |
| R-CHOP-14 versus CHOP-14 for 6 versus 8 cycles [34] | 1222 | EFS | Yes | Yes | Yes | R-CHOP-14 for 6 cycles |
| CEOP-14 versus CEOP-21, ±R [35] | 217 | OS | No | n/a | No | No difference |
| Escalated R-CEOP versus escalated CEOP [36] | 204 | DFS | No | n/a | No | No difference |
| Intensified CHOP-14 for 6 cycles versus standard CHOP-21 for 8 cycles [37] | 477 | OS | No | No | No | No difference |
| Mini-COEP versus P-VEBEC [38] | 232 | OS | No | No | No | No difference |
| R-CHOP versus CHOP, then R-maintenance versus nothing [39] | 632 | EFS | n/a | Yes | No | R-CHOP |
| R-CHOP-like versus CHOP-like chemotherapy [34] | 824 | EFS | Yes | Yes | Yes | R-CHOP like |
| PMitCEBO versus CHOP, ±GCSF [40] | 784 | EFS | Yes | No | No | No difference |
| Flexible versus fixed dosing of anthracycline in ProMECE-CytaBOM or ProMI-CytaBOM [41] | 356 | EFS | No | n/a | No | No difference |
| ProMECE-CytaBOM versus ProMI-CytaBOM, then maintenance chemotherapy [42] | 249 | OS | No | No | No | No difference |
| R-CHOP-14 versus CHOP-14 [43] | 243 | EFS | n/a | n/a | Yes | R-CHOP |
| CIOP versus CHOP [44] | 211 | OS | Yes | Yes | Yes | CHOP |
| Pirarubicin-COP versus CHOP (2/3 dose) versus pirarubicin-COPE [45] | 443 | OS | No | No | No | No difference |
| VEPA-B/FEPP-AB/M-FEPA every 10 weeks for 3 cycles versus VEPA-B/FEPP-B/M-FEPA every 14 weeks for 4 cycles [46] | 447 | OS | No | n/a | No | No difference |
| CHOP-14 versus CHOP-21, ±etoposide [47] | 689 | EFS | n/a | Yes | No | CHOP-14 |
| ACVBP versus CHOP [26] | 635 | EFS | No | Yes | Yes | ACVBP |
| CHOEP versus CHOP, every 14 versus 21 days [48] | 710 | EFS | n/a | Yes | No | CHOEP |
| CNOP versus CHOP, ±GCSF [49] | 458 | EFS | Yes | Yes | Yes | CHOP |
| R-CHOP versus CHOP [50] | 399 | EFS | Yes | Yes | Yes | R-CHOP |
| CNOP versus CEOP [51] | 249 | OS | No | No | No | No difference |
| PMitCEBO versus PAdriaCEBO [52] | 473 | OS | No | n/a | Yes | PMitCEBO |
| CHOP + IFN versus CHOP [53] | 435 | RR | No | n/a | No | No difference |
| PACEBOM versus CHOP [54] | 459 | OS | No | n/a | No | No difference |
| MACOP-B versus CHOP [55] | 374 | OS | No | No | No | No difference |
| CAPOMEt versus CHOP-MTX [56] | 281 | OS | No | n/a | No | No difference |
| MECOP-B versus MACOP-B [57] | 211 | OS | No | n/a | No | No difference |
| Alternating B-CHOP-M and PEEC-M versus B-CHOP-M [58] | 325 | OS | No | n/a | No | No difference |
| CTVmP versus CVmP [59] | 453 | OS | Yes | Yes | Yes | CTVmP |
| MACOP-B over CHOP [60] | 236 | CR | No | Yes | Yes | MACOP-B |
| ProMACE-MOPP versus MACOP-B [61] | 221 | OS | No | No | No | No difference |
| ProMECE-CytaBOM versus MACOP-B [62] | 210 | OS | No | No | No | No difference |
| m-BACOD versus CHOP versus ProMACE-CytaBOM versus MACOP-B [63] | 899 | OS | No | No | No | No difference |
| ProMACE-MOPP versus CHVmP-VB [64] | 430 | OS | n/a | No | No | No difference |
| Escalated BACOP versus BACOP [65] | 238 | OS | No | No | No | No difference |
| m-BACOD versus CHOP [66] | 325 | OS | No | No | No | No difference |
| F-MACHOP versus MACOP-B [67] | 286 | CR | No | n/a | No | No difference |
| Low-dose bleomycin + CHOP versus CHOP, then low versus high-dose MTX [68] | 177 | RR | No | No | No | No difference |
| Indolent histology lymphoma | ||||||
| R-CVP versus CVP [21] | 321 | EFS | Yes | Yes | Yes | R-CVP |
| R-CHVP + IFN versus CHVP-IFN [22] | 360 | EFS | Yes | Yes | No | R-CHVP + IFN |
| R-MCP versus MCP [20] | 358 | RR | Yes | Yes | Yes | R-MCP |
| CID versus CD [69] | 200 | EFS | No | Yes | No | CID |
| MCP versus CHOP [25] | 277 | CR | No | n/a | No | No difference |
| F versus CVP [23] | 381 | PFS | Yes | No | No | No difference |
| FMD versus CMD [70] | 400 | PFS | No | n/a | n/a | CMD |
| R-CHOP versus CHOP [71] | 428 | EFS | No | Yes | Yes | R-CHOP |
| CHOP + bleomycin versus cyclophosphamide [72] | 228 | OS | No | No | No | No difference |
| COPA + IFN versus COPA [73] | 291 | PFS | No | Yes | COPA + IFN | |
| PmM versus COP, then IFN-maintenance versus observation [74] | 246 | RR | Yes | n/a | n/a | Not available |
| CHOP versus chlorambucil + prednisone [24] | 259 | RR | n/a | Yes | No | No difference |
| CHVP + IFN versus CHVP [75] | 242 | PFS | No | Yes | Yes | CHVP + IFN |
| BOP versus COP [76] | 164 | CR | No | n/a | No | No difference |
| Cladribine versus CVP versus cladribine + C [77] | 197 | PFS | Yes | Yes | n/a | Cladribine |
