Summary
We examined the effects of gestational cocaine treatment on oxytocin levels in the whole hippocampus (HIP), ventral tegmental area (VTA), medial preoptic area (MPOA) and amygdala (AMY) in rat dams on postpartum days (PPDs) 1 and 2. Cocaine treatment significantly reduced oxytocin levels in the MPOA within 12–16 h of delivery (PPD 1), but had no significant effect on the other brain areas. Oxytocin was significantly reduced in the HIP and VTA but not in the AMY or MPOA on PPD 2. These data provide the first evidence for the reduction of oxytocin levels in the VTA, HIP and MPOA as a result of gestational cocaine treatment.
INTRODUCTION
When centrally administered, the neuropeptide oxytocin (OXY) rapidly induces specific species typical components of maternal behavior in rats such as nest building, pup retrieval and grouping, cleaning and licking of pups and crouching over pups in a nursing posture.1-3 Intracerebroventricular (i.c.v.) infusions of oxytocin antagonists or antiserum block or delay the onset of maternal behavior of parturient rats.4-6 It has also been shown that oxytocin infused into the central nucleus of the amygdala of hamsters markedly increases postpartum aggression.7 All of these reports support the hypothesis that the onset of maternal behavior, and subsequently maternal aggression, is at least partially regulated by oxytocin.
We and others,8-10 have reported that, when given throughout gestation, cocaine, a commonly abused drug,11 and dopamine and serotonin reuptake inhibitor,12 delays the initial activation of maternal behavior of rat dams and increases maternal aggression8,13 towards a male or female intruder.
We have hypothesized that cocaine, acting through dopaminergic and/or serotonergic systems, alters oxytocin neurotransmission, which in turn affects maternal behavior and maternal aggression.8,14 Oxytocin levels were found to be significantly reduced in the amygdala (AMY) of the aggressive cocaine-treated rat dams at 8 days postpartum.15 Conversely, elevated oxytocin levels were found in the AMYs of acutely treated unaggressive rat dams on postpartum days (PPDs) 6 and 10.16 These data support our hypothesis that cocaine is affecting at least one aspect of maternal behavior (aggression) through alterations of the oxytocinergic system in the AMY, a brain area thought to be involved in maternal aggressive behavior.7,8,15,16
Most recently, oxytocin has been shown to play a critical role in postpartum activation of maternal behavior in the ventral tegmental area (VTA) and medial preoptic area (MPOA).17 These brain areas, as well as the hippocampus (HIP), contain oxytocinergic, dopaminergic and/or serotonergic receptors, neurons or pathways, which have been strongly implicated in the regulation of maternal behavior.15,18-20
In the present study we assessed the effects of chronic gestational cocaine treatment on oxytocin levels in the AMY, MPOA, VTA and HIP on PPDsl and 2, a time when oxytocin is thought to play an important role in the initiation of maternal behaviors other than maternal aggression.6,17,18,21
METHODS
General
Gravid, nulliparous Sprague-Dawley rats (Charles River, Raleigh, NC) (250–275 g) received subcutaneous (s.c.) injections, twice daily at approximately 08:00 and 16:00 throughout gestation (gestation days [GDs] 1–20), normal saline (0.9%), or 15 mg/kg cocaine hydrochloride (HCL; Sigma, St Louis, MO) dissolved in normal saline. Rats were randomly assigned to the treatment groups or as a surrogate (no treatment) the morning a sperm plug was found (GD 0). Dams were housed singly on a 12:12-h reversed light–dark cycle with lights out at 09:00. Treatment and surrogate dams all had free access to food (rat chow) and water. Daily weight gain was measured for treatment subjects. Immediately following completion of delivery, the litter weight, pup sizes and number were recorded, and the natural litters were given to a surrogate. Each treatment dam was then given 4 surrogate pups (2 of each sex) born within 12–24 h of her own delivery. At approximately 09:00 on the first morning (PPD 1) following delivery (delivery generally occurred between 15:00 and 18:00, half the subjects in each treatment group were killed by decapitation. This procedure was repeated with the remaining subjects from both treatment groups on the second morning following delivery (PPD 2), also at 09:00. Immediately after decapitation, the whole HIP, MPOA, AMY and VTA of each subject were dissected out on ice, weighed, rapidly frozen and stored at −70°C for later radioimmunoassay. Procedures involving animals and their care were approved by, and conducted in conformity with, the institutional guidelines, which are in compliance with national and international laws and policies (NIH guide for the care and use of laboratory animals, NIH publication no. 85-23, 1985). University veterinarians supervised the care and use of these animals and no unhealthy animals were employed in this study.
