Abstract
TBR-652 is a novel CCR5 antagonist with potent in vitro anti-HIV activity. The objective of this study was to determine the pharmacokinetics (PK) and pharmacodynamics (PD) of TBR-652 in HIV-1-infected, antiretroviral treatment-experienced, CCR5 antagonist-naïve patients. A double-blind, placebo-controlled, randomized, dose-escalating study of TBR-652 monotherapy given once daily orally for 10 days was performed, followed by a 40-day follow-up period. Approximately 10 patients/dose level received 25, 50, 75, 100, and 150 mg TBR-652 or placebo (4:1). Blood was collected at different intervals for PK and HIV-1 RNA assessments. PK analysis of TBR-652 was performed using noncompartmental methods. PK/PD was modeled using a maximum inhibitory effect model (Emax) and 50% inhibitory concentrations (IC50). TBR-652 was well absorbed in the systemic circulation. TBR-652 concentration levels declined slowly, with mean elimination half-lives ranging from 22.5 to 47.62 h across dose levels. TBR-652 treatment resulted in potent, dose-dependent decreases in viral load, with statistically significant decreases in nadir HIV-1 RNA compared to baseline for all dose levels. Suppression of HIV-1 RNA persisted over the 40-day follow-up period. A steep exposure-effect relationship was observed, with an Emax of −1.43 log10 copies/ml and IC50 of 13.1 ng/ml. TBR-652 was generally safe and well tolerated at all dose levels studied. Short-term monotherapy treatments of TBR-652 in HIV-1-infected patients resulted in promising PK and PD results, with a clear exposure-response relationship at the current dose levels studied. Data from this study support further development of TBR-652 in HIV-infected patients.
INTRODUCTION
The search for an agent with broad inhibitory activity against HIV cell entry has been hampered by the difficulty in overcoming the high degree of sequence variability and evolutionary drift of the gp120 region(s) responsible for interacting with the CD4 cell surface receptor (15). With the discovery of chemokine receptor 5 (CCR5) and CXC chemokine receptor 4 (CXCR4), two discrete coreceptors required for the success of the three-step viral entry process, recent focus has shifted to inhibition of host cell coreceptors as targets for the development of new HIV therapeutics. Initial data from short-term (10- to 14-day) monotherapy studies in HIV-infected patients with the CCR5 antagonists maraviroc, vicriviroc, INCB009471, and aplaviroc were highly encouraging, producing declines of up to 1.8 log10 copies/ml in HIV-1 RNA levels relative to baseline (1, 4, 20). Long-term efficacy and safety data of CCR5 antagonists have been demonstrated with maraviroc in treatment-experienced and -naïve patients and with the current implementation of larger phase 3 studies for vicriviroc and ongoing postapproval studies of maraviroc, in which CCR5-containing highly active antiretroviral therapy (HAART) is given to treatment-experienced and treatment-naïve individuals (14).
A theoretical risk with CCR5 antagonists is that inhibition of the CCR5 coreceptor might induce a switch to CXCR4 coreceptor usage, which was seen during the clinical evaluation of the CCR5 antagonist maraviroc (21). This tropism shift from CCR5 to CXCR4 coreceptor usage has been shown to be associated with accelerated disease progression (16, 19); however, it is not known if this tropism shift is the cause or a consequence of HIV disease progression. It is possible that the detection of the more pathogenic CXCR4 virus may not reflect a shift in coreceptor tropism but rather may represent the emergence of CXCR4 virus from preexisting reservoirs, fluctuations in the amount of existing CXCR4 virus above and below the limit of quantitation of the tropism assay being used (7), or “unmasking” of a minor viral variant after viral suppression during effective CCR5 antagonist treatment (22). At present, coreceptor use is closely monitored for tropism shifts in clinical evaluations of CCR5 antagonists because the clinical consequences of selective inhibition of coreceptor usage are not yet fully understood. Although the development of mutations resulting in resistance to CCR5 antagonists has been reported in clinical trials of investigational coreceptor inhibitors, recent data suggest that this may be a very rare phenomenon (8, 18). In addition, virologic failure in this class has most often been associated with emergence of X4 virus (2). Analyses performed on maraviroc and vicriviroc mirror the results for all antivirals, that is, robust inhibition of replication is required for durable virus suppression and for avoiding the subsequent development of resistance. Data from maraviroc and vicriviroc trials show that CCR5 inhibitor drug levels exceeding the protein binding-adjusted 90% inhibitory concentration (IC90) of the minimum observed plasma concentration (Cmin) by severalfold are essential for durable viral suppression (1, 17).
TBR-652 is a novel CCR5 antagonist with potent in vitro anti-HIV activity as previously demonstrated by plasma protein binding analyses. The IC90 of TBR-652 was 0.25 nM, with a resulting 10-fold reduction in anti-HIV activity. TBR-652 was also found to be potent against chemokine receptor 2 (CCR2) in early in vitro studies, with an IC50 of 5.9 nM. The additional CCR2 antagonist activity of TBR-652 provides a unique feature as a CCR5 inhibitor. The pharmacokinetics (PK) of TBR-652 was evaluated in a multiple-ascending-dose study in healthy volunteers. TBR-652 was well absorbed following repeated dosing of 25 and 200 mg once a day (QD), with Cmin approximately 5- and 46-fold higher, respectively, than the in vitro 2 ng/ml target for TBR-652. Safety results from the multiple-ascending-dose study in healthy volunteers provided further data for risk assessment and supported the feasibility of once-daily oral dosing of TBR-652 in HIV-1-infected patients (12).
