Abstract
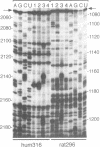
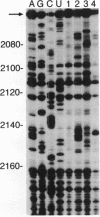
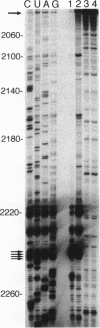
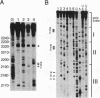
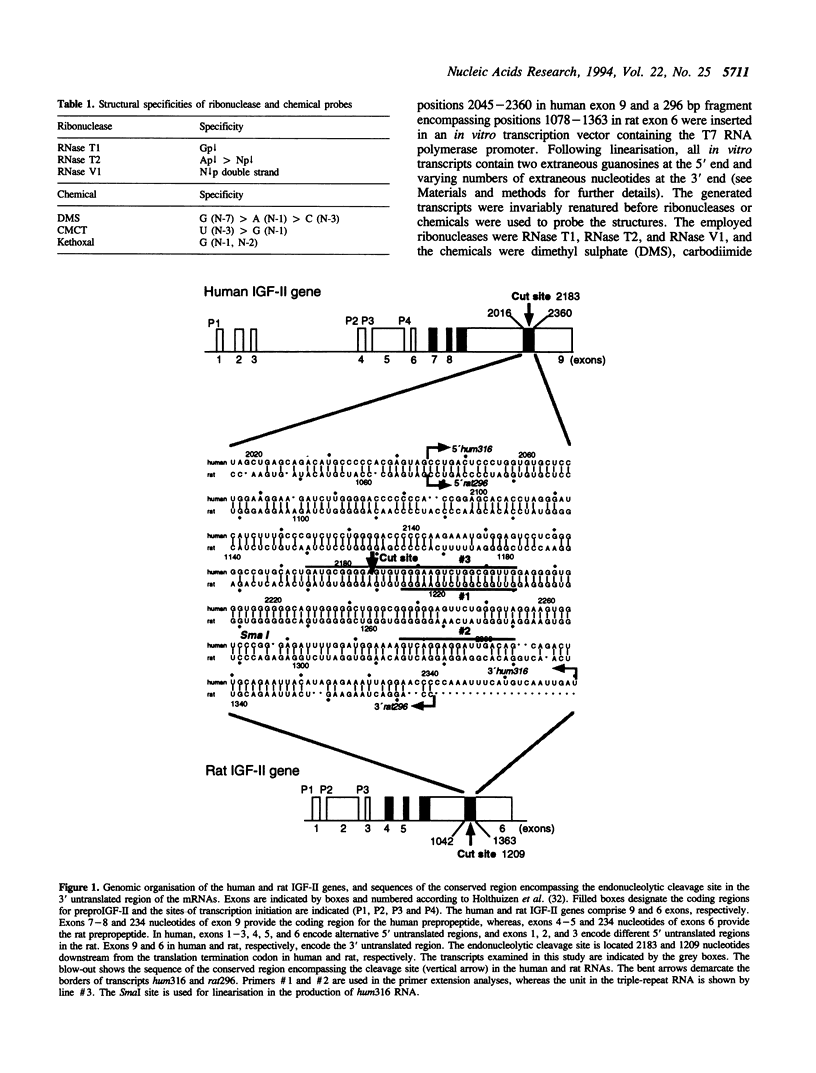
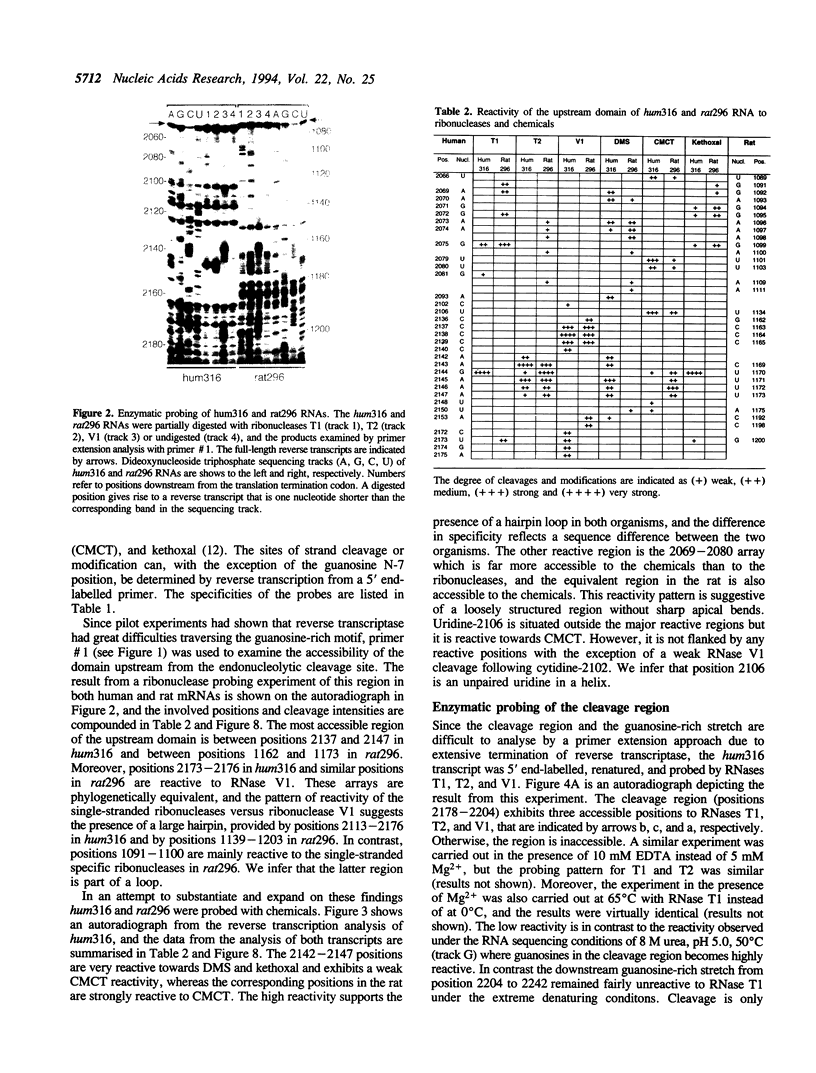
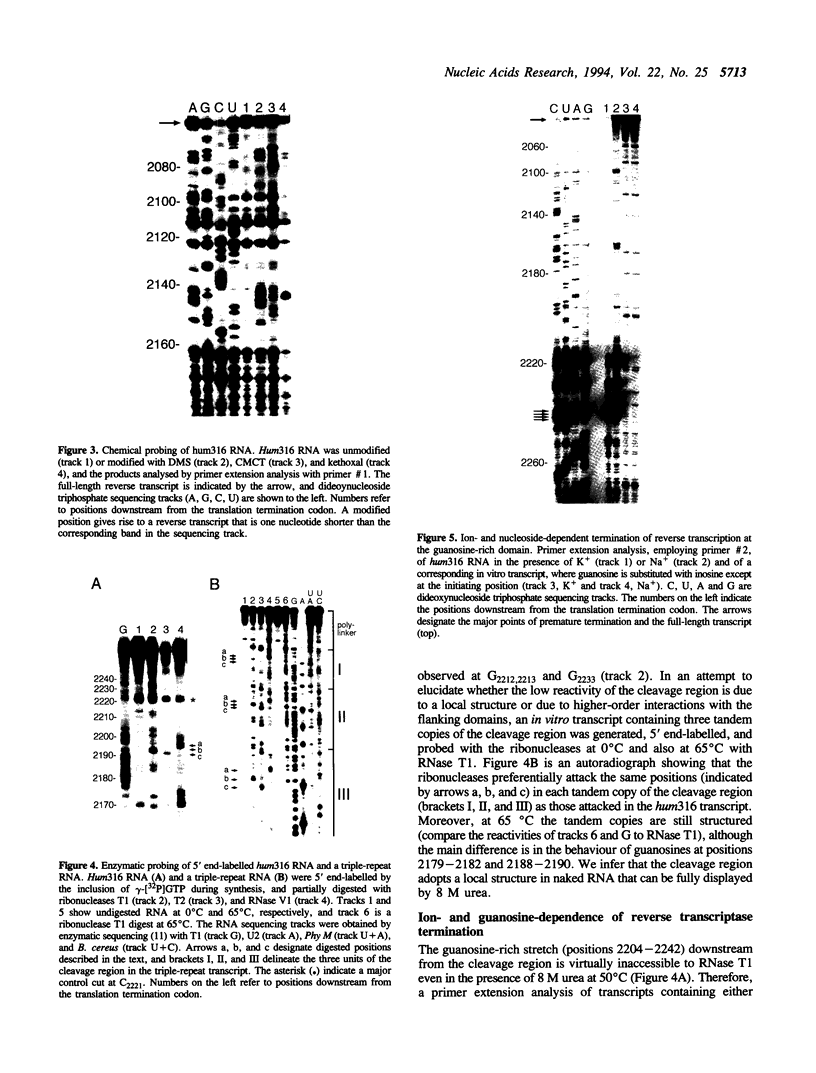
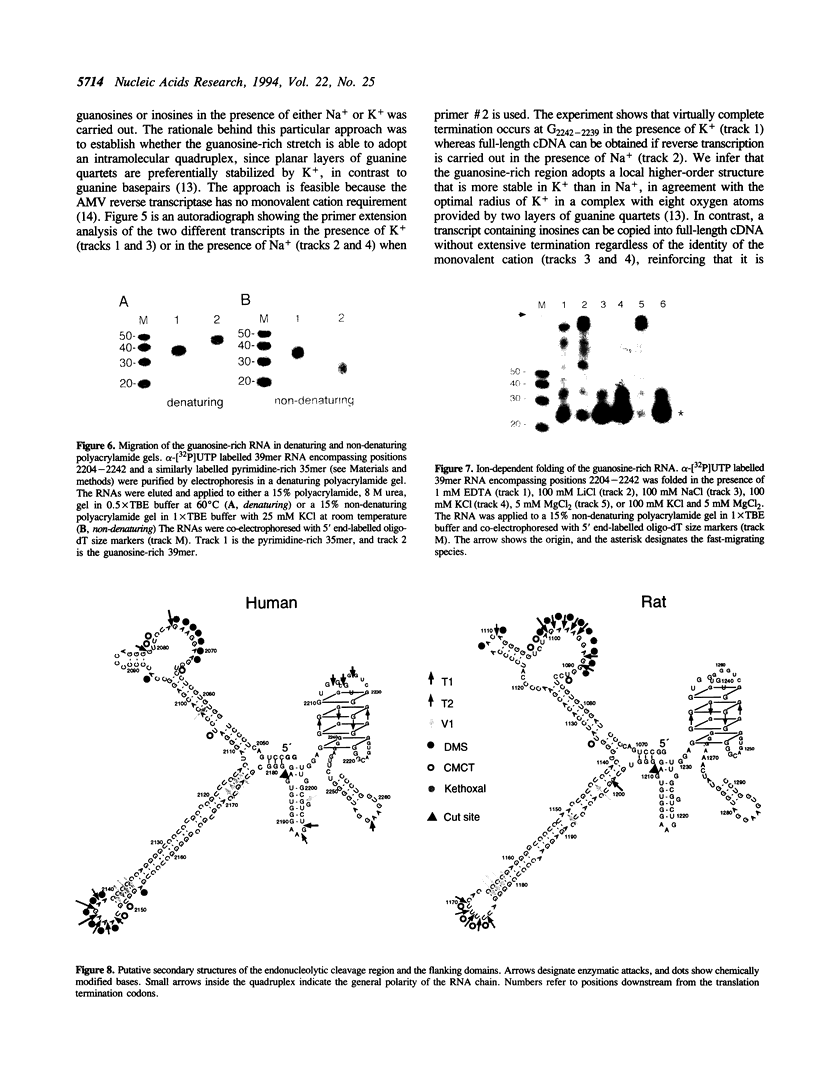
Insulin-like growth factor II (IGF-II) mRNAs are cleaved by an endonucleolytic event in a conserved part of their 3' untranslated region that is predicted to exhibit a complex higher-order RNA structure. In the present study, we have examined the putative secondary structures of in vitro transcripts from the conserved part of human and rat mRNAs by enzymatic and chemical probing. The results show that the cleavage site is situated between two highly structured domains. The upstream domain consists of two large hairpins, whereas the downstream domain is guanosine-rich. The guanosine-rich domain adopts a compact unimolecular conformation in Na+ or K+ but not in Li+, and it completely arrests reverse transcription in K+ but only partially in Na+, indicating the presence of an intramolecular guanosine quadruplex. The flanking higher-order structures may ensure that the cleavage site is not sequestered in stable RNA structures, thus allowing interactions with RNA or proteins at posttranscriptional stages of IGF-II expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder R., Horowitz J. A., Basilion J. P., Koeller D. M., Klausner R. D., Harford J. B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3' UTR and does not involve poly(A) tail shortening. EMBO J. 1994 Apr 