Dissection
Briefly, brains were coronally sectioned from the ventral side rostral to the optic chiasm [approximately A 7100 μm according to Konig & Klippel22] and just caudal to the optic chiasm (approximately A 5800 μm) to define the preoptic-anterior hypothalamic area. Vertical cuts ventral from the lines of the lateral ventricles and a horizontal slice through the anterior commissure were made to produce a block section of the MPOA. Brains were sectioned once again just caudal to the tuber cinereum (approximately A 3800 μm) and slightly above the cerebellum, and the AMY was removed in this section. The whole HIP was then removed from the caudal remainder of the brain, and the VTA was dissected from this portion by making dorsoventral cuts medial to the optic tracts with a dorsal cut at the ventral extent of the central gray.
Radioimmunoassay
Brain tissues were homogenized in cold buffer (19 mM monobasic sodium phosphate, 81 mM dibasic sodium phosphate, 0.05 M NaCl, 0.1% BSA, 0.1% Triton × 100, 0.1 % sodium oxide at pH 7.4) and centrifuged at 3000 × g for 30 min. Supernatants were analyzed for OXY immunoreactive content according to a protocol from Peninsula Laboratories, Inc. (Belmont, CA) for each brain area/animal. All supplies were obtained from Peninsula Labs.
Briefly, oxytocin-like immunoreactivity was assayed by incubating samples and standards (0.5–500 pg) in duplicate for 16–24 h at 0°C, with rabbit anti-oxytocin serum (Peninsula Lab, Belmont, CA, lot 023204-2) at a final dilution of 1:39 000. The assay samples and standards were then incubated with 125 I-oxytocin (specific activity 1286 Ci/mmol) for 16–24 h at 0°C. The bound oxytocin was separated from free by incubating with normal rabbit serum and goat anti-rabbit IgG serum and centrifugation for 40 min at 2500 rpm. Radioactivity was measured by an LKB Clinigamma counter which estimates B/Bo from the standard curve and estimates pg amounts from that determination for each sample. Cross-reactivity of arginine vasopressin and somatostatin was undetectable. The sensitivity of the assay was 13 pg/tube. An intra-assay coefficient of variation (CV) of <5% and an inter-assay CV of <14% at approximately 50% binding were determined. Oxytocin levels were analyzed by analyses of variance (ANOVA) followed by post-hoc tests comparing pg/mg wet tissue weight between groups on PPDs 1 and 2.
RESULTS
There were no significant differences between treatment groups in gestational weight gain, number of live pups, grams per pup or days of gestation (Table).
Table.
Gestational measures for chronic cocaine- and saline-treated rats
| Grp | Litters | Gestational dam weight gain (g) |
Gestation length (days) |
Live pups/ litter |
Pup weight (g) |
|---|---|---|---|---|---|
| Cocaine (SE) |
17 | 157.0 ±6.3 |
21.0 ±0.00 |
13.9 ±0.72 |
6.4 ±0.13 |
| Saline (SE) |
19 | 144.0 ±6.7 |
21.0 ±0.00 |
14.5 ±0.68 |
6.4 ±0.14 |
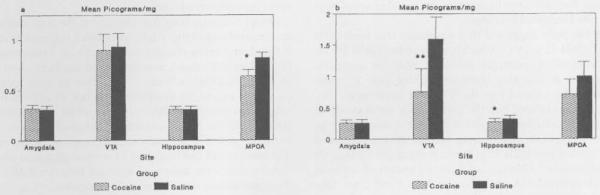
Gestational cocaine treatment significantly reduced oxytocin levels (pg/mg) in the medial preoptic area on PPD 1, (F(1, 14) = 4.59, P < 0.05, Figure) compared with saline-treated animals. Oxytocin levels in the VTA, HIP and AMY were not significantly different between groups, although cocaine treatment resulted in slightly lower levels of oxytocin in the ventral tegmental area. On PPD 2, oxytocin levels were significantly reduced in the VTA (F (1, 17) = 5.96, P < 0.03) and HIP (F (1, 18) = 9.87, P < 0.01) of cocaine-treated dams compared with saline-treated dams. Oxytocin levels were reduced, though not quite significantly, in the MPOA of cocaine-treated dams and there was no difference between cocaine- and saline-treated dams, on oxytocin levels in the AMY (Figure).