A double-blinded, randomized, placebo-controlled, dose-escalating study of TBR-652 was performed in HIV 1-infected, antiretroviral treatment-experienced, CCR5 antagonist-naïve patients. The objective of this study was to determine the PK, pharmacodynamics (PD), and exposure-response relationship, as well as safety and tolerability of TBR-652 following once-daily repeated oral administrations of 5 dose levels for 10 days as a monotherapy agent to ultimately support further development of TBR-652 for use in HIV-infected patients.
MATERIALS AND METHODS
Study design.
This was a double-blind, randomized, placebo-controlled, dose-escalating study to assess the PK, antiviral activity, safety, and tolerability of monotherapy with TBR-652, a CCR5 antagonist, dosed orally once daily for 10 days in HIV-1-infected, antiretroviral treatment-experienced, CCR5 antagonist-naïve patients. Patients received the drug once daily for 10 days as one 25-mg tablet, two 25-mg tablets, three 25-mg tablets, one 100-mg tablet, or six 25-mg tablets or the appropriate number of placebo matching tablets. The 100-mg tablet was administered as a new formulation (F2) compared to the 25-mg tablet formulation (F1). A total of 54 patients at 10 sites (9 in the United States and 1 in Argentina) were screened and entered into study 652-2-201. The protocol required that all patients in the study be treatment experienced and that prior antiretroviral therapy be discontinued at least 6 weeks before study entry to allow adequate drug washout. Prior antiretroviral therapy included the use of a combination of nucleoside/nucleotide reverse transcriptase inhibitors (RTIs), protease inhibitors, single-formulation nucleoside/nucleotide RTIs, prior nonnucleoside RTIs, or prior treatment with raltegravir. Patients were not to have had prior CCR5 antagonist therapy. The time off therapy varied from 6 weeks to 3 years. Reasons for discontinuation of prior therapy varied and included intolerance or failure of prior therapy regimens, discretionary drug holidays, and a preference for phase 1 study participation. All patients signed informed consent, which included a warning of the risk of developing drug resistance. At screening, each patient had to have a confirmed plasma HIV-1 RNA measurement of at least 5,000 copies/ml, a CD4+ cell count of at least 250 cells/mm3, and a CCR5-positive viral coreceptor tropism. Viral tropism was determined using the enhanced sensitivity Trofile assay (Monogram Biosciences, San Francisco, CA), and all patients were required to be CCR5-tropic by this assay. Within each dosing cohort, approximately 10 patients were randomized in a blinded fashion in a 4:1 ratio of active drug to placebo. It was anticipated that each cohort would enroll approximately 8 patients treated with TBR-652 and approximately 2 patients treated with placebo. An unblinded safety and activity review was performed when all patients in a given cohort completed 10 days of treatment. The safety data review included vital signs, safety laboratory values, 12-lead electrocardiograms (ECGs), and adverse events (AEs).
Inclusion and exclusion criteria.
Antiretroviral treatment-experienced, HIV-positive adult male and female subjects between 18 and 65 years old, inclusive, were eligible for study participation if they (i) had no clinically significant findings at screening (clinical, laboratory, and ECG), (ii) were antiretroviral treatment experienced but not treated with antiretroviral therapy for at least 6 weeks prior to the study, (iii) were CCR5 antagonist naïve, (iv) had a qualifying CD4 cell count of ≥250 cells/mm3 within 30 days prior to the study, (v) had two separate measurements of qualifying plasma HIV-1 RNA levels of ≥5,000 copies/ml within 45 days prior to the study, (vi) were females who had no reproductive potential or who had child-bearing potential but were willing to take appropriate precautions to avoid pregnancy or males who were willing to take appropriate precautions to avoid fathering a child (i.e., use two forms of barrier method contraception during the trial and for 2 months after stopping the medication if participating in activity that could lead to pregnancy), and (viii) were able to comprehend and willing to sign an informed consent form. Patients were excluded from participation in the study if any of the following criteria were met: (i) presence of CXCR4- or dual/mixed-tropic HIV-1, (ii) presence of an active Centers for Disease Control and Prevention category C disease, (iii) history of infection with hepatitis B or hepatitis C virus, history of cirrhosis, or any known active or chronic liver disease, (iv) serum alanine aminotransferase and aspartate aminotransferase values greater than grade 1 or bilirubin value greater than the upper limit of normal at screening, (v) history of HIV-2 and recent history of clinically significant infection, (vi) treatment with immunomodulating agents or with any agent with known anti-HIV activity within 30 days, (vii) treatment with any vaccine within 30 days, (viii) a positive prestudy drug screen, including amphetamines, barbiturates, cocaine, or phencyclidine (PCP), (ix) anticipated use of antacids during the trial and/or within 7 days prior the study, (x) current alcohol or drug use, (xi) inability to comply with the dosing schedule and protocol evaluations, (xii) use of any experimental medications within at least 4 weeks prior to screening, (xiii) current or anticipated use of antimetabolites, alkylating agents, or drugs, herbal preparations, and foods known to affect cytochrome P450 (CYP) enzymes or P-glycoprotein transporter activity, (xiv) history of clinically significant hepatic, metabolic, endocrine, renal, hematologic, pulmonary, gastrointestinal, or cardiovascular disorders, (xv) uncontrolled hypertension, (xvi) presence of a malabsorption syndrome, or (xvii) history of malignancy, with the exception of cured basal or squamous cell carcinoma of the skin. Also excluded were subjects who were pregnant of breastfeeding, who had undergone radiation therapy or taken cytotoxic chemotherapeutic agents and had not recovered from side effects, who had used any over-the-counter medications within 14 days prior to the study, and who had a history of or recent abnormal ECGs.
