Abstract
We present a 74-year old woman with inherited myoclonus-dystonia, with predominant myoclonus and a novel mutation in the ε-sarcoglycan gene. The patient reports a life-long history of rapid, jerking movements, most severe in the upper extremities as well as a postural and action tremor. Bilateral deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus was performed, and the patient demonstrated moderate clinical improvement in myoclonus. We studied the effects on myoclonus and tremor of varying DBS frequency and amplitude. The frequency tuning curve for myoclonus was similar to that of tremor, suggesting similar mechanisms by which DBS alleviates both disorders.
Keywords: stimulation frequency, stimulation amplitude, epsilon-sarcoglycan gene, thalamus, movement disorders
INTRODUCTION
Myoclonus refers to sudden, shock-like involuntary movements associated with brief bursts of muscle activity. Myoclonus is commonly inherited as an autosomal dominant trait, termed inherited myoclonus-dystonia (M-D) [1]. Clinical characteristics of M-D include myoclonic jerks affecting the neck, shoulders, and arms, and dystonic symptoms such as torticollis [1]. The M-D phenotype is clinically heterogeneous with variable expression of myoclonus and dystonia [1], and many cases are due to mutations in the ε-sarcoglycan (SGCE) gene [2, 3]. M-D has an onset in childhood or adolescence, a benign clinical course, and occurs in the absence of other neurological deficits [1, 4, 5].
Deep brain stimulation (DBS) is an effective treatment for several movement disorders. DBS of the internal segment of the globus pallidus is effective for generalized dystonia, [6–8] and alleviates dystonia and myoclonus in M-D [9–11]. Amelioration of myoclonic, but not dystonic, symptoms of M-D has been reported with DBS of the ventral intermediate nucleus (Vim) of the thalamus. In one case, a 61-year old man, involuntary movements were reduced by 65% with unilateral stimulation [12] and by 80% with bilateral stimulation [13], and in a second case myoclonus was reduced by 90% with unilateral thalamic stimulation in a 21-year old man [14].
We present a patient with an action and postural tremor and inherited M-D, with severe myoclonus and mild action-induced dystonia, resulting from a novel mutation in the SGCE gene. We quantify the effects of the frequency and amplitude of bilateral Vim DBS ON myoclonus and tremor. Tremor was suppressed and myoclonus was moderately controlled.
CASE REPORT
This was a 74-year old woman with a history of tremor and myoclonic jerks. Her myoclonus was most prominent in her head and upper extremities. In addition to prominent action myoclonus, she exhibited bilateral postural and action tremor in the upper extremities. Over the last five years, she had moderate worsening of symptoms including a pronounced and constant jerking of her mouth, upper extremities, neck, torso, and sometimes legs. She recently lost the ability to write and feed herself. Her involuntary movements were aggravated by stress and significantly alleviated by alcohol. She was otherwise neurologically normal.
She reported minimal benefit from primidone. Multiple pharmacological treatments produced no benefit, including clonazepam, diazepam, divalproex sodium, gabapentin, levetiracetam, and tizanidine. She also failed to benefit from anti-parkinsonian therapies including ropinirole, pramipexole, and carbidopa/levodopa. Botulinum toxin, type A, resulted in neck weakness, but no clinical benefit. Physical therapy and balance exercises provided minimal relief. Due to a lack of success of treatment with medications and a decreasing ability to complete daily tasks, she sought DBS for the treatment of her disabling involuntary movements.
Genetic Analysis
Genetic testing of the patient and one of her siblings revealed a mutation in the SGCE gene, supporting the diagnosis of M-D. After obtaining informed consent, DNA was extracted from white blood cells using standard methods. All 12 exons and flanking intronic regions of the SGCE gene were amplified by PCR using previously published primers and conditions [15]. The PCR products were tested using single-strand conformation polymorphism analysis, followed by standard dideoxy cycle sequencing of observed band shifts. Mutation screening of the SGCE gene revealed a novel single basepair deletion in exon 7 (c.940delT) resulting in a frameshift with subsequent addition of a stop codon in the protein (Y313fsX318).
Three of the patient’s four siblings and her father were also diagnosed with inherited M-D, though the phenotype was markedly different in several of the relatives, with a preponderance of slower, dystonic movements in the siblings, rather than the rapid myoclonic movements. The same SGCE mutation was found in one of the affected siblings from whom DNA was available.