Figure.
(A) The effect of chronic cocaine on mean levels of oxytocin (p/mg) in the AMY, VTA, HIP and MPOA on PPD 1. There were no significant differences between saline (n = 8) and cocaine (n = 8) treatment groups in the AMY (P < 0.86), HIP (P < 0.84) or VTA (P < 0.94; n = 7 cocaine, n = 8 saline). Levels of oxytocin were significantly reduced In the MPOA of the cocaine (n = 8 per group)-treated females (0.64 ± 0.06) compared with saline-treated females (0.82 ± 0.06; * P < 0.05, ANOVA). (B) The effect of chronic cocaine on mean levels of oxytocin (p/mg) in the AMY, VTA, MPOA and HIP on PPD2. There were no significant differences between saline (n = 9) and cocaine (n = 11) treatment groups in the amygdala (P < 0.93) or MPOA (P < 0.24). Levels of oxytocin were significantly reduced in the HIP (0.27 ± 0.01) and VTA (0.75 ± 0.24) of cocaine-treated females, compared with saline-treated females ([0.32 ± 0.01) HIP, *P < 0.01; [1.59 ± 0.25] VTA, **P < 0.03, (ANOVA).
DISCUSSION
We have demonstrated that chronic gestational cocaine treatment reduces oxytocin in brain sites thought to play a critical role in the initiation of maternal behavior at a time when oxytocin is thought to have a substantial impact on those behaviors. This is the first report showing that cocaine treatment reduces oxytocin in the MPOA, HIP and VTA at about the same time that early postpartum maternal behavior is altered by this same dose of cocaine (30 mg/kg). That gestational cocaine treatment did not reduce oxytocin in the AMY during this early postpartum period is consistent with the behavioral data. During the first 12–17 h following parturition, rat dams normally experience postpartum estrus and are usually not as aggressive towards a male intruder as they are during PPDs 3–12. We have previously reported that chronic gestational cocaine treatment, using the same dose and regimen that was employed in this study, both increased aggression towards an intruder and reduced oxytocin in the AMY on PPD 8.15 Recent studies16,23 have determined that acute, unlike chronic, cocaine treatment reduces postpartum maternal aggression. In one of these studies,16 oxytocin levels were significantly elevated in the AMY of the least aggressive rat dams. These correlational data support our hypodiesis that cocaine treatment alters maternal behavior at least partially through its effects on the oxytocin system. These results, taken together, suggest that oxytocin is involved in alterations of maternal aggression.
Several studies,14,24 have previously reported that acute and repeated cocaine treatment reduces oxytocin in the HIP of non-pregnant rats. Lesions of the dorsal HIP in postpartum rats have been shown to significandy disrupt maternal behaviors such as crouching, nest building and grouping of pups.19 We have reported that both crouching and nest building are disrupted or delayed in rat dams treated throughout gestation with cocaine.8 We have not, as yet, found a reduction in oxytocin levels in the HIP on PPDs 8 or 10 as a result of either acute postpartum or chronic gestational cocaine treatment at the time when maternal aggression is altered by cocaine. These findings suggest that the HIP may not be as important a site as the AMY in the regulation of maternal aggression. The present findings suggest that if behaviors are affected by the reduction of oxytocin in the HIP of cocaine-treated dams, they are more likely to be the initial onset of pup-directed maternal behaviors.
