Abstract
Objectives
Progression of Alzheimer’s dementia (AD) is highly variable. Most estimates derive from convenience samples from dementia clinics or research centers where there is substantial potential for survival bias and other distortions. In a population-based sample of incident AD cases, we examined progression of impairment in cognition, function, and neuropsychiatric symptoms, and the influence of selected variables on these domains.
Design
Longitudinal, prospective cohort study
Setting
Cache County (Utah)
Participants
328 persons diagnosed with Possible/Probable AD
Measurements
Mini-Mental State Exam (MMSE), Clinical Dementia Rating sum-of-boxes (CDR-sb), and Neuropsychiatric Inventory (NPI).
Results
Over a mean follow-up of 3.80 (range 0.07–12.90) years, the mean (S.D.) annual rates of change were −1.53 (2.69) on the MMSE, 1.44 (1.82) on the CDR-sb, and 2.55 (5.37) scale points on the NPI. Among surviving participants, 30–58% progressed less than one point/year on these measures, even 5–7 years after dementia onset. Rates of change were correlated between MMSE and CDR-sb (r=−0.62, df=201, p<0.001) and between the CDR-sb and NPI (r=0.20, df=206, p<0.004). Females (LR χ2=8.7, df=2, p=0.013) and those with younger onset (LR χ2=5.7, df=2, p=0.058) declined faster on the MMSE. Although one or more APOE ε4 alleles and ever-use of FDA-approved anti-dementia medications were associated with initial MMSE scores, neither was related to the rate of progression in any domain.
Conclusions
A significant proportion of persons with AD progresses slowly. The results underscore differences between population- vs. clinic-based samples and suggest ongoing need to identify factors that may slow the progression of AD.
Keywords: Alzheimer’s disease, dementia, cognition, neuropsychiatric symptoms, progression, decline
Alzheimer’s dementia (AD) is a significant cause of disability and mortality among the elderly. Some 26.6 million cases presently worldwide may increase to 106.2 million by 2050,(1) unless a means of prevention can be identified. Without a cure, better understanding of the clinical course and course-modifying factors is needed.
AD causes impairment not only in cognition and function, but also in behavior prompted by neuropsychiatric symptoms (NPS). Numerous studies report significant variability in the rate of cognitive and functional decline in AD. For example, a recent review reported that the mean annual rate of change (ARC) on the Mini-Mental State Exam (MMSE), a global measure of cognition, varied from 0.8 to 4.4 points.(2) Similar variability is seen in functional decline,(3) although comparisons across studies are impeded by differences in instrumentation. NPS in AD are marked by increasing incidence over time and by an episodic course.(4)
These studies of the natural history of AD share several limitations. Most come from observations in clinics or clinical research centers. Compared to panels of AD cases ascertained from populations, clinic AD patients are up to 20 years younger, have higher educational and occupational attainment, are more often married and living with a spouse,(5) are more likely to be carriers of the Apolipoprotein E (APOE) ε4 allele,(6) and tend to suffer from fewer co-morbid conditions.(7) The few available population-based studies report lower ARCs in cognition or function.(3) Also, most studies of AD progression describe the course of prevalent cases. Rate of decline is known to vary by stage of dementia severity,(8),(9) so that survival bias may produce different estimates in prevalent vs. incident samples.(10) Furthermore, few studies have examined cognition, function, and NPS simultaneously, so their descriptions of AD progression are incomplete. Finally, many studies encompass limited time of follow-up in their descriptions of dementia course.
Here, we describe results from the Cache County Dementia Progression Study (DPS), an ongoing population-based study of AD that characterizes the course of symptoms in the domains of cognition, function and NPS from a point near the onset of dementia. We also assess the influence of several variables reported to affect progression, including age of onset, gender, education, and APOE genotype.(11),(12)
METHODS
The DPS was derived from the longitudinal, population-based Cache County Study on Memory in Aging (CCSMA), which has examined the prevalence, incidence, and risk factors for dementia in a U.S. county recognized for its residents’ longevity.(13) In its first wave, CCSMA enrolled 90% of the 5677 county residents who were aged 65 years or older. Three subsequent triennial waves of case detection have been completed. As described below, most individuals with incident dementia have been followed prospectively by the DPS. Those with diagnoses of Possible or Probable AD were included in the present analyses.
Participants and Dementia Diagnoses
The multi-stage case identification procedures of the CCSMA have been reported elsewhere.(13) Briefly, participants were screened for cognitive disorders using the Modified Mini-Mental State Exam,(14) as adapted for epidemiological studies.(15) Those who screened positive, as well as members of a weighted, stratified population subsample (irrespective of screening results) were studied further using an informant-based telephone interview. This interview queried cognitive and functional impairments typical in dementia.(16) Participants whose interviews were suggestive of dementia or its prodrome, and those of the population subsample, were invited to undergo a clinical assessment (CA) by a trained research nurse and psychometric technician. The CA included a structured physical and neurological examination and a battery of neuropsychological tests.(17) A knowledgeable informant provided information regarding the participant’s history of cognitive or functional impairment, medical history, and psychiatric symptoms.