PK sample collection.
Venous blood samples (9 ml each) were collected by individual venipuncture or via an indwelling catheter at the following time points: day 8 (predose), day 9 (predose), and day 10 (predose and 0.5, 1, 2, 3, 4, 6, 8, 24, 96, and 120 h postdose). After collection, blood samples were gently mixed thoroughly with the anticoagulant by slowly inverting the collection tube several times. Samples were then placed in an ice-water bath. Within 45 min of sample collection, tubes were centrifuged at approximately 2,000 × g for 15 min at approximately 5°C. Plasma was separated into 2 aliquots of approximately equal volumes and stored at approximately −70°C. Frozen samples were shipped on dry ice to the bioanalytical laboratory for analysis. Concentrations of TBR-652 in human plasma (containing K2EDTA as an anticoagulant) were assessed by high-performance liquid chromatography (HPLC) with liquid chromatography/tandem mass spectrometry (LC/MS/MS) detection. The analytical method was developed at AAI Pharma (Shawnee, KS). Assay validation was performed at low (0.0500 to 9.60 ng/ml), middle (1.00 to 192 ng/ml), and high (5.00 to 960 ng/ml) analytical ranges. Briefly, TBR-652 and the internal standard (TBR-652-d7) were extracted from human plasma using either liquid-liquid extraction (low range) or protein precipitation (middle and high ranges). The residue (liquid-liquid extraction) or supernatant (protein precipitation) was chromatographed using reverse-phase HPLC. The analytical column was a Betasil C18 5μ (50 by 2.1 mm). TBR-652 and TBR-652-d7 were detected by monitoring the precursor and product ions (m/z changes of 697.4 to 574.4 for TBR-652 and 704.4 to 574.4 for TBR-652-d7) using an Applied Biosystems API3000 or API4000 LC/MS/MS. The quantification was based on setting a calibration graph using the internal standard method. The graph was established by a weighted linear regression (1/x2) of the peak area ratio (analyte/internal standard) versus the concentration of the analyte.
Human plasma lots, free of significant interference, were pooled and used to prepare calibration standard and quality control (QC) samples. Linearity was assessed by plotting area ratios versus standard concentrations and using a linear regression weighted by 1/concentration2. Calibration standards ranging in concentration from 1.00 to 192 ng/ml and quality control pools were prepared by spiking appropriate levels of TBR-652 into blank human plasma with K2EDTA as the anticoagulant. Based on QC samples prepared at low, middle, and high analytical ranges during method validation, interassay precision (percent coefficient of variation [CV%]) results ranged between 1.8% and 6.5% and interassay accuracy (percentage of theoretical) results ranged between −4.5% and 10.3%. Intra-assay precision (CV%) based on QC samples prepared at low, middle, and high analytical ranges during method validation ranged between 0.8% and 10.7%, and intra-assay accuracy (percentage of theoretical) results ranged between −5.8% and 11.4%.
Viral load.
Blood samples for HIV-1 RNA analysis were collected on the following days: 1, 2, 3, 4, 7, 8, 10, 15, 24, and 40. Viral load was assessed using the Cobas assay, with a lower limit of quantitation (LLOQ) of 400 copies/ml. For HIV-1 RNA levels that were below the LLOQ of the assay or expected to be below the LLOQ based on previous samples, the sample was tested using an ultrasensitive PCR, with an LLOQ of 50 copies/ml.
Pharmacokinetics.
PK parameters of TBR-652 were calculated using noncompartmental analysis with WinNonlin Enterprise, version 5.2 (Pharsight). The following PK parameters were calculated: area under the plasma concentration-time curve from time zero to 24 h (AUC0–24), calculated using a linear trapezoidal rule; maximum plasma concentration (Cmax); minimum plasma concentration (Cmin); average concentrations under steady-state conditions (Css); time to maximum plasma concentration (Tmax); apparent elimination half-life (t1/2; calculated as ln 2/λz); apparent elimination rate constant (λz); apparent clearance (CL/F; calculated as dose/AUC0–24). Differences in trough concentrations of TBR-652 on days 8, 9, and 10 were assessed using Helmert contrasts from a repeated measurement analysis of variance (ANOVA) model in order to determine the time to steady state. Time was modeled as a fixed effect, and the correlation within each subject was modeled using various variance-covariance matrix structures. Helmert contrasts were constructed such that the least-square mean (LSM) for each predose value was compared to the mean for subsequent time points. Steady state was concluded if Helmert contrasts were not statistically significant at a two-sided alpha level of 5%. The Helmert contrast analysis was performed using SAS version 9.1.3.