It has been suggested that a pathway that leads from the MPOA to the VTA, which appears to be critical for the expression of maternal behavior, may be oxytocinergic.25,26 It has also been determined that oxytocin antagonists infused into the VTA and oxytocin and VI antagonists infused into the MPOA block the initiation of maternal behavior.17 Oxytocin binding is also higher in both these brain areas during mid-parturition compared with other pre- (GDs 15–17) and postpartum (PPDs 5–7) time periods.17 Although we have not examined oxytocin levels in the MPOA other than on PPDs 1 and 2, we have found no cocaine-induced reductions (acute or chronic) in the VTA from PPDs 6–10. We have reported slight elevations of oxytocin in the VTA following acute cocaine treatment of ovariectomized, estrogen-treated rats.4
While it is not yet clear where the oxytocin neurons that project to the MPOA and VTA lie, the dorsolateral preoptic area, rostral paraventricular nucleus and anterior commissural nucleus have been implicated.27 We have previously suggested a possible neurochemical pathway by which cocaine may be interacting with the oxytocinergic system.8,14 The ascending mesolimbic dopaminergic system (A10), which originates in the VTA, projects to both the HIP and the AMY.28 Receptors that bind oxytocin, dopamine or cocaine are found in several brain structures of the limbic system including the VTA, HIP and AMY.20,28-31 Cocaine, a dopaminergic (as well as serotonergic and noradrenergic) reuptake inhibitor, may alter oxytocin levels indirectly via its actions on the dopaminergic system in the VTA32,33 Manipulations of the dopaminergic system have been reported to alter some of the same behaviors that are altered by both cocaine and oxytocin.34,35 However, since gestational cocaine treatment alters maternal behavior and only slightly increases locomotor and stereotyped behaviors, the dopaminergic system is probably not the only neurotransmitter system implicated in the effects of cocaine on maternal behavior.
The present findings provide further support for a cocaine–oxytocin interaction which appears to be mediated, in some part, through oxytocin levels being altered in structures (VTA, HIP) that are a part of the dopaminergic mesolimbic pathway. Changes in oxytocin levels first appear in the MPOA, then soon afterward in the VTA and HIP. It is not yet clear how the MPOA fits into the overall scheme, but determination of the location of the oxytocin neurons projecting to the MPOA should be helpful. Cocaine could be acting directly on oxytocin neurons in the MPOA or acting through dopamine receptors which alter oxytocin levels. There are also both dopaminergic and noradrenergic projections from the VTA to the MPOA, through which cocaine could have a secondary affect on oxytocin release.36
We are not suggesting that oxytocin is the only important neurochemical that might be involved in the cocaine-induced disruption of maternal behavior. A vasopressin (VI) antagonist infused into the MPOA has been shown to block maternal behavior.17 The effect of gestational cocaine treatment on vasopressin has not, to our knowledge, been elucidated. Others have reported that opioids infused into the MPOA can inhibit maternal behavior.37 Prolactin, which is normally under tonic inhibition by dopamine, could also be altered by cocaine treatment and thereby alter some aspects of maternal behavior.38 All of these possibilities require further investigation.
Future experiments will include the examination of cocaine-related changes in oxytocin, dopamine and serotonin receptor binding in these, and perhaps other, brain structures. We hope to determine soon if an oxytocin antagonist infused into the amygdala on PPDs 6–10 alters maternal aggression as does cocaine. It will also be important to determine through which neurotransmitter systems cocaine exerts its effects to alter oxytocin levels and/or receptors. These findings have implications for our understanding of how cocaine and oxytocin interact at a time when oxytocin appears to be necessary for the initiation of normal maternal behavior.
ACKNOWLEDGEMENTS
This study was supported by the National Institutes on Drug Abuse (grant #R29–DA08456 to J.M.J.), an NIH drug abuse predoctoral training grant #DA 07244 (D.A.L.) and the Stanley Foundation (K.E.M.). Assistance with the assays was provided by the Mental Health Clinical Research Center of the Psychiatry Department.