| FM versus mini-CHVdP [78] | 155 | CR | Yes | Yes | No | FM |
| FND versus alternating triple therapy (CHOD-bleomycin, ESHAP, and NOPP) [79] | 142 | RR | No | Yes | No | No difference |
| CHVmP + IFN versus F [80] | 131 | EFS | No | n/a | n/a | CHVP + IFNb |
| CVP + IFN versus CVP, then IFN-maintenance versus observation [81] | 155 | RR | n/a | Yes | No | No difference |
| IFN versus prednimustine versus observation [59] | 193 | OS | No | No | No | No difference |
Bolded items represent the two comparator arms used for analysis in the following format: experimental versus standard arm.
Conclusion based on 2-year follow-up data.
RR, response rate; CR, complete response; EFS, Event-Free Survival; PFS, Progression-Free Survival; OS, overall survival; n/a, not applicable; CHOEP, cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CEOP, cyclophosphamide, epirubicin, vincristine, prednisone; R, rituximab; 14, cycle given every 14 days; 21, cycle given every 21 days; P-VEBEC, prednisone, vinblastine, epirubicin, bleomycin, etoposide, cyclophosphamide; PMitCEBO, prednisolone, mitoxantrone, cyclophosphamide, etoposide, bleomycin, vincristine; ProMECE-CytaBOM, prednisone, cyclophosphamide, etoposide, epidoxorubicin, cytarabine, bleomycin, vincristine, methotrexate with leucovorin; ProMICE-CytaBOM, prednisone, cyclophosphamide, etoposide, idarubicin, cytarabine, bleomycin, vincristine, methotrexate with leucovorin; CIOP, cyclophosphamide, idarubicin, vincristine, prednisone; COP or CVP, cyclophosphamide, vincristine, prednisolone (or prednisone); COPE, cyclophosphamide, vincristine, prednisolone, etoposide; VEPA-B/FEPP-AB/M-FEPA and VEPA-B/FEPP-B/M-FEPA both contain vincristine, cyclophosphamide, prednisolone, doxorubicin, bleomycin, etoposide, procarbazine, methotrexate, leucovorin, vindesine (at differing doses and schedules of administration); ACVBP, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone; CNOP, cyclophosphamide, mitoxantrone, vincristine, prednisone; PAdriaCEBO, prednisolone, adriamycin, cyclophosphamide, etoposide, bleomycin, vincristine; IFN, interferon; GCSF, granulocyte colony stimulating factor; MTX, methotrexate; PACEBOM, prednisolone, doxorubicin, cyclophosphamide, etoposide, bleomycin, vincristine, methotrexate; MACOP-B, methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone, bleomycin; CAPOMEt, weekly alternating cyclophosphamide and doxorubicin, vincristine and prednisolone, methotrexate with leucovorin and etoposide; MECOP-B, methotrexate, epirubicin, cyclophosphamide, vincristine, prednisolone, and bleomycin; B-CHOP-M, bleomycin, cyclophosphamide, doxorubicin, vincristine, prednisolone and methotrexate; PEEC-M, methylprednisolone, vindesine, etoposide, chlorambucil and methotrexate; CVmP, cyclophosphamide, teniposide, prednisone; CTVmP, cyclophosphamide, pirarubicin, teniposide, prednisone; ProMACE-MOPP, procarbazine, methotrexate with leucovorin, doxorubicin, cyclophosphamide, and etoposide; m-BACOD, low-dose methotrexate with leucovorin rescue, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone; ProMACE-CytaBOM, prednisone, doxorubicin, cyclophosphamide, and etoposide, followed by cytarabine, bleomycin, vincristine, and methotrexate with leucovorin rescue; CHVmP-VB, cyclophosphamide, doxorubicin, teniposide, prednisone and vincristine, bleomycin; BACOP, bleomycin, doxorubicin, cyclophosphamide, vincristine, prednisone; m-BACOD, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone, methotrexate with leucovorin; F-MACHOP, 5-fluorouracil, methotrexate with leucovorin, cytarabine, cyclophosphamide, doxorubicin, vincristine, prednisone; CHVP, cyclophosphamide, doxorubicin, etoposide, prednisolone; MCP, mitoxantrone; chlorambucil, prednisolone; CID, chlorambucil, idarubicin, dexamethasone; CD, chlorambucil, dexamethasone; F, fludarabine; CMD, chlorambucil, mitoxantrone, dexamethasone; FMD, fludarabine, mitoxantrone, dexamethasone; COPA, cyclophosphamide, doxorubicin, vincristine and prednisone every 28 days; PmM, prednimustine, mitoxantrone; BOP, bendamustine, vincristine, prednisone; FM, fludarabine, mitoxantrone; CHVdP, cyclophosphamide, doxorubicin, vindesine, prednisone; FND, fludarabine, mitoxantrone, dexamethasone; CHOD, cyclophosphamide, doxorubicin, vincristine, dexamethasone, bleomycin; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; NOPP, mitoxantrone, vincristine, procarbazine, prednisone.