Pharmacodynamics.
The plasma HIV-1 RNA level (log10 copies/ml) at day 1 predose (baseline) and median change from baseline on the day 2, 3, 4, 7, 8, 10, 11, 15, 24, and 40 study visits were summarized and analyzed. The nadir HIV-1 RNA changes from baseline were also summarized and analyzed using an analysis of covariance (ANCOVA) model, including the baseline value as a covariate. Patients achieving HIV-1 RNA levels <400 copies/ml or <50 copies/ml were also summarized. Determination of the change from baseline in the inflammatory marker monocyte chemotactic protein 1 (MCP-1) was performed based on data collected on day 10.
Pharmacokinetic/pharmacodynamic modeling.
The relationship between antiviral activity (log10 copies/ml) and drug exposure was described by testing various PK/PD models such as the maximum inhibitory effect model (Emax), with and without sigmoidal factor (gamma), as well as linear models, where viral inhibition was driven by the observed exposure of TBR-652 (i.e., AUC0–24, Cmax, and CSS). PK/PD modeling was performed using Phoenix WinNonlin (version 6.1). An exploratory analysis was performed by assessing the relationship between drug exposure and change from baseline in the inflammatory marker MCP-1. PK/PD model selection was based on the minimization of the Akaike information criteria (AIC) and visual evaluation of goodness of fit. The standard errors (SE) around Emax and IC50 parameters of TBR-652 were derived to assess the precision of key PD parameters.
Safety and tolerability.
Safety and tolerability assessment included review of medical/medication history, a physical examination, measurement of vital signs, height, and weight, determination of body mass index (BMI), 12-lead electrocardiograms including corrected QT (QTc) measurements, and clinical laboratory tests (serum chemistry, hematology, complete urinalysis, coagulation profile, drug screen, and tests for hepatitis antibody/antigen and HIV antibody). Adverse events were monitored, and severity was rated according to the grading table of the Division of AIDS (DAIDS), National Institute of Allergy and Infectious Diseases.
RESULTS
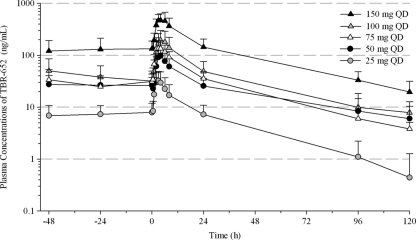
Demographic characteristics of patients are summarized by treatment group in Table 1. A total of 87% of the population was male, and 96.3% of the patients were enrolled in the United States. The two patients from Argentina were both enrolled in the 100-mg dose group (cohort 4). The mean overall age was 38.9 years. The predominant races were Caucasian (56%) and African-American (26%). Mean BMI was greatest in the 50-mg QD group (30.51 kg/m2), which also had a higher mean body weight than the other treatment groups. The other groups had mean BMI values that ranged from 23.52 to 26.50 kg/m2 and were similar to the overall mean of 25.65 kg/m2. Mean plasma concentration-time profiles of TBR-652 following multiple oral administrations of 25, 50, 75, 100, and 150 mg QD are presented on semi-log scale in Fig. 1. TBR-652 was well absorbed following repeated dosing, and all plasma concentrations of TBR-652 were above the LLOQ of the assay. Mean plasma concentrations of TBR-652 declined in a multiexponential manner. Descriptive statistics of PK parameters of TBR-652 are presented in Table 2. One subject (no. 2007) randomly assigned to cohort 2 (50 mg/day of TBR-652) mistakenly received 25 mg TBR-652 during the study. As a result, PK and PD data for this subject were assessed as part of cohort 1 (25 mg/day of TBR-652; n = 9). Mean oral clearance (CL/F) values for TBR-652 at the 50-, 75-, and 100-mg QD dose levels were relatively constant, with mean values of 49.26, 42.17, and 48.06 liters/h, respectively. Mean CL/F values at the 25- and 150-mg QD dose levels (i.e., 81.31 and 22.95 liters/h, respectively) appeared to be markedly different from those observed at the 50- and 100-mg QD dose levels. Two patients enrolled at the clinical site in Argentina displayed TBR-652 systemic exposure more than 2-fold higher than those observed in other subjects in cohort 4 (100 mg QD). This difference may suggest the presence of a food effect on the PK of TBR-652 since patients at the clinical site in Argentina received meals with a fat content higher than that of meals administered in the U.S. clinics. Mean terminal elimination half-lives of TBR-652 across the 25- to 150-mg QD dose levels ranged from 22.50 to 47.62 h. Dose-adjusted exposure parameters of TBR-652 (AUC0–24 and Cmax) were calculated to assess the dose range over which PK parameters increased in a dose-proportional manner. Dose-adjusted AUC0–24 values for the 25-, 50-, 75-, 100-, and 150-mg QD dose levels were 15.3, 24.9, 25.5, 26.6, and 48.5, respectively. Dose-adjusted Cmax for the 25-, 50-, 75-, 100-, and 150-mg QD dose levels were 1.40, 2.05, 2.09, 1.97, and 3.39, respectively. Overall, the above results suggest that AUC0–24 and Cmax of TBR-652 increased in a dose-proportional manner from 50 to 100 mg QD. The AUC0–24 and Cmax of TBR-652 for the 150-mg dose appeared to increase in a more-than-proportional manner compared to those for other dose levels. Time to steady state was evaluated using a Helmert contrast approach. Steady-state concentrations of TBR-652 were observed on day 8 for all dose levels since mean concentration levels were not statistically significantly different from those observed on days 9 and 10. These results suggest that PK parameters of TBR-652 calculated on day 10 in HIV-infected patients were assessed under steady-state conditions.