REFERENCES
- 1.Pedersen CA, Prange AJ., Jr. Induction of maternal behavior in virgin rats after intracerebroventrioilar administration of oxytocin. Proc Natl Acad Sci USA. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr. Oxytocin induces maternal behaviour in virgin female rats. Science. 1982;216:648–649. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 3.Fahrbach SE, Morrell JI, Pfaff DW. Oxytocin induction of short-latency maternal behavior, in nulliparous, estrogen-primed female rats. Horm Behav. 1984;18:267–286. doi: 10.1016/0018-506x(84)90016-3. [DOI] [PubMed] [Google Scholar]
- 4.Fahrbach SE, Morrell JI, Pfaff DW. Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology. 1985;40:526–532. doi: 10.1159/000124125. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ., Jr. Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides. 1985;6:175–182. doi: 10.1016/0143-4179(85)90108-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Leengoed E, Kerker E, Swanson HH. Inhibition of postpartum maternal behavior in the rat by injecting an oxytocin antagonist into the cerebral ventricles. J Endocrinol. 1987;112:275–282. doi: 10.1677/joe.0.1120275. [DOI] [PubMed] [Google Scholar]
- 7.Ferris CF, Foote KB, Meltser HM, Plenby MG, Smith KL, Insel TR. Pedersen CA, Caldwell JD, Jirikowski GF, Insel TR, editors. Oxytocin in the amygdala facilitates maternal aggression. Oxytocin in maternal, sexual, and social behaviors Ann NY Acad Sci. 1992;652:456–457. doi: 10.1111/j.1749-6632.1992.tb34382.x. [DOI] [PubMed] [Google Scholar]
- 8.Johns JM, Zimmerman LI, Noonan LR, Li L, Pedersen CA. Effects of chronic and acute cocaine treatment on maternal behavior and aggression. Behav Neurosci. 1994;108:107–112. doi: 10.1037//0735-7044.108.1.107. [DOI] [PubMed] [Google Scholar]
- 9.Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47:857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 10.Nelson CJ, Ayers A, Meter KE, Walker CH, Johns JM. Chronic cocaine treatment alters maternal behavior in a dose response manner in Sprague Dawley rats. Soc Neurosci Abs. 1996;22:738.8. [Google Scholar]
- 11.Chasnoff IJ, Burns WJ, Schnoll SH, Burns KA. Cocaine use in pregnancy. N Engl J Med. 1985;313:666–669. doi: 10.1056/NEJM198509123131105. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn CM, Little PJ. Neuroendocrine effects of cocaine. In: Hammer RP, editor. The neurobiology of cocaine: cellular and molecular mechanisms. CRC Press; Boca Raton: 1995. pp. 49–63. [Google Scholar]
- 13.Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurobehav Toxicol Teratol. 1992;14:415–422. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- 14.Johns JM, Caldwell JD, Pedersen CA. Acute cocaine treatment decreases oxytocin levels in the rat hippocampus. Neuropeptides. 1993;24:165–169. doi: 10.1016/0143-4179(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 15.Johns JM, Faggin BM, Noonan LR, Li L, Zimmerman LI, Pedersen CA. Chronic cocaine treatment decreases oxytocin levels in the amygdala and increases maternal aggression in Sprague-Dawley rats. Soc Neurosci Abs. 1995;21 #1954. [Google Scholar]
- 16.Johns JM, Ayers A, Couch CD, Nelson CJ, Meter KE, Walker CH. Acute cocaine treatment decreases maternal aggression in a dose response manner in Sprague-Dawley rats. Soc Neurosci Abs. 1996;22 #738.7. [Google Scholar]
- 17.Pedersen CA, Caldwell JD, Walker CH, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:107–112. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 18.Fahrbach SF, Morrell JI, Pfaff DW. Role of oxytocin in onset of estrogen-facilitated maternal behavior. In: Amico J, Robinson AG, editors. Oxytocin: clinical and laboratory studies. Elsevier; Amsterdam: 1985. pp. 372–388. [Google Scholar]