Deep Brain Stimulation Implant
Quadripolar electrodes (Model 3387, Medtronic, Inc.) were implanted bilaterally in Vim in a single session. The surgical procedure was performed under local anesthesia using the Leksell stereotactic frame and stereotactic MRI scanning for AC/PC localization. The electrodes were targeted for 5 mm anterior to the posterior commisure, 15 mm lateral to midline, and the dorsal-ventral target was on the AC-PC line on both sides. Electrode targeting was confirmed using microelectrode recordings which demonstrated active units in the Vim with burst firing in synchrony with the patient’s tremor [16]. A dual-channel pulse generator (Kinetra Model 7428, Medtronic, Inc.) was connected to the leads and implanted subclavicularly in the right chest wall during the same surgical procedure. Following the DBS implant, the patient did not take any medications for her myoclonus or tremor and was not taking medications during the following evaluations.
Evaluation Methods
The effect of Vim DBS ON myoclonus was evaluated using accelerometry and the Unified Myoclonus Rating Scale (UMRS) [17] at nine months post-implant. The UMRS tests were video-taped with DBS ON and 30 minutes after DBS was turned off. The videos were presented to three raters in a blinded fashion.
The effects of DBS amplitude and frequency on myoclonus and tremor in the more affected left arm were quantified using accelerometry and clinical ratings while the contralateral stimulator was turned off. Six frequencies (10, 15, 60, 90, 130, and 185 Hz) and 3 amplitudes (1.5, 3.0, and 4.5 V) were tested with the electrode geometry (5− 4+) selected during the patient’s clinical tuning session. The 18 combinations of frequency and amplitude were tested in a random order, 2–3 times each in three blocks. For each frequency-amplitude combination, stimulation was turned on, accelerometry was recorded for 20 seconds, and then stimulation was turned off for one minute before the next trial began. Trials were video-taped and presented to a rater in a blinded fashion. The frequency and amplitude of the myoclonic jerks were rated on a 0–4 scale, and the myoclonus clinical rating for each trial was the product of the frequency and amplitude scores [17]. The tremor severity was also rated on a 0–4 scale.
An accelerometer was placed on the dorsal surface of the left hand, and accelerometry data were collected while the patient held her arm in the wing-beating posture. To quantify the amplitude of myoclonus and tremor, the three accelerometry signals (ax, ay, and az) were combined into one signal ( ), and the power spectral density, which quantifies the amount of signal power at each frequency, was calculated. Subsequently, the amplitude of myoclonus in each trial was quantified by summing the signal power over frequencies from 0 to 3 Hz. This range was selected after review of video demonstrated that jerks occurred within this frequency range. Tremor amplitude was quantified by summing the signal power over frequencies from 3 to 6 Hz.
RESULTS
Myoclonus was alleviated, but not completely suppressed, and tremor was completely suppressed by intraoperative high frequency stimulation of the Vim, and the response to stimulation was immediate. Two weeks post-implant initial stimulation parameters were selected for the left (Contacts 0−1+ with 1.5 V, 60 μs, and 185 Hz) and right (Contacts 5−4+ with 2.8 V, 90 μs, and 185 Hz) electrodes, with continuous bilateral stimulation. Paresthesias in the contralateral hands were transient and myoclonic jerking was moderately controlled. Control of myoclonic jerking remained constant at 3, 5, and 9 month follow-ups with increases in stimulation amplitude (1.5 to 3.6 V on the left, 2.8 to 3.8 V on the right).
High frequency DBS reduced the clinical myoclonus. The effect of stimulation on myoclonus was immediate, and myoclonic jerking was visibly reduced with DBS ON when the arm was moving in finger-to-nose testing and when held in a sustained wing-beating posture. At nine months post-implant, the UMRS action and functional scores were 53 and 14% lower, respectively, with DBS ON as compared to DBS OFF.
Spectral analysis (fig. 1, right column) and clinical ratings were used to quantify the effects of 18 combinations of frequency and amplitude on myoclonic jerking and tremor. Spectral analysis revealed that the effect on myoclonus of stimulation amplitude was not significant (p = 0.252, ANOVA), but the effect of stimulation frequency (fig. 1d) was significant (p<0.0001, ANOVA). High frequency (90–185 Hz) stimulation suppressed (fig. 1b) the myoclonic jerking from the no stimulation condition (fig. 1a). Conversely, low frequency (10–15 Hz) stimulation increased the amplitude of the myoclonic jerks (fig. 1c). The clinical ratings of myoclonus, which were the product of the ratings for myoclonic frequency and myoclonic amplitude, revealed the same trend (fig. 1e). At low stimulation frequencies, there was a small increase in the myoclonus rating, and at high stimulation frequencies the myoclonus rating was reduced from baseline levels. Remarkably, the effects of stimulation frequency on postural tremor, quantified using accelerometry (fig. 1f) and clinical ratings (fig. 1g), paralleled the effects of DBS frequency on myoclonus.