Table 1.
Demographic characteristics of HIV-1 patients
| Characteristic | Value for: |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 10) | Cohort 1 (25 mg; n = 8) | Cohort 2 (50 mg; n = 8) | Cohort 3 (75 mg; n = 9) | Cohort 4 (100 mg; n = 10) | Cohort 5 (150 mg; n = 9) | Overall (n = 54) | |
| No. (%) of: | |||||||
| Males | 9 (90) | 8 (100) | 7 (87.5) | 9 (100) | 8 (80) | 6 (66.7) | 47 (87) |
| Females | 1 (10) | 0 | 1 (12.5) | 0 | 2 (20) | 3 (33.3) | 7 (13) |
| Mean age (yr) (SD) | 33.9 (5.45) | 41.0 (7.95) | 40.8 (7.85) | 41.0 (5.92) | 38.1 (7.53) | 40.0 (11.47) | 38.9 (7.95) |
| No. (%) of: | |||||||
| African-Americans | 4 (40.0) | 0 | 2 (25.0) | 1 (11.1) | 4 (40.0) | 3 (33.3) | 14 (25.9) |
| Asians | 0 | 0 | 0 | 0 | 0 | 1 (11.1) | 1 (1.9) |
| Caucasians | 4 (40.0) | 7 (87.5) | 4 (50.0) | 6 (66.7) | 6 (60.0) | 3 (33.3) | 30 (55.6) |
| Hispanics | 2 (20.0) | 1 (12.5) | 2 (25.0) | 1 (11.1) | 0 | 1 (11.1) | 7 (13.0) |
| Others | 0 | 0 | 0 | 1 (11.1) | 0 | 1 (11.1) | 2 (3.7) |
| No. (%) of patients from: | |||||||
| United States | 10 (100) | 8 (100) | 8 (100) | 9 (100) | 8 (100) | 9 (100) | 52 (96.3) |
| Argentina | 0 | 0 | 0 | 0 | 2 (20) | 0 | 2 (3.7) |
| Mean ht (cm) (SD) | 178.88 (7.785) | 178.13 (8.870) | 179.14 (8.845) | 178.92 (8.962) | 174.31 (12.588) | 171.33 (9.915) | 176.71 (9.685) |
| Mean wt (kg) (SD) | 80.95 (9.660) | 83.71 (7.308) | 93.87 (29.378) | 79.63 (11.382) | 75.55 (20.429) | 69.50 (11.734) | 79.81 (17.078) |
| Mean BMI (kg/m2) (SD) | 25.39 (3.268) | 26.50 (2.999) | 30.51 (11.041) | 24.84 (2.607) | 24.56 (4.935) | 23.52 (2.733) | 25.65 (5.322) |
Fig. 1.
Mean (+SD) plasma concentration-time profiles of TBR-652 on day 10.
Table 2.
Summary of pharmacokinetic parametersa of TBR-652 on day 10
| Treatment (mg QD) (n) | AUC0–24 (ng · h/ml) | CSS (ng/ml) | Cmax (ng/ml) | Cmin (ng/ml) | Tmaxb (h) | t1/2 (h) | CL/F (liters/h) |
|---|---|---|---|---|---|---|---|
| 25 (9) | 382.0 (52.8) | 15.92 (52.8) | 35.11 (46.9) | 6.883 (55.7) | 2.98 [1.00–4.03] | 22.50 (42.7) | 81.31 (45.3) |
| 50 (7) | 1,245 (40.4) | 51.89 (40.4) | 102.4 (41.9) | 21.47 (44.9) | 4.00 [2.00–6.00] | 47.62 (4.5) | 49.26 (55.3) |
| 75 (8) | 1,916 (30.6) | 79.82 (30.6) | 157.0 (41.0) | 30.06 (36.7) | 3.50 [2.00–6.17] | 29.78 (12.4) | 42.17 (27.8) |
| 100 (10) | 2,659 (52.8) | 110.8 (52.8) | 196.5 (54.3) | 30.00 (68.6) | 4.00 [3.00–6.00] | 32.06 (23.1) | 48.06 (51.2) |
| 150 (9) | 7,272 (36.8) | 303.0 (36.8) | 508.1 (34.9) | 128.0 (40.4) | 4.00 [3.00–8.00] | 41.26 (42.1) | 22.95 (32.3) |
Except where indicated otherwise, values are arithmetic means (%CV).
Values are medians [minimums–maximums].