A study geropsychiatric psychiatrist and neuropsychologist next reviewed data from the CA and assigned preliminary diagnoses of dementia according to DSM-III-R criteria.(18) An age of onset was estimated as the age when the participant unambiguously met DSM-III-R criteria for dementia. Dementia severity was rated using the Clinical Dementia Rating (CDR;(19) see below) and health status as assessed with the General Medical Health Rating.(20) Participants with suspected dementia were asked to undergo neuroimaging and laboratory studies as well as a geropsychiatric physician’s exam to provide differential diagnoses of dementia. Participants were also recruited for a post-mortem brain autopsy program. A panel of experts in neurology, geropsychiatry, neuropsychology, and cognitive neuroscience reviewed all available clinical and neuropathological data and assigned diagnoses of AD and other forms of dementia according to standard protocols (e.g., NINCDS-ADRDA research criteria for AD (21)). All with suspected dementia or a dementia prodrome were invited for an 18-month follow-up CA, the results of which were reviewed by the expert panel who rendered final diagnoses. Participants with dementia newly diagnosed at Waves 2–4 were invited to join the DPS (see Figure 1). All study procedures were approved by the institutional review boards of Utah State, Duke, and the Johns Hopkins Universities.
Figure 1.
displays individuals identified with dementia from the Cache County Study on Memory in Aging (CCSMA) to the present. Not shown are the CCSMA subjects lost to follow-up between study waves.
Measures of Dementia Progression
The Mini-Mental State Exam (MMSE), a measure of global cognitive functioning,(22) was administered by trained neuropsychological technicians. A study neuropsychologist trained these individuals and periodically reviewed audiotaped test sessions to ensure consistent techniques of standardized administration. As in the CCSMA,(13) we calculated an adjusted MMSE score by discarding items missed because of sensory or motor impairment (e.g., severe vision or hearing loss, motor weakness, tremor, etc.), noting the percent correct, and rescaling the final score on a 30-point scale. Participants whose sensory or motor impairments affected more than 3 points were excluded from the analyses (n=30, 9%).
The Clinical Dementia Rating (CDR(19)) is a measure of functional ability in six areas: memory, orientation, judgment/problem solving, community affairs, participation in home/hobbies, and personal care. An ordinal scale is used to reflect degree of impairment: 0 = no impairment; 0.5 = questionable impairment; 1 = mild impairment; 2 = moderate impairment; 3 = severe impairment; 4 = profound impairment; 5 = terminal. The CDR was scored by a trained research nurse at each visit, considering the caregiver’s report of symptoms and the participant’s neuropsychological test performance. A geriatric psychiatrist conducted the initial training and performed periodic reviews of the RN ratings. For analyses, the ratings in each category were summed (CDR-sb).
The Neuropsychiatric Inventory (NPI) assesses NPS that commonly occur in dementia including delusions, hallucinations, agitation-aggression, depression-dysphoria, apathy-indifference, elation-euphoria, anxiety, disinhibition, irritability-lability, and aberrant motor behavior. A trained research nurse administered the NPI (23) to the caregiver. The instrument screens for the presence of each symptom and follows positive responses with a series of standardized questions to characterize the symptom, its frequency, severity, and degree of change from premorbid characteristics. The NPI frequency and severity ratings were multiplied to yield a summary score for each symptom, and then summed across all 10 symptom types (range: 0–120).
Predictor Variables
Variables available from the CCSMA included age of dementia onset, gender, education, and presence of one or two APOE ε4 alleles, determined from buccal DNA.(24)
Analyses
To illustrate an individual participant’s course of decline in cognition, function, and NPS, we calculated an annual rate of change (ARC) or linear slope for each outcome for those with at least two measurements. Subjects were categorized into groups based on whether their slopes were above or below the group median on the MMSE. Participants’ trajectories of scores on the MMSE, CDR-sb and NPI were plotted, with blue lines representing subjects whose MMSE slopes were above the median and red lines representing subjects below the median.
To model non-linear effects, we examined average change from dementia onset for each outcome, using mixed effects models, treating subject-specific intercepts and linear change with time as random effects. This approach allowed us to account for the dependence between within-subject repeated measures and for non-linear change with respect to time by incorporating time-squared effects. We then calculated the average change in MMSE, CDR-sb and NPI from dementia onset to specific time points over the course of dementia, providing means and standard errors of estimated change for each measure for selected time points. To estimate the proportion of individuals with a slowly progressive course, we used a threshold of less than a one-point/year decline on the MMSE or similar magnitude increase on the CDR-sb or NPI.
To examine the association between predictor variables and change in each outcome, we built upon the base mixed model by adding each predictor followed by its interaction with time and time squared. A predictor was retained in the model if the individual term had an associated Wald statistic with p<0.05 or if the likelihood ratio (LR) chi-square test of models with and without the new terms yielded a p<0.05. To consider the effects of incomplete follow-up or dementia duration before diagnosis, we repeated these analyses for participants with at least two follow-up visits whose dementia diagnoses were made within three years after onset. Finally, in secondary analyses, we examined whether differences in rate of change could be attributable to use of FDA approved medications for AD at any time in the course of the illness following two approaches: 1) we added a term for medication use, contrasting those who were ever or never treated with anti-dementia medications; and 2) we repeated analyses excluding those ever treated with these medications. Analyses were completed using SAS Version 9.1.