15;13(8):1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. D., Zipkin I. D., Harland R. M. Sequence-specific endonucleolytic cleavage and protection of mRNA in Xenopus and Drosophila. Genes Dev. 1993 Aug;7(8):1620–1631. doi: 10.1101/gad.7.8.1620. [DOI] [PubMed] [Google Scholar]
- Cheong C., Moore P. B. Solution structure of an unusually stable RNA tetraplex containing G- and U-quartet structures. Biochemistry. 1992 Sep 15;31(36):8406–8414. doi: 10.1021/bi00151a003. [DOI] [PubMed] [Google Scholar]
- Decker C. J., Parker R. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 1993 Aug;7(8):1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann C., Baudin F., Mougel M., Romby P., Ebel J. P., Ehresmann B. Probing the structure of RNAs in solution. Nucleic Acids Res. 1987 Nov 25;15(22):9109–9128. doi: 10.1093/nar/15.22.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Parsons J. T., Beard J. W., Beard D., Green M. Mechanism of carcinogenesis by RNA tumor viruses. 3. Formation of RNA, DNA complex and duplex DNA molecules by the DNA polymerase (s) of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1432–1439. doi: 10.1073/pnas.67.3.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavis E. R., Lehmann R. Translational regulation of nanos by RNA localization. Nature. 1994 May 26;369(6478):315–318. doi: 10.1038/369315a0. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Holthuizen P., van der Lee F. M., Ikejiri K., Yamamoto M., Sussenbach J. S. Identification and initial characterization of a fourth leader exon and promoter of the human IGF-II gene. Biochim Biophys Acta. 1990 Nov 30;1087(3):341–343. doi: 10.1016/0167-4781(90)90010-y. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang X., Ratliff R., Moyzis R., Rich A. Crystal structure of four-stranded Oxytricha telomeric DNA. Nature. 1992 Mar 12;356(6365):126–131. doi: 10.1038/356126a0. [DOI] [PubMed] [Google Scholar]
- Lee R. C., Feinbaum R. L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993 Dec 3;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Liu Z., Gilbert W. The yeast KEM1 gene encodes a nuclease specific for G4 tetraplex DNA: implication of in vivo functions for this novel DNA structure. Cell. 1994 Jul 1;77(7):1083–1092. doi: 10.1016/0092-8674(94)90447-2. [DOI] [PubMed] [Google Scholar]
- Meinsma D., Holthuizen P. E., Van den Brande J. L., Sussenbach J. S. Specific endonucleolytic cleavage of IGF-II mRNAs. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1509–1516. doi: 10.1016/0006-291x(91)91743-v. [DOI] [PubMed] [Google Scholar]
- Meinsma D., Scheper W., Holthuizen P. E., Van den Brande J. L., Sussenbach J. S. Site-specific cleavage of IGF-II mRNAs requires sequence elements from two distinct regions of the IGF-II gene. Nucleic Acids Res. 1992 Oct 11;20(19):5003–5009. doi: 10.1093/nar/20.19.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. F., Uhlenbeck O. C. Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 1989;180:51–62. doi: 10.1016/0076-6879(89)80091-6. [DOI] [PubMed] [Google Scholar]
- Nielsen F. C., Christiansen J. Endonucleolysis in the turnover of insulin-like growth factor II mRNA. J Biol Chem. 1992 Sep 25;267(27):19404–19411. [PubMed] [Google Scholar]