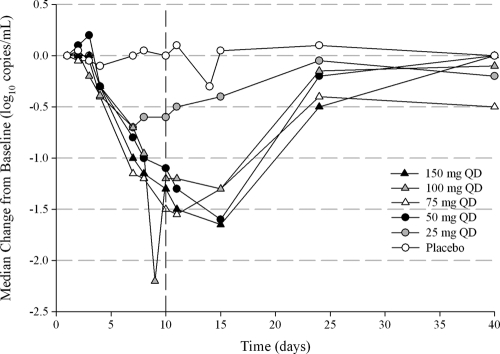
Median change in HIV-1 RNA levels over the 10-day study and during the study or during follow-up through day 40 are presented in Fig. 2. Descriptive statistics of HIV-1 RNA levels are also presented in Table 3.
Fig. 2.
Median change in HIV-1 RNA levels from baseline over time.
Table 3.
Summary of HIV-1 RNA levels
| Study day and parametersa | Valueb for: |
|||||
|---|---|---|---|---|---|---|
| All cohorts, placebo (n = 10) | Cohort 1, 25 mg (n = 9) | Cohort 2, 50 mg (n = 7) | Cohort 3, 75 mg (n = 8) | Cohort 4, 100 mg (n = 10) | Cohort 5, 150 mg (n = 8) | |
| 1 (baseline) | ||||||
| n | 10 | 9 | 7 | 8 | 10 | 8 |
| Median | 4.2 | 4.2 | 4.5 | 4.6 | 4.4 | 4.0 |
| Minimum | 3.2 | 3.1 | 3.9 | 4.3 | 3.9 | 3.6 |
| Maximum | 5.1 | 6.0 | 4.7 | 5.3 | 5.7 | 4.9 |
| 11 | ||||||
| n | 10 | 9 | 7 | 8 | 10 | 8 |
| Median | 4.3 | 4.0 | 3.2 | 3.2 | 3.3 | 2.9 |
| Minimum | 3.4 | 2.4 | 2.6 | 2.8 | 2.6 | 2.0 |
| Maximum | 4.9 | 5.5 | 3.9 | 3.5 | 4.6 | 3.5 |
| Median change from baseline | +0.1 | −0.5 | −1.3 | −1.6 | −1.2 | −1.5 |
| 15 | ||||||
| n | 10 | 8 | 7 | 8 | 9 | 8 |
| Median | 4.4 | 3.9 | 2.9 | 3.4 | 3.3 | 2.9 |
| Minimum | 3.6 | 2.2 | 2.2 | 3.1 | 2.1 | 1.7 |
| Maximum | 5.0 | 5.7 | 4.4 | 3.4 | 4.7 | 3.8 |
| Median change from baseline | +0.1 | −0.4 | −1.6 | −1.3 | −1.3 | −1.7 |
| 24 | ||||||
| n | 9 | 8 | 7 | 7 | 10 | 8 |
| Median | 4.3 | 4.7 | 4.3 | 4.1 | 4.4 | 3.6 |
| Minimum | 3.9 | 3.2 | 2.5 | 3.2 | 3.1 | 2.1 |
| Maximum | 5.0 | 6.0 | 5.2 | 5.0 | 5.7 | 5.2 |
| Median change from baseline | +0.1 | −0.1 | −0.2 | −0.4 | −0.2 | −0.5 |
| 40 | ||||||
| n | 9 | 8 | 6 | 8 | 9 | 7 |
| Median | 4.4 | 4.6 | 4.5 | 4.5 | 4.3 | 4.2 |
| Minimum | 2.6 | 3.8 | 1.8 | 3.5 | 4.1 | 3.6 |
| Maximum | 5.9 | 5.6 | 5.0 | 5.0 | 5.4 | 4.9 |
| Median change from baseline | 0.0 | −0.2 | 0.0 | −0.5 | −0.1 | 0.0 |
Median, minimum, maximum, and median change from baseline refer to HIV-1 RNA levels.
HIV-1 RNA levels are in units of log10 copies/ml.
One subject (no. 5002) assigned to cohort 5 (150 mg/day of TBR-652) was found to have a tropism shift from CCR5 to CXCR4 coreceptor at baseline and should not have been eligible for the study. There were no shifts in viral tropism among the other patients, who provided usable samples for testing at study termination. Ad hoc retesting of patient 5002's baseline sample was performed at another laboratory (BC Centre for Excellence in HIV/AIDS, Vancouver, Canada) using the geno2pheno algorithm to infer tropism from triplicate population sequencing of the V3 loop (6, 10). Results confirmed that the patient had a preponderance of CXCR4-tropic virus at entry, which made this patient ineligible for the study. As a result, this patient was omitted from antiviral efficacy analyses. TBR-652 showed strong antiviral activity after 10 days of once-daily dosing. The median changes in HIV-1 RNA from baseline on day 11 for the placebo and 25-, 50-, 75-, 100-, and 150-mg dose levels were +0.1, −0.5, −1.3, −1.6, −1.2, and −1.5 log10 copies/ml, respectively. The mean HIV-1 RNA reductions from baseline achieved statistical significance by day 4 for all doses and by day 7 reached P values of <0.002 for the 25-mg QD dose and <0.001 for the 50-, 75-, 100-, and 150-mg QD dose levels. This level of significance persisted through day 15 for the 50- to 150-mg QD dosing groups. HIV-1 RNA reductions were still significant on day 24 in the 150-mg dose group (P = 0.03). Median nadir HIV-1 RNA values in the 25-, 50-, 75-, 100-, and 150-mg dose groups were −0.7, −1.6, −1.8, −1.4, and −1.7 log10 copies/ml, respectively.