Of the 14 indolent lymphoma trials that had paired results for CR and 3-year EFS/PFS, eight had concordant conclusions (five reported statistically significant differences for both end points and two trials reported no difference at either end points, Table 4). Among the six trials with discordant results, five had no difference in CR but did have a significant difference in 3-year EFS/PFS, while only one trial had a difference in CR but no difference in 3-year EFS/PFS.
discussion
Despite the lack of validated surrogate end points in NHL, our review reveals that time-to-event end points are increasingly used in place of OS as the primary end point in recent phase III clinical trials. There was a trend toward increasing use of EFS and TTF, respectively, in RCTs of aggressive and indolent histology disease. Improvements in CR strongly predicted for improvements in 3-year EFS in both aggressive and indolent histology lymphomas but were not predictive of OS. In aggressive histology lymphoma, 3-year PFS/EFS were strongly correlated with 5-year OS, and statistically significant differences in PFS/EFS observed at 3 years predicted for differences in OS after 5 years of follow-up. However, considerable inconsistency exists both in the reporting and definition of failure or event end points. Our results suggest that 3-year PFS should be further explored as a candidate surrogate end point in RCTs of aggressive NHL using individual patient data.
A validated surrogate end point for 5-year OS offers potential advantages for conducting clinical trials more efficiently and expediting development of new treatments. In contrast to OS, however, time-to-event end points are poorly defined and may suffer from bias in ascertainment [16–18]. For example EFS is a composite end point consisting of objective measures, such as death and progression, in addition to more subjective components (i.e. investigator decision to initiate new treatment). Guidelines for the harmonization of response assessment in clinical trials of lymphoma provide a clear definition and methodology for assessing progression but do not address the definition and assessment of nonprogression events [15, 19]. Consequently, PFS may be a better candidate surrogate since the specificity of included ‘events’ has implications for the power of a trial, the likelihood of a significant result, and ability to conduct cross-trial comparisons [18].
Recognizing that the majority of RCTs were initiated before the publication of guidelines to harmonize end points [15, 19], PFS and EFS were combined in our analysis. For both histologic subgroups of lymphoma, initial CR predicted for lack of events at 3 years, but the correlation with OS at either 3 or 5 years was not strong, implying that attainment of CR does not provide information about longer term outcomes. While CR and 3-year EFS were strongly correlated, this may be partially attributed to the actual definition of event, which encompassed lack of response or absence of CR in some trials. In indolent lymphoma, a similar relationship between higher CR rates and improved EFS/PFS has been demonstrated in individual trials of rituximab-based treatment [20–22] but was not consistently seen in trials evaluating cytotoxic chemotherapy [23–25]. However, attainment of CR did not predict for improved OS, which was likely due to the availability of effective treatments for relapsed or refractory disease and the relatively short duration of follow-up of 5 years, which may be inadequate for evaluation of OS given the long natural history of indolent lymphomas.
For aggressive NHL, the strong relationship between CR and EFS/PFS is not surprising. Achievement of CR may be associated with lower relapse rates [26] and failure to achieve CR is an indication for high-dose chemotherapy and ASCT for young fit patients. The correlation between CR and OS was moderate at 3 years but was not apparent at 5 years of follow-up. This dissociation between CR and OS at 5 years may reflect the cumulative impact of relapse over time [27, 28]. In contrast, a significant number of deaths within the first 3 years are likely attributed to those without initial CR (i.e. those with refractory or residual disease). Although these patients may be offered ASCT, they tend to have worse outcomes [29], so the potentially confounding effect of ASCT on 3-year survival is minimal.