RESULTS
The CCSMA identified 328 individuals with incident AD. The majority were female (66%) and Caucasian (99%). Table 1 displays sample characteristics at the diagnosis visit. Participants were observed at times between 0.07–12.9 years after onset. Sixty-three percent died while being followed, and 4% either refused further participation or moved out of the area. The remaining 33% were active participants at the time of analysis. The mean (SD) duration of dementia from onset to the last observation was 3.80 (2.58) years. Individuals who lacked any follow-up numbered 112 (34%), in most instances because of death (n=88, 79%). These 112 individuals were significantly older (t=3.59, df=326, p<0.0001) and scored lower on the MMSE at diagnosis (t=3.09, df=295, p=0.002) than those with follow-up data. However, years of education and proportion of males/females did not differ between these groups.
Table 1.
| Demographic and Baseline Characteristics | |
|---|---|
| Male N (%) | 112 (34) |
| Female N (%) | 216 (66) |
| Age M (SD) | 85.92 (6.34) |
| Years of Education M (SD) | 13.20 (3.01) |
| Caucasian N (%) | 325 (99) |
| APOE E4 carrier N (%) | 147 (45) |
| Dementia Duration M (SD) | 1.71 (1.26) |
| Residence: Assisted Living N (%) | 41 (12) |
| Residence: Nursing Home N (%) | 22 (7) |
| Ever use of anti-dementia medications N (%) | 73 (22) |
| MMSE M (SD) | 21.92 (4.60) |
| CDR, global M (SD) | 1.06 (0.59) |
| NPI, any behavior N (%) | 165 (50) |
| NPI, total M (SD) | 4.30 (8.30) |
| General Health | |
| Excellent N (%) | 39 (12) |
| Good N (%) | 178 (54) |
| Fair/Poor N (%) | 109 (34) |
| Number Follow-ups M (SD) | 1.97 (2.09) |
| Duration Follow-ups M (SD) | 3.80 (2.58) |
MMSE = Mini-Mental State Exam; CDR (Clinical Dementia Rating); NPI (Neuropsychiatric Inventory)
Course of Dementia
Over time, the severity of cognitive, functional and behavioral symptoms increased (MMSE LR χ2=128.7, df=2, p<0.0001; CDR-sb LR χ2=137.6, df=2, p<0.0001; NPI LR χ2=77.1, df=2, p< 0.0001). The mean (SD), measure-specific ARCs were −1.53 (2.69) for the MMSE, +1.44 (1.82) for the CDR-sb, and +2.55 (5.37) for the NPI. Fifty percent of participants experienced NPS at baseline, most commonly depression (26%), irritability or apathy (17% each). Most NPI symptoms increased over time such that 89% of survivors were experiencing symptoms by the final visit. However, for hallucinations, anxiety, and irritability, the percent of those affected declined at the final visit, possibly reflecting the fluctuating nature of NPS (see Figure 2), differential survival of those without symptoms, or other factors that diminished the occurrence of symptoms over time. The pattern of NPS also shifted over time as apathy became the most commonly reported symptom by Visit 4. Table 2 displays the one-month prevalence of NPS at each visit.
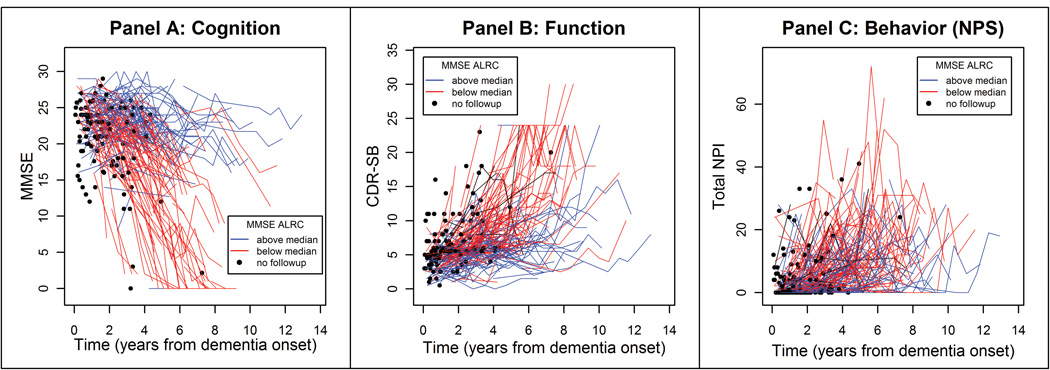
Figure 2.
displays the trajectories of cognitive (Panel A), functional (Panel B) and NPS (Panel C) domains of dementia. Trajectories in blue represent those whose MMSE slopes fall above the median and red are those that fall below the median. Filled black circles represent individuals with no follow-up. Note the individual in Panel A whose slope falls above the median, but MMSE score is stable at 0. This reflects the relative insensitivity of the MMSE to change in very severe dementia. Inspection of the plots suggests a significant number of individuals decline slowly, with MMSE values at 20 or above at the final observation. The plots also suggest an association between cognitive and functional domains, but little to no association between cognitive and NPS domains. ARC = Annual rate of change.