- Nielsen F. C. The molecular and cellular biology of insulin-like growth factor II. Prog Growth Factor Res. 1992;4(3):257–290. doi: 10.1016/0955-2235(92)90023-b. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- Qian Z. W., Wilusz J. An RNA-binding protein specifically interacts with a functionally important domain of the downstream element of the simian virus 40 late polyadenylation signal. Mol Cell Biol. 1991 Oct;11(10):5312–5320. doi: 10.1128/mcb.11.10.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastinejad F., Conboy M. J., Rando T. A., Blau H. M. Tumor suppression by RNA from the 3' untranslated region of alpha-tropomyosin. Cell. 1993 Dec 17;75(6):1107–1117. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- Sachs A. B. Messenger RNA degradation in eukaryotes. Cell. 1993 Aug 13;74(3):413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Smith F. W., Feigon J. Quadruplex structure of Oxytricha telomeric DNA oligonucleotides. Nature. 1992 Mar 12;356(6365):164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Heaphy S. Evidence for interstrand quadruplex formation in the dimerization of human immunodeficiency virus 1 genomic RNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3393–3397. doi: 10.1073/pnas.90.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993 Dec 3;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Williamson J. R., Cech T. R., Prescott D. M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991 Apr 25;350(6320):718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- Zuker M. Computer prediction of RNA structure. Methods Enzymol. 1989;180:262–288. doi: 10.1016/0076-6879(89)80106-5. [DOI] [PubMed] [Google Scholar]