It is striking that a 3-year time-to-event end point such as PFS may be predictive of 5-year OS in aggressive histology lymphoma, considering the diversity of treatments investigated in the trials that we evaluated. The correlation between PFS and OS in aggressive but not indolent NHL is consistent with the observation that the relationship between these two end points is influenced by expected survival post-progression (SPP) time [30]. A significant difference in PFS is more likely to predict for significant OS difference in a disease with a shorter expected SPP such as aggressive lymphoma where median survival following relapse is 9 months [27] compared with follicular lymphoma where median OS is not reached even after 6 years of follow-up [31].
Our literature-based analysis is the first to examine end points in RCTs of untreated aggressive and indolent lymphoma spanning a 25-year period. One previous meta-analysis of follicular lymphoma trials reported a correlation between higher CR rate and reduction in hazard rate for PFS [32]. However, that analysis included single-arm phase II trials and effectively compared overall rates of CR and PFS associated with individual treatment arms. By including only RCTs, we were able to compare the actual impact of different treatments on these end points.
This study does have some limitations. First, our analysis was conducted using published trial-level data. To confirm the validity of 3-year PFS as a surrogate for 5-year OS in aggressive lymphoma, it is necessary to assess their correlation using individual patient data. Second, as the total number of trials included for each histologic subgroup of lymphoma was small, the power to evaluate these relationships was limited by the actual reporting of the published studies. To test for correlations, a complete set of data for both the candidate surrogate and true end points is required [14] and only half of all trials had quantitative estimates for both PFS/EFS and OS. While hazard ratios would have provided a better comparison of the overall effect of treatment on survival over time, these were reported in <20% of all trials. Furthermore, in contrast to trials of metastatic cancer [3, 7], median time-to-event was often not reached in trials of primary chemotherapy for NHL thereby rendering it difficult to evaluate the relationship between time-to-progression and median OS end points, either at the trial level as differences between treatment arms or to determine the correlation between these two end points within individual treatment arms. Finally, since we only included RCTs of untreated NHL in this study, estimates of these relationships are not applicable to RCTs of relapsed or refractory disease or of maintenance strategies.
In this study, we determined correlations as well as estimated relationships between different end points in RCTs of untreated aggressive and indolent NHL. Definition of these relationships may improve the design of clinical trials in lymphoma. Estimates between response and efficacy end points may be helpful for designing randomized phase III RCTs based on randomized phase II data. Our findings suggest that 3-year PFS may be an appropriate surrogate end point for 5-year OS in clinical trials of aggressive NHL and provides the preliminary evidence necessary to further evaluate the strength of this relationship using a meta-analysis with individualized patient data. Use of PFS rather than OS would lead to considerable lead time advantage in the evaluation of clinical trials for aggressive lymphoma, but acceptance of PFS as a surrogate end point is required in order to expedite approval of novel agents.
disclosure
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We would like to thank Dr Lillian Siu for her valuable comments during the conception of this study and Esther Atkinson for assistance with the systematic literature review.
References
- 1.Ng R, Pond GR, Tang PA, et al. Correlation of changes between 2-year disease-free survival and 5-year overall survival in adjuvant breast cancer trials from 1966 to 2006. Ann Oncol. 2008;19:481–486. doi: 10.1093/annonc/mdm486. [DOI] [PubMed] [Google Scholar]
- 2.Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;26:1987–1992. doi: 10.1200/JCO.2007.10.8407. [DOI] [PubMed] [Google Scholar]
- 3.Hotta K, Fujiwara Y, Matsuo K, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:311–317. doi: 10.1097/JTO.0b013e3181989bd2. [DOI] [PubMed] [Google Scholar]