Table 2.
| One-month Prevalence of NPS by Visits | |||||||
|---|---|---|---|---|---|---|---|
| Visits | |||||||
| Dx V | FV 1 | FV 2 | FV 3 | FV 4 | FV 5 | FV 6 | |
| N | 328 | 216 | 140 | 110 | 84 | 60 | 35 |
| Yrs from Dementia | 1.71 | 3.28 | 4.47 | 5.20 | 5.64 | 6.55 | 7.72 |
| Onset M (SD) | (1.26) | (1.47) | (1.92) | (1.89) | (1.83) | (1.95) | (2.24) |
| Any Behavior | 50% | 73% | 78% | 82% | 85% | 93% | 89% |
| Delusions | 15% | 26% | 36% | 33% | 41% | 35% | 39% |
| Hallucinations | 5% | 10% | 15% | 18% | 24% | 25% | 12% |
| Agitation | 10% | 17% | 27% | 23% | 30% | 37% | 46% |
| Depression | 26% | 36% | 38% | 41% | 41% | 37% | 37% |
| Apathy | 17% | 32% | 45% | 47% | 57% | 63% | 63% |
| Elation | 0.6% | 1% | 0.7% | 2% | 2% | 7% | 3% |
| Anxiety | 13% | 24% | 30% | 27% | 29% | 37% | 17% |
| Disinhibition | 7% | 14% | 16% | 18% | 24% | 23% | 31% |
| Irritability | 17% | 26% | 25% | 18% | 25% | 32% | 23% |
| Aberrant Motor Behavior | 8% | 19% | 21% | 24% | 26% | 30% | 33% |
Dx V = Diagnosis Visit; FV = Follow-up Visit
Person-specific longitudinal scores on the MMSE, CDR-sb and NPI are plotted in Panels A–C of Figure 2. Inspection of the plots shows a substantial number of individuals declining slowly. There was a strong association between slopes on the MMSE and CDR-sb (r=−0.62, df=201, p<0.001), but none between the MMSE and NPI (r=0.052, df=195, p=0.469), and only a weak association between the CDR-sb and NPI (r=0.20, df=206, p=0.004).
Mixed effects models revealed a significant non-linear component in trajectories for MMSE and CDR-sb (MMSE LR χ2=17.5, df=1, p<0.0001 for quadratic time; CDR LR χ2=12.3, df=1, p=0.0005), suggesting acceleration in the rate of change over time. Nonlinearity of change was slight on the NPI (LR χ2=1.82, df=1, p=0.18). Table 3 displays the estimated mean (se) annual change at selected time points for each measure. Some 30–58% of the survivors, (5–10% of the entire cohort), declined slowly (less than one point/year), even at 5–7 years after onset. Table 4 displays the percentages of those with a slow course in each of the three domains.
Table 3.
displays the estimated average annual rate of change for each measure from a series of linear mixed effects models. The increase in absolute values over time reflects the slight acceleration in decline (MMSE) or impairment (CDR-sb) estimated by mixed effects models incorporating terms for time and time2. The estimates of annual change for the subsample diagnosed with AD within three years of their dementia onset were similar to the values displayed above and therefore are not provided.
| Summary of Estimated Annual Rate of Change in Three Dementia Trajectories from Mixed Effects Models | |||
|---|---|---|---|
| Cognitive ΔMMSE (se)* |
Functional ΔCDR-sb (se)* |
Behavioral ΔNPI total (se)* |
|
| All Subjects (N) | 203 | 214 | 209 |
| One Yr Post onset | −1.50 (0.14) | 1.00 (0.10) | 2.20 (0.23) |
| Three Yrs Post onset | −1.60 (0.12) | 1.13 (0.09) | 1.93 (0.17) |
| Five Yrs Post onset | −1.76 (0.12) | 1.30 (0.09) | 1.70 (0.14) |
| Seven Yrs Post onset | −1.90 (0.13) | 1.44 (0.10) | 1.47 (0.16) |
Standard error (s.e.) represents the standard deviation of the estimated rate of change computed from the fitted model.
Table 4.
The percentages of persons with Possible/Probable AD with a slow course from age of dementia onset to time points 1, 3, 5, and 7 years post onset are displayed. Slow course is defined as an average annual decline of no more than one point on MMSE (or average annual increase of no more than one point for CDR-sb and NPI total). The numbers and percentages of survivors represent those who survived up to each time point whereas those of the total represent the entire sample of 328 persons with AD.
| % of Survivors with Slow Course | % of Entire Sample (N = 328) with Slow Course | ||||||
|---|---|---|---|---|---|---|---|
| Onset to |
N Surviving |
Cognitive (MMSE) |
Functional (CDR-sb) |
NPS (NPI-total) |
Cognitive (MMSE) |
Functional (CDR-sb) |
NPS (NPI-total) |
| 1 Yr | 282 | 37.9 | 46.1 | 34.8 | 32.6 | 39.6 | 29.9 |
| 3 Yrs | 185 | 36.2 | 55.1 | 36.2 | 20.4 | 31.1 | 20.4 |
| 5 Yrs | 98 | 30.0 | 58.2 | 36.7 | 8.8 | 17.4 | 11.0 |
| 7 Yrs | 46 | 34.8 | 41.3 | 45.7 | 4.9 | 5.8 | 6.4 |
Note: a small number of subjects who scored 0 (floor) at their first observation were excluded from the analyses on the MMSE.