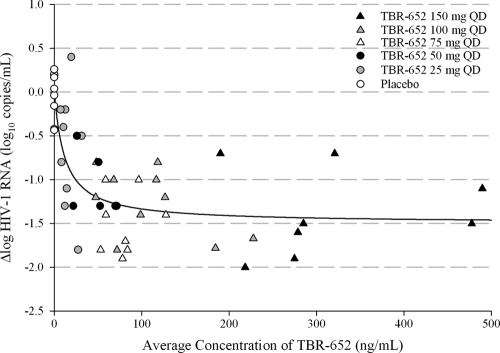
A simple Emax model was used to describe the relationship between PK and PD of TBR-652 based on the minimization of the AIC. Results obtained from the simple Emax model are presented in Fig. 3. The Emax and IC50 of TBR-652 were −1.43 log10 copies/ml and 13.1 ng/ml, respectively. Standard errors (SE) of Emax and IC50 parameters of TBR-652 were 0.138 log10 copies/ml and 6.53 ng/ml, respectively. Based on the PK/PD model, the average steady-state concentration levels of TBR-652 for the 25-, 50-, 75-, 100-, and 150-mg doses are expected to result in 54.5%, 79.6%, 85.7%, 89.3%, and 95.8% of the maximum inhibitory effect (Emax) of TBR-652, respectively. The above results suggest that TBR-652 doses of 75, 100, and 150 mg QD are expected to result in robust antiviral activity, with PD effects greater than 80% of the maximum inhibition (Imax) of TBR-652 in HIV-1-infected patients.
Fig. 3.
Correlation between average concentrations of TBR-652 and Δlog HIV-1 RNA on day 11.
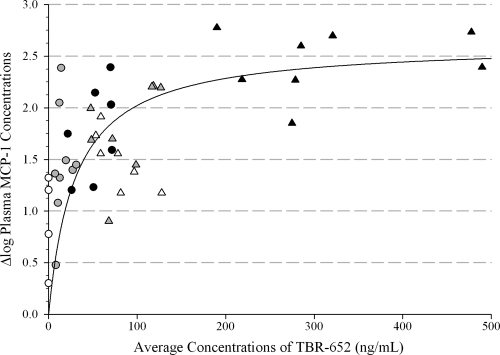
The relationship between the exposure of TBR-652 and MCP-1 plasma concentrations was described using a simple Emax model. Results obtained with the Emax model for MCP-1 are presented in Fig. 4. The Emax and IC50 of TBR-652 were 2.63 log10 and 29.8 ng/ml, respectively. SE of Emax and IC50 parameters of TBR-652 were 0.233 log10 pg/ml and 9.45 ng/ml, respectively. Based on the PK/PD model, MCP-1 concentrations increase rapidly with higher dose levels and reach a plateau at exposure levels of TBR-652 associated with the 150-mg dose level. The above results suggest a strong CCR2 blockade at the higher dose levels of TBR-652. Additional studies will be required to assess the longer-term effect of TBR-652 on MCP-1.
Fig. 4.
Correlation between average concentrations of TBR-652 and Δlog MCP-1 concentrations (pg/ml).
Adverse events were reported in a total of 34 patients, 30 of whom received TBR-652 treatments. Of these 34 patients, 20 patients on TBR-652 treatment had adverse events considered possibly or probably related to the drug. A total of 40% of patients on placebo reported at least one adverse event; the greatest number of patients reporting an adverse event were in the 150-mg dose group (100%), but the least number were in the 75-mg dose group (22%). Overall, adverse events were generally mild, and there were no indications of a relationship with TBR-652 dose. The most frequent adverse events were gastrointestinal (63%) and general disorders (37%). Nearly all patients with AEs were considered to have a grade 1 or 2 event except one patient each on placebo and the 75-mg dose who had grade 3 adverse events. The latter was an abscess that was judged nonserious and unrelated to study drug and resolved without sequelae. There were no grade 4 AEs, no serious AEs (SAEs), no deaths, and no study discontinuations because of an AE.
DISCUSSION
The current placebo-controlled, dose escalation study in HIV-1-infected, antiretroviral treatment-experienced, CCR5 antagonist-naïve patients was designed as a proof-of-concept study to demonstrate the antiviral activity of TBR-652. The short-duration trial was performed using monotherapy treatments of TBR-652 to minimize drug resistance risk for other viral targets and possibly other drugs in the same drug class while allowing assessment of antiviral potency without confounding antiviral activity and without the confounding pharmacologic interactions of other antiviral agents (5).