- 4.Methy N, Bedenne L, Conroy T, et al. Surrogate end points for overall survival and local control in neoadjuvant rectal cancer trials: statistical evaluation based on the FFCD 9203 trial. Ann Oncol. 2010;21:518–524. doi: 10.1093/annonc/mdp340. [DOI] [PubMed] [Google Scholar]
- 5.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 6.Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25:5218–5224. doi: 10.1200/JCO.2007.11.8836. [DOI] [PubMed] [Google Scholar]
- 7.Tang PA, Bentzen SM, Chen EX, Siu LL. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol. 2007;25:4562–4568. doi: 10.1200/JCO.2006.08.1935. [DOI] [PubMed] [Google Scholar]
- 8.Gill S, Sargent D. End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? Oncologist. 2006;11:624–629. doi: 10.1634/theoncologist.11-6-624. [DOI] [PubMed] [Google Scholar]
- 9.Knox JJ. Progression-free survival as endpoint in metastatic RCC? Lancet. 2008;372:427–429. doi: 10.1016/S0140-6736(08)61040-5. [DOI] [PubMed] [Google Scholar]
- 10.Appelbaum FR, Rosenblum D, Arceci RJ, et al. End points to establish the efficacy of new agents in the treatment of acute leukemia. Blood. 2007;109:1810–1816. doi: 10.1182/blood-2006-08-041152. [DOI] [PubMed] [Google Scholar]
- 11.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 12.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Buyse M, Molenberghs G, Burzykowski T, et al. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;1:49–67. doi: 10.1093/biostatistics/1.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Leung DHY. On the use of surrogate end points in randomized trials. J R Stat Soc Ser A Stat Soc. 2000;163:15–28. [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16.Panageas KS, Ben-Porat L, Dickler MN, et al. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99:428–432. doi: 10.1093/jnci/djk091. [DOI] [PubMed] [Google Scholar]
- 17.Saad ED, Katz A, Hoff PM, Buyse M. Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 18.Mathoulin-Pelissier S, Gourgou-Bourgade S, Bonnetain F, Kramar A. Survival end point reporting in randomized cancer clinical trials: a review of major journals. J Clin Oncol. 2008;26:3721–3726. doi: 10.1200/JCO.2007.14.1192. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 20.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: an East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 21.Marcus R, Imrie K, Solal-Celigny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26:4579–4586. doi: 10.1200/JCO.2007.13.5376. [DOI] [PubMed] [Google Scholar]
- 22.Salles G, Mounier N, De Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 23.Hagenbeek A, Eghbali H, Monfardini S, et al. Phase III intergroup study of fludarabine phosphate compared with cyclophosphamide, vincristine, and prednisone chemotherapy in newly diagnosed patients with stage III and IV Low-grade malignant non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:1590–1596. doi: 10.1200/JCO.2005.03.7952. [DOI] [PubMed] [Google Scholar]
- 24.Kimby E, Bjorkholm M, Gahrton G, et al. Chlorambucil/prednisone vs. CHOP in symptomatic low-grade non-Hodgkin's lymphomas: a randomized trial from the Lymphoma Group of Central Sweden. Ann Oncol. 1994;5:67–71. [PubMed] [Google Scholar]
- 25.Nickenig C, Dreyling M, Hoster E, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer. 2006;107:1014–1022. doi: 10.1002/cncr.22093. [DOI] [PubMed] [Google Scholar]
- 26.Tilly H, Lepage E, Coiffier B, et al. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102:4284–4289. doi: 10.1182/blood-2003-02-0542. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein SH, Unger JM, Leblanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin's lymphoma: a 20-year follow-up analysis of SWOG 8516—the Southwest Oncology Group. J Clin Oncol. 2009;27:114–119. doi: 10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larouche J-F, Berger F, Chassagne-Clement C, et al. Lymphoma recurrence 5 years or later following diffuse large B-Cell lymphoma: clinical characteristics and outcome. J Clin Oncol. 2010;28:2094–2100. doi: 10.1200/JCO.2009.24.5860. [DOI] [PubMed] [Google Scholar]
- 29.Guglielmi C, Gomez F, Philip T, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–3269. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 30.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Oers MH, Van Glabbeke M, Giurgea L, et al. Rituximab maintenance treatment of relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol. 2010;28:2853–2858. doi: 10.1200/JCO.2009.26.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saville MW, Leonard JP, Hainsworth JD, et al. Role of different frontline regimens in achieving complete response in follicular lymphoma: a meta-analysis of CR rate and its relation to hazard rate for disease progression. Blood (ASH Annual Meeting Abstracts) 2006;108 (Abstr 2754) [Google Scholar]