- 19.Kimble DP, Rogers I, Hendrickson CW. Hippocampal lesions disrupt maternal, not sexual behavior in the albino rat. J Comp Physiol Psychol. 1967;63:401–407. doi: 10.1037/h0024605. [DOI] [PubMed] [Google Scholar]
- 20.Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]
- 21.Insel TR, Harbaugh CR. Lesions of the hypothalamic paraventricular nucleus disrupt the initiation of maternal behavior. Physiol Behav. 1989;45:1033–1041. doi: 10.1016/0031-9384(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 22.Konig JFR, Klippel RA. The rat brain: a stereotaxic atlas of the forebrain and lower parts of the brain stem. Krieger; New York: 1963. [Google Scholar]
- 23.Vernotica EM, Rosenblatt JS, Morrell JI. Acute cocaine alters all components of established postpartum maternal behavior in the rat. Soc Neurosci Abs. 1996;22 #738.6. [Google Scholar]
- 24.Sarnyai Z, Biro E, Babarczy E, et al. Oxytocin modulates behavioural adaptation to repeated treatment with cocaine in rats. Neuropharmacology. 1992;31:593–598. doi: 10.1016/0028-3908(92)90192-r. [DOI] [PubMed] [Google Scholar]
- 25.Numan M, Smith Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 26.Numan M. Maternal behavior. In: Knobil F, Neill JD, editors. The physiology of reproduction. 2nd ed. Raven Press; New York: 1994. pp. 264–275. [Google Scholar]
- 27.Jirikowski GF, Caldwell JD, Pedersen CA, Strumpf WE. Estradiol influences oxytocin-immunoreactive brain systems. Neuroscience. 1988;25:237–248. doi: 10.1016/0306-4522(88)90022-x. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. 7th ed. Oxford University Press; New York: 1996. [Google Scholar]
- 29.Insel TR. Oxytocin – A neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- 30.Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, Ricliard P. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- 31.Tribollet E, Charpak S, Schmidt A, Dubois-Dauphin M, Dreifuss JJ. Appearance and transient expression of oxytocin receptors in fetal, infant and peripubertal rat brain studied by autoradiography and electrophysiology. J Neurosci. 1989;9:1764–1773. doi: 10.1523/JNEUROSCI.09-05-01764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodie MS, Dunwiddie TV. Cocaine effects in the ventral tegmental area: evidence for an indirect dopaminergic mechanism of action. Arch Pharmacol. 1990;342:660–665. doi: 10.1007/BF00175709. [DOI] [PubMed] [Google Scholar]
- 33.Sarnyai Z, Babarczy E, Krivan M, et al. Selective attenuation of Cocaine-induced stereotyped behaviour by oxytocin: putative role Of basal forebrain target sites. Neuropeptides. 1991;19:51–56. doi: 10.1016/0143-4179(91)90073-r. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs GL, Faludi M, Falkay G, Telegdy G. Peripheral oxytocin treatment modulates central dopamine transmission in the mouse limbic structures. Neurochem Int. 1986;9:481–485. doi: 10.1016/0197-0186(86)90138-5. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs GL, Sarnyai Z, Babarczi E, Szabo G, Telegdy G. The role of oxytocin–dopamine interactions in cocaine-induced locomotor hyperactivity. Neuropharmacology. 1990;29:365–368. doi: 10.1016/0028-3908(90)90095-9. [DOI] [PubMed] [Google Scholar]
- 36.Willis WD, Grossman RG. Medical neurobiology. 3rd ed. C. V. Mosby; St Louis: 1981. p. 429. [Google Scholar]
- 37.Bridges RS. Endocrine regulation of parental behavior in rodents. In: Krasnegor NA, Bridges RS, editors. Mammalian parenting. Oxford University Press; Oxford: 1990. pp. 93–117. [Google Scholar]
- 38.Bridges RS, Mann PE. Prolactin–brain interactions in the induction of maternal behavior in rats. Psychoneuroendocrinology. 1994;19:611–622. doi: 10.1016/0306-4530(94)90045-0. [DOI] [PubMed] [Google Scholar]