Association between Predictors and Rate of Change
MMSE
On average, males (LRχ2=14.1, df=1, p=0.002), APOE ε4 carriers (LR χ2=4.5, df=1, p=0.035, 1 df) and those with older onset ages (LR χ2=20.9, p<0.0001, df=1) and fewer years of education (LR χ2=21.2, p< 0.0001, df=1) scored worse at dementia onset. Females declined more rapidly than males (LR χ2=8.7, p=0.013, df=2), with an average additional decline of 2.9 points over 3 years, 3.8 points over 5 years, and 4.1 points over 7 years. Those with younger onset ages also declined faster (LR χ2=5.7, df=2, p=0.058). Notably, neither APOE genotype nor education influenced the rate of decline. There were no appreciable differences in results in analyses restricted to those with more complete follow-up and whose dementia was diagnosed within three years of onset (results not shown).
CDR-sb
On average, older onset age (LR χ2=6.8, df=1, p=0.0096) and female gender (LR χ2=7.9, p=0.0053, df=1) were associated with greater impairment at onset. For each added year of age, there was a 0.06 (se=0.02) point higher score, and females scored 0.80 points higher on average, than males. Neither education nor APOE genotype was associated with rate of change. There were no appreciable differences in results in analyses restricted to those with more complete follow-up and whose dementia was diagnosed within three years of onset (results not shown).
Total NPI
On average, individuals with younger onset ages had higher total NPI scores (LR χ2=3.6, df=1, p = 0.060). In separate models considering the time elapsed between dementia onset and diagnoses, participants with older onset ages had higher total NPI scores but only among those diagnosed within three years of onset (LR χ2=3.2, df=1 p interaction=0.076). APOE genotype, gender, and education were not associated with NPI. Table 5 displays the results of the multivariable models for the MMSE, CDR-sb and NPI.
Table 5.
Parameter Estimates from Mixed Effects Models
| MMSE Trajectory | ||||
|---|---|---|---|---|
| Effect | Estimate | Standard Error | T statistic | P value |
| Intercept | 40.3284 | 4.5858 | 8.79 | <.0001 |
| Time | −7.4168 | 2.6980 | −2.75 | 0.0065 |
| Time2 | 0.6064 | 0.2970 | 2.04 | 0.0419 |
| Age of Onset | −0.2467 | 0.05397 | −4.57 | <.0001 |
| Male Gender | −2.4857 | 0.6619 | −3.76 | 0.0002 |
| APOE E4 Present | −0.8584 | 0.4045 | −2.12 | 0.0345 |
| Education | 0.3092 | 0.06720 | 4.60 | <.0001 |
| Time*Onset age | 0.07266 | 0.03261 | 2.23 | 0.0264 |
| Time2*Onset age | −0.00834 | 0.003686 | −2.26 | 0.0243 |
| Time*Male Gender | 1.2689 | 0.4354 | 2.91 | 0.0038 |
| Time2*Male Gender | −0.09864 | 0.05310 | −1.86 | 0.0640 |
| CDR-SB Trajectory | ||||
| Intercept | −0.5369 | 2.1333 | −0.25 | 0.8015 |
| Time | 0.8317 | 0.1601 | 5.20 | <.0001 |
| Time2 | 0.06423 | 0.01866 | 3.44 | 0.0006 |
| Age of Onset | 0.06135 | 0.02357 | 2.60 | 0.0096 |
| Male Gender | −0.8024 | 0.2864 | −2.80 | 0.0053 |
| APOE E4 Present | −0.09872 | 0.2862 | 0.34 | 0.7303 |
| Education | −0.03024 | 0.04748 | −0.64 | 0.5244 |
| NPS Trajectory | ||||
| Intercept | 14.4520 | 5.7428 | 2.52 | 0.0123 |
| Time | 1.2848 | 0.4187 | 3.07 | 0.0024 |
| Time2 | 0.07639 | 0.04918 | 1.55 | 0.1211 |
| Age of Onset | −0.1191 | 0.06318 | −1.89 | 0.0601 |
| Male Gender | −0.5906 | 0.7772 | −0.76 | 0.4478 |
| APOE E4 Present | 0.07593 | 0.7758 | −0.10 | 0.9221 |
| Education | −0.1277 | 0.1280 | −1.00 | 0.3191 |
The results of mixed effects models in the three dementia domain trajectories are shown. Parameter estimates, standard errors, T statistics (assuming a t distribution) and their associated p-values are provided. Not shown are the results of mixed effects models of the subset of individuals diagnosed within three years of dementia onset and with more than two follow-up visits. The results of analyses did not differ except in the NPS trajectory where a shorter duration between dementia onset and diagnosis was associated with a lower NPI score. Among those diagnosed within three years of onset, older individuals had higher average NPI scores.
Effect of Anti-dementia Medications
Twenty-two percent of participants had used anti-dementia medications at some point over the course of dementia. Such medication use was associated with higher MMSE scores at onset (LRT χ2=3.83, df=1, p=0.051), but did not significantly influence rate of decline in the MMSE, CDR-sb, or NPI. However, analyses that excluded those treated with anti-dementia medications no longer showed association of younger onset age with more rapid decline in MMSE.