Figure 1.
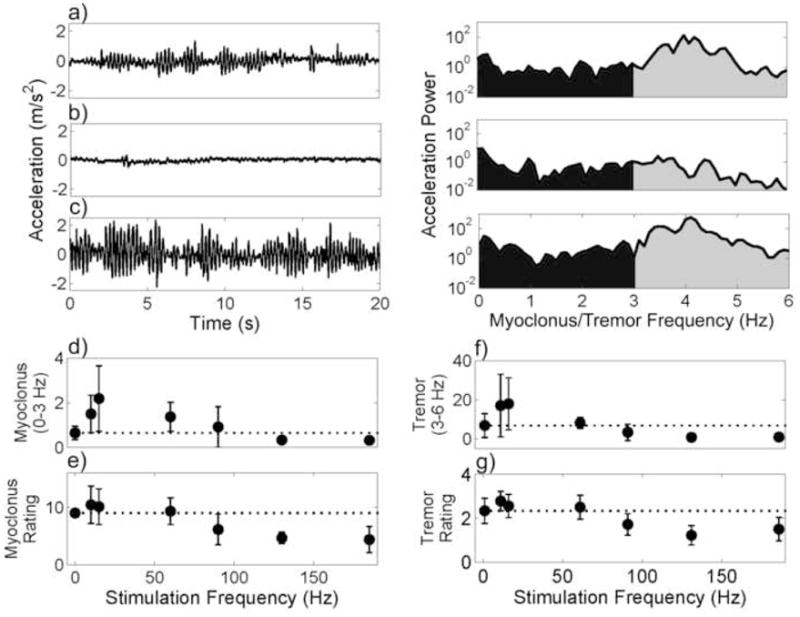
Effect of DBS amplitude and frequency on myoclonic jerking and tremor. a–c) Raw accelerometry (left) and power spectrums (right) for a) DBS OFF, b) 130 Hz, 3.0 V, 60 μs stimulation, and c) 15 Hz, 1.5 V, 60 μs stimulation. Myoclonus (d–e) and tremor (f–g) as a function of stimulation frequency, averaged across stimulation amplitudes (1.5, 3.0, and 4.5 V). Standard deviations are shown. d, f) Myoclonus and tremor were quantified by summing the acceleration power over 0–3 Hz (black) and 3–6 Hz (gray), respectively. e, g) Myoclonus and tremor were rated by a clinician blinded to the stimulation condition. The myoclonus rating (0–16) was the product of the rating for myoclonus amplitude (0–4) and frequency (0–4) [17]. Tremor was rated on a 0–4 scale.
DISCUSSION
This patient has inherited M-D and a postural and action tremor. A diagnosis of M-D was genetically supported with the identification of a novel deletion mutation in the SGCE gene. Due to a poor response to medication, bilateral Vim DBS was performed and the effect of DBS was quantified. Vim was selected as the anatomical target for this case because the patient had tremor and a myoclonus predominant M-D phenotype. Vim DBS has been shown to improve myoclonus [12–14] and is a well established treatment for tremor [18].
This case demonstrates moderate improvement in myoclonus after Vim DBS. The reduction in myoclonus has allowed the patient to feed herself, drink using a straw, and control a computer mouse, all of which she was unable to do prior to surgery. However, DBS has not completely suppressed the myoclonic jerking, and she still cannot perform certain activities that she would like, including writing legibly.
Although the mechanisms underlying myoclonus are not understood, several studies suggest that dysfunction of the Vim thalamus plays a role in the generation of myoclonic jerks. Myoclonic jerks were induced in monkeys with the injection of bicuculline into the thalamic caudal ventrolateral nuclei, the homologue of the human Vim [19]. In another study, myoclonic jerks were induced in the upper limb contralateral to stimulation in patients with Parkinson’s disease or essential tremor by low frequency (10–25 Hz) stimulation in the Vim [20].
The response of both myoclonus and tremor to DBS was immediate, and clinical variations in stimulus parameters were not able to improve further the control of the myoclonus. The effect of stimulation amplitude on myoclonus was not significant, and this is likely due to the range (1.5–4.5 V) of stimulation amplitudes tested. Amplitudes used clinically for tremor range from 1 to 3.5 V [21, 22]; the range of amplitudes tested in this study were likely all suprathreshold for the effects of DBS.