- 33.Pfreundschuh M, Zwick C, Zeynalova S, et al. Dose-escalated CHOEP for the treatment of young patients with aggressive non-Hodgkin's lymphoma: II. Results of the randomized high-CHOEP trial of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL) Ann Oncol. 2008;19:545–552. doi: 10.1093/annonc/mdm514. [DOI] [PubMed] [Google Scholar]
- 34.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 35.Economopoulos T, Psyrri A, Dimopoulos MA, et al. CEOP-21 versus CEOP-14 chemotherapy with or without rituximab for the first-line treatment of patients with aggressive lymphomas: results of the HE22A99 trial of the Hellenic Cooperative Oncology Group. Cancer J. 2007;13:327–334. doi: 10.1097/PPO.0b013e3181570170. [DOI] [PubMed] [Google Scholar]
- 36.Aviles A, Nambo MJ, Castaneda C, et al. Rituximab and escalated chemotherapy in elderly patients with aggressive diffuse large-cell lymphoma: a controlled clinical trial. Cancer Biother Radiopharm. 2007;22:194–199. doi: 10.1089/cbr.2006.360. [DOI] [PubMed] [Google Scholar]
- 37.Verdonck LF, Notenboom A, De Jong DD, et al. Intensified 12-week CHOP (I-CHOP) plus G-CSF compared with standard 24-week CHOP (CHOP-21) for patients with intermediate-risk aggressive non-Hodgkin lymphoma: a phase 3 trial of the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) Blood. 2007;109:2759–2766. doi: 10.1182/blood-2006-07-035709. [DOI] [PubMed] [Google Scholar]
- 38.Merli F, Bertini M, Luminari S, et al. Long term results of a randomized study performed by Intergruppo Italiano Linfomi comparing Mini-CEOP vs P-VEBEC in elderly patients with diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48:367–373. doi: 10.1080/10428190601078100. [DOI] [PubMed] [Google Scholar]
- 39.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 40.Burton C, Linch D, Hoskin P, et al. A phase III trial comparing CHOP to PMitCEBO with or without G-CSF in patients aged 60 plus with aggressive non-Hodgkin's lymphoma. Br J Cancer. 2006;94:806–813. doi: 10.1038/sj.bjc.6602975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Federico M, Luminari S, Gobbi PG, et al. The length of treatment of aggressive non-Hodgkin's lymphomas established according to the international prognostic index score: long-term results of the GISL LA03 study. Eur J Haematol. 2006;76:217–229. doi: 10.1111/j.1600-0609.2005.00609.x. [DOI] [PubMed] [Google Scholar]
- 42.Federico M, Clo V, Brugiatelli M, et al. Efficacy of two different ProMACE-CytaBOM derived regimens in advanced aggressive non-Hodgkin's lymphoma. Final report of a multicenter trial conducted by GISL. Haematologica. 1998;83:800–811. [PubMed] [Google Scholar]
- 43.Sonneveld P, van Putten W, Biesma D, et al. Phase III trial of 2-weekly CHOP with rituximab for aggressive B-cell non-Hodgkin's lymphoma in elderly patients. Blood ASH Annual Meeting Abstracts. 2006;108 (Abstr 210) [Google Scholar]
- 44.Burton C, Smith P, Vaughan-Hudson G, et al. Comparison of CHOP versus CIOP in good prognosis younger patients with histologically aggressive non-Hodgkin lymphoma. Br J Haematol. 2005;130:536–541. doi: 10.1111/j.1365-2141.2005.05640.x. [DOI] [PubMed] [Google Scholar]
- 45.Mori M, Kitamura K, Masuda M, et al. Long-term results of a multicenter randomized, comparative trial of modified CHOP versus THP-COP versus THP-COPE regimens in elderly patients with non-Hodgkin's lymphoma. Int J Hematol. 2005;81:246–254. doi: 10.1532/IJH97.03147. [DOI] [PubMed] [Google Scholar]
- 46.Kinoshita T, Hotta T, Tobinai K, et al. A randomized controlled trial investigating the survival benefit of dose-intensified multidrug combination chemotherapy (LSG9) for intermediate- or high-grade non-Hodgkin's lymphoma: Japan Clinical Oncology Group Study 9002. Int J Hematol. 2004;80:341–350. doi: 10.1532/ijh97.04085. [DOI] [PubMed] [Google Scholar]
- 47.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634–641. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 48.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104:626–633. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 49.Osby E, Hagberg H, Kvaloy S, et al. CHOP is superior to CNOP in elderly patients with aggressive lymphoma while outcome is unaffected by filgrastim treatment: results of a Nordic lymphoma group randomized trial. Blood. 2003;101:3840–3848. doi: 10.1182/blood-2002-10-3238. [DOI] [PubMed] [Google Scholar]