DISCUSSION
This study of a population-based, incident cohort of persons with AD found: first, that 30–58% of those who survived 5–7 years after dementia onset declined slowly; second, that AD progressed faster in women than in men; third, that number and severity of NPS increased over time, but the course was variable and episodic; and fourth, that rate of change in NPS was correlated weakly, if at all, with rate of change in cognition or function.
Several studies have noted a contrast between “fast” and “slow” progressors in AD (e.g.,(25),(26)), but studies of incident cases from populations are lacking. Approximately one-third to one-half of persons in the Cache County DPS fell into the slow progression category. By contrast, the multi-center French Network on Alzheimer Disease (REAL-FR) consisting of a volunteer sample of 686 individuals reported that 23% of their sample could be characterized as “slow” progressors.(27) The French study also reported that 89% of their participants were receiving treatment for AD (cf., 22% of DPS participants). The lower figure is similar to estimates (26%) reported among Medicare beneficiaries with dementia.(28) Nonetheless, our analyses suggest that slow dementia progression is not attributable to treatment with anti-dementia medications.
In the DPS sample, the mean ARC on the MMSE was considerably lower than was found in clinical or other convenience samples. For example, a mean ARC of −3.9 (SD=3.7) has been reported from the multicenter Consortium to Establish a Registry in AD,(8) and rates of −2.97 (SD=4.26) for Possible AD and −3.05 (SD=3.86) for Probable AD in patients at California AD Centers.(29) A meta-analysis of studies primarily from clinical/university research centers or hospitals reported a pooled ARC on the MMSE of of −3.3 (95% CI:−2.9 to −3.7).(30) To our knowledge, the Kungsholmen project is the only population-based study that has reported an ARC on the MMSE. This was somewhat greater than in the DPS (−2.75 at the study’s first 3-year follow-up and −3.03 at the second follow-up after 3–7 more years).(31) We speculate that the Kungsholmen cases may not have entered the longitudinal analysis as shortly after diagnosis as the DPS cases, and that their case cohorts may therefore show some of the same phenomena (survival bias, entry into study when MMSE decline was more rapid) as is likely in convenience samples.
Functional change in DPS participants was also quite variable. The REAL-FR study reported a mean change in CDR-sb of 4.17 over two years (2.09/year),(27) an approximately 0.65 point faster rate of progression than was observed in DPS. However, differences in CDR versions used between studies make comparisons problematic.
In the behavioral domain, we observed increasing occurrence, rate, and overall severity of NPS over time, consistent with other studies (reviewed in (4)). Change in severity of symptoms in the DPS was higher than that reported in REAL-FR. However, again, comparisons between studies are hampered due to differences in the NPI versions and baseline differences in NPI scores (mean=4.30 in DPS vs. 15.11 in REAL-FR). In the DPS, rate of change in NPS was marginally associated with change in CDR-sb, but not with change in MMSE. Although the lack of correspondence between dementia domains is consistent with other reports,(33) these results may also reflect the crude measurements of change employed here. Alternate methods that characterize the non-linear nature of progression in each domain may reveal stronger associations.(34) We also note that the occurrence of NPS varies with severity of dementia,(35) creating problems for cross-study comparisons, and that symptoms tend to be correlated.(36) Hence, a global summary score may not be optimal for examining associations between NPS and other clinical features of AD.
Among the variables examined, there was no consistent set of factors that influenced change across domains. In cognition, carriers of the APOE ε4 allele performed worse at baseline than did non-carriers but APOE status did not affect rate of decline. Studies examining the effect of APOE after dementia onset have found inconsistent results. Our findings are consistent with recent work suggesting that APOE ε4 exerts deleterious effects early in the disease course.(12) In DPS, education was associated with higher MMSE scores at onset, but not with decline on any of the outcomes. This finding contrasts with studies reporting more rapid decline among those with more years of education,(37) but is consistent with higher education conferring advantages early in the disease course.(38) Differences in results may also reflect sample differences in years of education and the timing of observations along the course of dementia.
Older age was associated with worse cognition and function at baseline, while women declined more rapidly (in cognition) than men. More rapid decline among women with AD has been reported in some,(31) but not all studies.(32) The reasons for gender differences on rates of decline in AD are unclear and warrant further study.
Among the study limitations are the use of single measures of cognition, function and NPS. Some measures (e.g., MMSE) have been criticized for differential performance in classifying the cognitive status of individuals from different ages and educational backgrounds, and for significant floor effects when studying persons with severe dementia (reviewed in (43)). We do not believe these issues substantially affected the results as a somewhat more sensitive measure, the 3MS, was employed in dementia screening in the Cache County population, and dementia diagnoses were based on rigorous clinical examination. Additionally, because we followed individuals with incident dementia, the majority (89%) of our participants did not reach the floor of this measure over the period of observation.
Other limitations included the missing MMSE scores at baseline and/or follow-up owing to sensory/motor impairments among 9% of the sample, the lack of follow-up among 29% of the sample (mostly due to death(44)), and our cursory examination of the effects of anti-dementia medications on dementia progression. Here, we did not consider duration or consistency of medication use; a thorough examination of the effects of anti-dementia medications will be the topic of a subsequent paper on dementia treatments. Finally, the Cache County population is primarily Caucasian and of northern European descent. Thus, the results obtained here may not generalize to populations with different ethnic representation.