Stimulation with high frequencies (90–185 Hz) alleviated myoclonic jerking and completely suppressed tremor. Low frequency (10, 15 Hz) stimulation exacerbated both the myoclonic jerking and tremor. The mechanism through which DBS alleviates symptoms of movement disorders is unknown, but the similarity in the frequency tuning curves for tremor and myoclonus suggests common mechanisms. Computational models showed that DBS produced frequency-depended modulation of the variability in the neuronal output [23]. High frequency stimulation regularized the output of the stimulated nucleus, thereby masking the underlying pathological burst activity, and low frequency stimulation produced firing superimposed on the intrinsic pathological activity. Computational [24] and experimental data [25] indicate that pauses in thalamic inputs lead to burst responses in thalamus. The interspike pauses in low frequency stimulation may have led to additional burst activity and subsequent symptom aggravation.
Supplementary Material
Patient with inherited myoclonus-dystonia, with predominant myoclonus, and a postural and action tremor. With Vim deep brain stimulation, there is a decrease in the amplitude of the myoclonic jerks. Tremor is almost completely suppressed.
Acknowledgments
This work was supported by NIH Grant R01-NS40894.
Thanks to Dr. Burton Scott, Dr. Valerie Street and Dr. David Sommer for performing the blinded ratings.
Footnotes
Disclosure: The authors have reported no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furukawa Y, Rajput AH. Inherited myoclonus-dystonia: How many causative genes and clinical phenotypes. Neurology. 2002;59:1130–1131. doi: 10.1212/wnl.59.8.1130. [DOI] [PubMed] [Google Scholar]
- 2.Montcel STd, Clot F, Vidailhet M, Roze E, Damier P, Jedynak CP, Camuzat A, Lagueny A, Vercueil L, Doummar D, Guyant-Marechal L, Houeto JL, Ponsot G, Thobois S, Cournelle MA, Durr A, Durif F, Echenne B, Hannequin D, Tranchant C, Brice A the French Dystonia N. Epsilon sarcoglycan mutations and phenotype in French patients with myoclonic syndromes. J Med Genet. 2006;43:394–400. doi: 10.1136/jmg.2005.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimprich A, Grabowski M, Asmus F, Naumann M, Berg D, Bertram M, Scheidtmann K, Kern P, Winkelmann J, Muller-Myhsok B, Riedel L, Bauer M, Muller T, Castro M, Meitinger T, Strom TM, Gasser T. Mutations in the gene encoding epsilon-sarcoglycan cause myoclonus-dystonia syndrome. Nature Genetics. 2001;29:66–69. doi: 10.1038/ng709. [DOI] [PubMed] [Google Scholar]
- 4.Quinn NP. Essential myoclonus and myoclonic dystonia. Mov Disord. 1996;11:119–124. doi: 10.1002/mds.870110202. [DOI] [PubMed] [Google Scholar]
- 5.Brown P. Myoclonus. In: Sawle G, editor. Movement Disorders in Clinical Practice. Oxford: Isis Medical Media Ltd; 1999. pp. 147–157. [Google Scholar]
- 6.Kupsch A, Kuehn A, Klaffke S, Meissner W, Harnack D, Winter C, Haelbig TD, Kivi A, Arnold G, Einhaupl KM, Schneider GH, Trottenberg T. Deep brain stimulation in dystonia. J Neurol. 2003;250 (Suppl 1):I47–52. doi: 10.1007/s00415-003-1110-2. [DOI] [PubMed] [Google Scholar]
- 7.Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider G-H, Poewe W, Eisner W, Wolters A, Muller J-U, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J the Deep-Brain Stimulation for Dystonia Study G. Pallidal Deep-Brain Stimulation in Primary Generalized or Segmental Dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 8.Vidailhet M, Vercueil L, Houeto J-L, Krystkowiak P, Benabid A-L, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P the French Stimulation du Pallidum Interne dans la Dystonie Study G. Bilateral Deep-Brain Stimulation of the Globus Pallidus in Primary Generalized Dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 9.Cif L, Valente EM, Hemm S, Coubes C, Vayssiere N, Serrat S, Giorgio AD, Coubes P. Deep brain stimulation in myoclonus-dystonia syndrome. Movement Disorders. 2004;19:724–727. doi: 10.1002/mds.20030. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Griffin IC, Parkin SG, Miall RC, Rowe JG, Gregory RP, Scott RB, Aziz TZ, Stein JF. Involvement of the medial pallidum in focal myoclonic dystonia: A clinical and neurophysiological case study. Movement Disorders. 2002;17:346–353. doi: 10.1002/mds.10038. [DOI] [PubMed] [Google Scholar]