- 50.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 51.Economopoulos T, Dimopoulos MA, Mellou S, et al. Treatment of intermediate- and high-grade non-Hodgkin's lymphoma using CEOP versus CNOP. Eur J Haematol. 2002;68:135–143. doi: 10.1034/j.1600-0609.2002.01620.x. [DOI] [PubMed] [Google Scholar]
- 52.Mainwaring PN, Cunningham D, Gregory W, et al. Mitoxantrone is superior to doxorubicin in a multiagent weekly regimen for patients older than 60 with high-grade lymphoma: results of a BNLI randomized trial of PAdriaCEBO versus PMitCEBO. Blood. 2001;97:2991–2997. doi: 10.1182/blood.v97.10.2991. [DOI] [PubMed] [Google Scholar]
- 53.Giles FJ, Shan J, Advani SH, et al. A prospective randomized study of Chop versus Chop plus Alpha-2B interferon in patients with Intermediate and High Grade non-Hodgkin's lymphoma: the International Oncology Study Group NHL1 study. Leuk Lymphoma. 2001;40:95–103. doi: 10.3109/10428190009054885. [DOI] [PubMed] [Google Scholar]
- 54.Linch DC, Smith P, Hancock BW, et al. A randomised British National Lymphoma Investigation trial of CHOP vs. a weekly multi-agent regimen (PACEBOM) in patients with histologically aggressive non-Hodgkin's lymphoma. Ann Oncol. 2000;11:S87–S90. [PubMed] [Google Scholar]
- 55.Jerkeman M, Anderson H, Cavallin-Stahl E, et al. CHOP versus MACOP-B in aggressive lymphoma—a Nordic Lymphoma Group randomised trial. Ann Oncol. 1999;10:1079–1086. doi: 10.1023/a:1008392528248. [DOI] [PubMed] [Google Scholar]
- 56.Bailey NP, Stuart NSA, Bessell EM, et al. Five-year follow-up of a prospective randomised multi-centre trial of weekly chemotherapy (CAPOMEt) versus cyclical chemotherapy (CHOP-Mtx) in the treatment of aggressive non-Hodgkin's lymphoma. Central Lymphoma Group. Ann Oncol. 1998;9:633–638. doi: 10.1023/a:1008276700860. [DOI] [PubMed] [Google Scholar]
- 57.Nair R, Ramakrishnan G, Nair NN, et al. A randomized comparison of the efficacy and toxicity of epirubicin and doxorubicin in the treatment of patients with non-Hodgkin's lymphoma. Cancer. 1998;82:2282–2288. doi: 10.1002/(sici)1097-0142(19980601)82:11<2282::aid-cncr26>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 58.Cameron DA, White JM, Proctor SJ, et al. CHOP-based chemotherapy is as effective as alternating PEEC/CHOP chemotherapy in a randomised trial in high-grade non-Hodgkin's lymphoma. Eur J Cancer. 1997;33:1195–1201. doi: 10.1016/s0959-8049(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 59.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15:1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 60.Wolf M, Matthews JP, Stone J, et al. Long-term survival advantage of MACOP-B over CHOP in intermediate-grade non-Hodgkin's lymphoma. Ann Oncol. 1997;8:S71–S75. [PubMed] [Google Scholar]
- 61.Sertoli MR, Santini G, Chisesi T, et al. MACOP-B versus ProMACE-MOPP in the treatment of advanced diffuse non-Hodgkin's lymphoma: results of a prospective randomized trial by the Non-Hodgkin's Lymphoma Cooperative Study Group. J Clin Oncol. 1994;12:1366–1374. doi: 10.1200/JCO.1994.12.7.1366. [DOI] [PubMed] [Google Scholar]
- 62.Silingardi V, Federico M, Cavanna L, et al. ProMECE-CytaBOM vs MACOP-B in advanced aggressive non-Hodgkin's lymphoma: long term results of a multicenter study of the Italian lymphoma study group (GISL) Leuk Lymphoma. 1995;17:313–320. doi: 10.3109/10428199509056837. [DOI] [PubMed] [Google Scholar]
- 63.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 64.Somers R, Carde P, Thomas J, et al. EORTC study of non-Hodgkin's lymphoma: phase III study comparing CHVmP-VB and ProMACE-MOPP in patients with stage II, III, and IV intermediate- and high-grade lymphoma. Ann Oncol. 1994;5:85–89. doi: 10.1093/annonc/5.suppl_2.s85. [DOI] [PubMed] [Google Scholar]
- 65.Meyer RM, Quirt IC, Skillings JR, et al. Escalated as compared with standard doses of doxorubicin in BACOP therapy for patients with non-Hodgkin's lymphoma. N Engl J Med. 1993;329:1770–1776. doi: 10.1056/NEJM199312093292404. [DOI] [PubMed] [Google Scholar]
- 66.Gordon LI, Harrington D, Andersen J, et al. Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin's lymphoma. N Engl J Med. 1992;327:1342–1349. doi: 10.1056/NEJM199211053271903. [DOI] [PubMed] [Google Scholar]
- 67.Mazza P, Zinzani PL, Martelli M, et al. MACOP-B vs F-MACHOP regimen in the treatment of high-grade non-Hodgkin's lymphomas. Leuk Lymphoma. 1995;16:457–463. doi: 10.3109/10428199509054434. [DOI] [PubMed] [Google Scholar]