The study strengths include its population base, its focus on incident cases, the characterization of course in the three domains of dementia, the extended follow-up after dementia onset, and the high participation rates observed in dementia ascertainment and over the period of observation.
In conclusion, a significant proportion of individuals with AD exhibit a slowly progressive course. The present results in general suggest important differences between population- vs. clinic-based samples. As the DPS continues to accrue additional observations, we will focus our efforts on identifying factors that moderate dementia progression, in addition to those we have described earlier (e.g.,(39),(40),(41),(42)).
Acknowledgments
Supported by NIH grants: R01AG21136, R01AG11380, R01AG18712, R01HG02213, P30AG028377, K24AG027841, P30AG13846
The authors are indebted to Dr. Ronald Munger for his unqualified support of the DPS. We also acknowledge the contributions of the following individuals whose activities have helped to ensure the success of the project: Cara Brewer, B.A., Tony Calvert, R.N., B.A., Michelle Carlson, Ph.D., Elizabeth Fauth, Ph.D., Kimberly Graham, B.A., Kyle Hess, M.S., Hochang Ben Lee, M.D., Carol Leslie, M.S., Lawrence S. Mayer, Ph.D., Chiadi U. Onyike, M.D., Roxane Pfister, M.S., Georgiann Sanborn, M.S., Nancy Sassano, Ph.D., Ingmar Skoog, M.D., Heidi Wengreen, Ph.D.,RD, James Wyatt, and Peter P. Zandi, Ph.D., M.P.H. Finally, we thank the participants and their families for their participation and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in preliminary form: International Neuropsychological Society Conference Portland, OR (2007); American Association for Geriatric Psychiatry Conference, Orlando, FL (2008)
Author Disclosures:
Peter V. Rabins
Legal testimony for Janssen Pharmaceutica
Martin Steinberg:
Grant support from NIA and Elan Pharmaceuticals
Kathleen A. Welsh-Bohmer:
1. Scientific advisory board for Medivation, Inc.
2. Received funding for travel and speaker honoraria from Medivation, Inc. and Elan Corporation/Wyeth
3. Has served/serves as an Associate Editor of Neuropsychology Review, on the editorial boards of Alzheimer's & Dementia, the Journal International Neuropsychological Society, and the Journal of Experimental & Clinical Neuropsychology, and as a consulting editor for Aging, Neuropsychology, and Cognition and Neuropsychology
4. Holds US Patent #6867236 (issued 2005):Nonsteroidal Anti-inflammatory drugs for the treatment of Alzheimer’s disease
5. Receives royalties from the publication of Geriatric Neuropsychology: Assessment and Intervention (Guildford Publications, 2006)
6. Receives research support from the NIH (NIA AG11380 [PI] and NIA AG028377 [PI]).
Constantine Lyketsos:
1. Grant support (research or CME)
– NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis
2. Consultant/Advisor
– Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Genentech
3. Honorarium or travel support
– Pfizer, Forest, Glaxo-Smith Kline, Health Monitor
JoAnn Tschanz, Chris Corcoran, Sarah Schwartz, Katherine Treiber, Robert Green, Maria Norton, Michelle Mielke, Kathleen Piercy, Jeanne-Marie Leoutsakos, and John C.S. Breitner have no disclosures.
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, et al. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Behl P, Stefurak TL, Black SE. Progress in clinical neurosciences: cognitive markers of progression in Alzheimer's disease. The Canadian journal of neurological sciences. 2005;32:140–151. doi: 10.1017/s0317167100003917. [DOI] [PubMed] [Google Scholar]
- 3.Aguero-Torres H, Fratiglioni L, Winblad B. Natural history of Alzheimer's disease and other dementias: review of the literature in the light of the findings from the Kungsholmen Project. Int J Geriatr Psychiatry. 1998;13:755–766. doi: 10.1002/(sici)1099-1166(1998110)13:11<755::aid-gps862>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer's disease: characteristics and treatment. Neurologic clinics. 2000;18:829–846. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 5.Kokmen E, Ozsarfati Y, Beard CM, et al. Impact of referral bias on clinical and epidemiological studies of Alzheimer's disease. Journal of clinical epidemiology. 1996;49:79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang D, Kukull W, Sheppard L, et al. Impact of sample selection on APOE epsilon 4 allele frequency: a comparison of two Alzheimer's disease samples. Journal of the American Geriatrics Society. 1996;44:704–707. doi: 10.1111/j.1532-5415.1996.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 7.Massoud F, Devi G, Stern Y, et al. A clinicopathological comparison of community-based and clinic-based cohorts of patients with dementia. Archives of neurology. 1999;56:1368–1373. doi: 10.1001/archneur.56.11.1368. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, Edland S, Clark C, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part IV. Rates of cognitive change in the longitudinal assessment of probable Alzheimer's disease. Neurology. 1993;43:2457–2465. doi: 10.1212/wnl.43.12.2457. [DOI] [PubMed] [Google Scholar]
- 9.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. The American journal of psychiatry. 1994;151:390–396. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