- 11.Magarinos-Ascone CM, Regidor I, Martinez-Castrillo JC, Gomez-Galan M, Figueiras-Mendez R. Pallidal stimulation relieves myoclonus-dystonia syndrome. J Neurol Neurosurg Psychiatry. 2005;76:989–991. doi: 10.1136/jnnp.2004.039248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kupsch A, Trottenberg T, Meissner W, Funk T. Neurostimulation of the ventral intermediate thalamic nucleus alleviates hereditary essential myoclonus. J Neurol Neurosurg Psychiatry. 1999;67:415–416. doi: 10.1136/jnnp.67.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trottenberg T, Meissner W, Kabus C, Arnold G, Funk T, Einhaupl KM, Kupsch A. Neurostimulation of the ventral intermediate thalamic nucleus in inherited myoclonus-dystonia syndrome. Movement Disorders. 2001;16:769–771. doi: 10.1002/mds.1119. [DOI] [PubMed] [Google Scholar]
- 14.Suchowersky O, Davis JL, Furtado S, Wilkinson M, Kreek J. Thalamic Surgery for Essential Myoclonus Results in Clinical but not Functional Improvement (P332). Mov Disord; 6th International Congress of Parkinson’s Disease and Movement Disorders; Barcelona, Spain. June 11–15, 2000; 2000. pp. 1–308. [Google Scholar]
- 15.Klein C, Liu L, Doheny D, Kock N, Müller B, Aguiar PDC, Leung J, Leon DD, Bressman SB, Silverman J, Smith C, Danisi F, Morrison C, Walker RH, Velickovic M, Schwinger E, Kramer PL, Breakefield XO, Brin MF, Ozelius LJ. Epsilon-sarcoglycan mutations found in combination with other dystonia gene mutations. Annals of Neurology. 2002;52:675–679. doi: 10.1002/ana.10358. [DOI] [PubMed] [Google Scholar]
- 16.Garonzik IM, Hua SE, Ohara S, Lenz FA. Intraoperative microelectrode and semi-microelectrode recording during the physiological localization of the thalamic nucleus ventral intermediate. Mov Disord. 2002;17:S135–S144. doi: 10.1002/mds.10155. [DOI] [PubMed] [Google Scholar]
- 17.Frucht SJ, Leurgans SE, Hallett M, Fahn S. The Unified Myoclonus Rating Scale. Adv Neurol. 2002;89:361–376. [PubMed] [Google Scholar]
- 18.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 19.Guehl D, Burbaud P, Boraud T, Bioulac B. Bicuculline injections into the rostral and caudal motor thalamus of the monkey induce different types of dystonia. Eur J Neurosci. 2000;12:1033–1037. doi: 10.1046/j.1460-9568.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 20.Bejjani BP, Arnulf I, Vidailhet M, Pidoux B, Damier P, Papadopoulos S, Bonnet AM, Cornu P, Dormont D, Agid Y. Irregular jerky tremor, myoclonus, and thalamus: a study using low-frequency stimulation. Mov Disord. 2000;15:919–924. doi: 10.1002/1531-8257(200009)15:5<919::aid-mds1024>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Moro E, Esselink RJA, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology. 2002;59:706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 22.O’Suilleabhain P, Frawley W, Giller C, Dewey RBJ. Tremor response to polarity, voltage, pulsewidth and frequency of thalamic stimulation. Neurology. 2003;60:786–790. doi: 10.1212/01.wnl.0000044156.56643.74. [DOI] [PubMed] [Google Scholar]
- 23.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 24.Babadi B. Bursting as an effective relay mode in a minimal thalamic model. J Comput Neurosci. 2005;18:229–243. doi: 10.1007/s10827-005-6560-5. [DOI] [PubMed] [Google Scholar]
- 25.Person AL, Perkel DJ. Unitary IPSPs Drive Precise Thalamic Spiking in a Circuit Required for Learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient with inherited myoclonus-dystonia, with predominant myoclonus, and a postural and action tremor. With Vim deep brain stimulation, there is a decrease in the amplitude of the myoclonic jerks. Tremor is almost completely suppressed.