- 68.Gottlieb AJ, Anderson JR, Ginsberg SJ, et al. A randomized comparison of methotrexate dose and the addition of bleomycin to CHOP therapy for diffuse large cell lymphoma and other non-Hodgkin's lymphomas. Cancer and Leukemia Group B study 7851. Cancer. 1990;66:1888–1896. doi: 10.1002/1097-0142(19901101)66:9<1888::aid-cncr2820660906>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 69.Taylor PR, White JM, Prescott RJ, et al. The addition of oral idarubicin to a chlorambucil/dexamethasone combination has a significant impact on time to treatment failure but none on overall survival in patients with low grade non-Hodgkin's lymphoma: results of the Scotland and Newcastle Lymphoma Group randomized NHL VIII trial. Leuk Lymphoma. 2006;47:2321–2330. doi: 10.1080/10428190600881256. [DOI] [PubMed] [Google Scholar]
- 70.Haynes A, McMillan A, Jack A, et al. A prospective, randomised trial of chlorambucil, mitoxantrone and dexamethasone (CMD) versus fludarabine, mitoxantrone and dexamethasone (FMD) for advanced follicular lymphoma (real grades I–III, stages III/IV) ASH Annual Meeting Abstracts. 2006;108 (Abstr 534) [Google Scholar]
- 71.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 72.Peterson BA, Petroni GR, Frizzera G, et al. Prolonged single-agent versus combination chemotherapy in indolent follicular lymphomas: a study of the cancer and leukemia group B. J Clin Oncol. 2003;21:5–15. doi: 10.1200/jco.2003.05.128. [DOI] [PubMed] [Google Scholar]
- 73.Smalley RV, Weller E, Hawkins MJ, et al. Final analysis of the ECOG I-COPA trial (E6484) in patients with non-Hodgkin's lymphoma treated with interferon alfa (IFN-alpha2a) plus an anthracycline-based induction regimen. Leukemia. 2001;15:1118–1122. doi: 10.1038/sj.leu.2402161. [DOI] [PubMed] [Google Scholar]
- 74.Unterhalt M, Herrmann R, Tiemann M, et al. Prednimustine, mitoxantrone (PmM) vs cyclophosphamide, vincristine, prednisone (COP) for the treatment of advanced low-grade non-Hodgkin's lymphoma. Leukemia. 1996;10:836–843. [PubMed] [Google Scholar]
- 75.Solal-Celigny P, Lepage E, Brousse N, et al. Recombinant interferon alfa-2b combined with a regimen containing doxorubicin in patients with advanced follicular lymphoma. N Engl J Med. 1993;329:1608–1614. doi: 10.1056/NEJM199311253292203. [DOI] [PubMed] [Google Scholar]
- 76.Herold M, Schulze A, Niederwieser D, et al. Bendamustine, vincristine and prednisone (BOP) versus cyclophosphamide, vincristine and prednisone (COP) in advanced indolent non-Hodgkin's lymphoma and mantle cell lymphoma: results of a randomised phase III trial (OSHO# 19) J Cancer Res Clin Oncol. 2006;132:105–112. doi: 10.1007/s00432-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 77.Kalinka-Warzocha E, Wajs J, Lech-Maranda E, et al. Randomized comparison of cladribine alone or in combination with cyclophosphamide, and cyclophosphamide, vincristine and prednisone in previously untreated low-grade B-cell non-Hodgkin lymphoma patients: final report of the polish lymphoma research group. Cancer. 2008;113:367–375. doi: 10.1002/cncr.23558. [DOI] [PubMed] [Google Scholar]
- 78.Foussard C, Colombat P, Gressin R, et al. Long-term follow-up of a randomized trial of fludarabine-mitoxantrone, compared with cyclophosphamide, doxorubicin, vindesine, prednisone (CHVP), as first-line treatment of elderly patients with advanced, low-grade non-Hodgkin's lymphoma before the era of monoclonal antibodies. Ann Oncol. 2005;16:466–472. doi: 10.1093/annonc/mdi091. [DOI] [PubMed] [Google Scholar]
- 79.Tsimberidou AM, McLaughlin P, Younes A, et al. Fludarabine, mitoxantrone, dexamethasone (FND) compared with an alternating triple therapy (ATT) regimen in patients with stage IV indolent lymphoma. Blood. 2002;100:4351–4357. doi: 10.1182/blood-2001-12-0269. [DOI] [PubMed] [Google Scholar]
- 80.Coiffier B, Neidhardt-Berard EM, Tilly H, et al. Fludarabine alone compared to CHVP plus interferon in elderly patients with follicular lymphoma and adverse prognostic parameters: a GELA study. Groupe d'Etudes des Lymphomes de l'Adulte. Ann Oncol. 1999;10:1191–1197. doi: 10.1023/a:1008347425795. [DOI] [PubMed] [Google Scholar]
- 81.Arranz R, Garcia-Alfonso P, Sobrino P, et al. Role of interferon alfa-2b in the induction and maintenance treatment of low-grade non-Hodgkin's lymphoma: results from a prospective, multicenter trial with double randomization. J Clin Oncol. 1998;16:1538–1546. doi: 10.1200/JCO.1998.16.4.1538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.