- 10.Ruitenberg A, Kalmijn S, de Ridder MA, et al. Prognosis of Alzheimer's disease: the Rotterdam Study. Neuroepidemiology. 2001;20:188–195. doi: 10.1159/000054786. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Gilley DW, Bennett DA, et al. Person-specific paths of cognitive decline in Alzheimer's disease and their relation to age. Psychol Aging. 2000;15:18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70:1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 14.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 15.Tschanz JT, Welsh-Bohmer KA, Plassman BL, et al. An adaptation of the modified mini-mental state examination: analysis of demographic influences and normative data: the cache county study. Neuropsychiatry, neuropsychology, and behavioral neurology. 2002;15:28–38. [PubMed] [Google Scholar]
- 16.Kawas C, Segal J, Stewart WF, et al. A validation study of the Dementia Questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 17.Tschanz JT, Welsh-Bohmer KA, Skoog I, et al. Dementia diagnoses from clinical and neuropsychological data compared: the Cache County study. Neurology. 2000;54:1290–1296. doi: 10.1212/wnl.54.6.1290. [DOI] [PubMed] [Google Scholar]
- 18.Diagnostic and Statistical Manual of Mental Disorders. 3rd ed-rev. American Psychiatric Association; 1987. [Google Scholar]
- 19.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. The British journal of psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 20.Lyketsos CG, Galik E, Steele C, et al. The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc. 1999;47:487–491. doi: 10.1111/j.1532-5415.1999.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 24.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 25.Johnsen S, Hughes S, Bullock R, et al. Prediction of the rate of decline in cognitive function in Alzheimer's disease: a model based on simple demographic data and widely used rating scales. Dementia and geriatric cognitive disorders. 2003;16:276–282. doi: 10.1159/000072813. [DOI] [PubMed] [Google Scholar]
- 26.Marra C, Silveri MC, Gainotti G. Predictors of cognitive decline in the early stage of probable Alzheimer's disease. Dementia and geriatric cognitive disorders. 2000;11:212–218. doi: 10.1159/000017239. [DOI] [PubMed] [Google Scholar]
- 27.Cortes F, Nourhashemi F, Guerin O, et al. Prognosis of Alzheimer's disease today: a two-year prospective study in 686 patients from the REAL-FR Study. Alzheimers Dement. 2008;4:22–29. doi: 10.1016/j.jalz.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman IH, Ryder PT, Simoni-Wastila L, et al. Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. J Gerontol B Psychol Sci Soc Sci. 2008;63:S328–S333. doi: 10.1093/geronb/63.5.s328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corey-Bloom J, Galasko D, Hofstetter CR, et al. Clinical features distinguishing large cohorts with possible AD, probable AD, and mixed dementia. Journal of the American Geriatrics Society. 1993;41:31–37. doi: 10.1111/j.1532-5415.1993.tb05944.x. [DOI] [PubMed] [Google Scholar]
- 30.Han L, Cole M, Bellavance F, et al. Tracking cognitive decline in Alzheimer's disease using the mini-mental state examination: a meta-analysis. Int Psychogeriatr. 2000;12:231–247. doi: 10.1017/s1041610200006359. [DOI] [PubMed] [Google Scholar]
- 31.Aguero-Torres H, Fratiglioni L, Guo Z, et al. Prognostic factors in very old demented adults: a seven-year follow-up from a population-based survey in Stockholm. Journal of the American Geriatrics Society. 1998;46:444–452. doi: 10.1111/j.1532-5415.1998.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 32.Swanwick GR, Coen RF, Coakley D, et al. Assessment of progression and prognosis in 'possible' and 'probable' Alzheimer's disease. International journal of geriatric psychiatry. 1998;13:331–335. doi: 10.1002/(sici)1099-1166(199805)13:5<331::aid-gps769>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Mohs RC, Schmeidler J, Aryan M, et al. Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer's disease. Stat Med. 2000;19:1401–1409. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1401::aid-sim432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 34.Corcoran C, Pieper C, Zandi P, et al. Modeling Dementia Trajectories: An Application of Dynamical Correlations to Age-Related Traits in the Cache County Dementia Progression Study. Alzheimers Dement. 2008;4:T332. [Google Scholar]
- 35.Lopez OL, Becker JT, Sweet RA, et al. Psychiatric symptoms vary with the severity of dementia in probable Alzheimer's disease. The Journal of neuropsychiatry and clinical neurosciences. 2003;15:346–353. doi: 10.1176/jnp.15.3.346. [DOI] [PubMed] [Google Scholar]
- 36.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer's disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 37.Stern Y, Albert S, Tang MX, et al. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 38.Koepsell TD, Kurland BF, Harel O, et al. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology. 2008;70:1732–1739. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- 39.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg PB, Mielke MM, Tschanz J, et al. Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:883–892. doi: 10.1097/JGP.0b013e318181276a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton MC, Piercy KW, Rabins PV, et al. Caregiver-recipient closeness and symptom progression in Alzheimer disease. The Cache County Dementia Progression Study. J Gerontol B Psychol Sci Soc Sci. 2009;64:560–568. doi: 10.1093/geronb/gbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treiber KA, Lyketsos CG, Corcoran C, et al. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. Int Psychogeriatr. 2008;20:538–553. doi: 10.1017/S1041610208006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 44.Tschanz JT, Corcoran C, Skoog I, et al. Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology. 2004;62:1156–1162. doi: 10.1212/01.wnl.0000118210.12660.c2. [DOI] [PubMed] [Google Scholar]