The pharmacokinetics of TBR-652 on day 10 was well characterized for all dose levels, with plasma concentrations well above the lower limit of quantitation of the assay. TBR-652 was well absorbed in the circulation, and systemic concentrations declined slowly, with mean elimination half-lives ranging from 22.5 to 47.62 h across dose levels. The favorable elimination half-life of TBR-652 was therefore appropriate for once daily dosing, and systemic concentrations of TBR-652 reached steady-state concentrations after 7 days of dosing. As expected, minimum steady-state concentrations (Cmin) of TBR-652 following 25- to 150-mg QD dosing were approximately 3.4- and 64-fold higher, respectively, than the in vitro-estimated therapeutic concentration of 2 ng/ml for TBR-652. Mean values of oral clearance of TBR-652 for the 50-, 75-, and 100-mg QD dose levels were consistent: 49.26, 42.17, and 48.06 liters/h, respectively. The mean CL/F value for TBR-652 at the 150-mg QD dose level (22.95 liters/h) appeared to be lower than those observed at the 25- and 150-mg QD dose levels. Consistent with the above information, mean values of AUC0–24 and Cmax of TBR-652 increased in a dose-proportional manner from 50 to 100 mg QD.
TBR-652 showed a potent dose-response effect on HIV-1 RNA levels that persisted after discontinuation of treatment. The mean HIV-1 RNA reductions from baseline achieved statistical significance for all dose levels. Median nadir HIV-1 RNA values in the 25-, 50-, 75-, 100-, and 150-mg dose groups were −0.7, −1.6, −1.8, −1.4, and −1.7 log10 copies/ml, respectively. Overall, nadir changes HIV-1 RNA were greater in the 50-, 75-, and 150-mg dose groups, for which the doses were administered using the 25-mg tablet formulations (F1). Furthermore, the 100-mg dose group was provided with a single daily tablet of 100 mg in a new formulation (F2), and these patients had a median change from baseline in HIV-1 RNA of −1.2 log10 copies/ml and a nadir change from baseline of −1.4 log10 copies/ml. Difference in formulations may be responsible for the slightly lower antiviral activity of the 100-mg dose level compared to those observed at the 50-, 75-, and 150-mg dose levels.
The relationship between TBR-652 exposure and antiviral activity was adequately described using an inhibitory Emax model. The Emax of TBR-652 derived with the PK/PD model was −1.43 log10 copies/ml. The steady-state plasma concentration of TBR-652 associated with 50% of the Emax (IC50) was 13.1 ng/ml. The above PK/PD results suggest that dose levels of 75, 100, and 150 mg QD would be expected to provide robust antiviral activity, with PD effect greater than 80% of the Emax of TBR-652 in HIV-1-infected patients. Dosing strategies of TBR-652 will be developed to maximize the probability of concentrations above the IC50 of TBR-652. The above PD values of TBR-652 are consistent with those previously reported for maraviroc, the first CCR5 antagonist approved by the FDA, since repeated oral administrations of 100 to 300 mg maraviroc for 10 days resulted in Emax values ranging from −1.13 to −1.45 log10 copies/ml, with an IC50 of 8 ng/ml (13). Furthermore, exploratory analysis suggests that TBR-652 displays significant dose-dependent increases in MCP-1 levels, confirming that TBR-652 is a potent CCR2 antagonist in HIV-1-infected patients.
Safety was monitored throughout the trial. In addition, viral tropism shifts and emergence of resistance were carefully monitored in the current study since these have been observed for the CCR5 inhibitor class of drugs and for antiretroviral agents of all drug classes (3, 11, 15). No serious adverse events or deaths occurred in the current study. A single patient withdrew consent after a single dose of the study drug due to difficulty with phlebotomy. The most commonly reported adverse events were headache, nausea, constipation, diarrhea, and sinusitis. No QTc prolongation or clinically significant ECG changes were observed. Overall, TBR-652 was well tolerated at all dose levels in the current dose escalation study. A thorough description of adverse events observed in the current study was presented by Lalezari et al. (9).
Based on the above results, monotherapy TBR-652 treatments resulted in promising PK and PD results and lack of significant side effects in HIV-1-infected, antiretroviral treatment-experienced and CCR5 antagonist-naïve patients. The dose and exposure-response relationship of TBR-652 observed in the current study will be used to support further development of TBR-652 following concomitant administration with highly active antiretroviral therapy (HAART) to HIV-infected, treatment naïve and CCR5-tropic patients.
Footnotes
Published ahead of print on 12 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Cohen C., et al. 2007. Potent antiretroviral activity of the once-daily CCR5 antagonist INCB009471 over 14 days of monotherapy, abstr. TUAB106. Abstr. 4th Int. AIDS Soc. Conf. HIV Pathogenesis Treatment Prev [Google Scholar]
- 2. Craig C., et al. 2010. Mechanisms of virologic failure with maraviroc in treatment-naïve HIV-1-infected patients through 96 weeks, poster 536. Abstr. 17th Conf. Retrovir. Opportunistic Infect [Google Scholar]
- 3. Esté J. A. 2002. Sch-351125 and Sch-350634. Schering Plough. Curr. Opin. Invest. Drugs 3:379–383 [PubMed] [Google Scholar]
- 4. Fätkenheuer G., et al. 2005. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV 1. Nat. Med. 11:1170–1172 [DOI] [PubMed] [Google Scholar]
- 5. Feinberg J., Japour A. J. 2003. Scientific and ethical considerations in trial design for investigational agents for the treatment of human immunodeficiency virus infection. Clin. Infect. Dis. 36:201–206 [DOI] [PubMed] [Google Scholar]
- 6. Harrigan P. R., et al. 2009. Screening for HIV tropism using population-based V3 genotypic analysis: a retrospective virological outcome analysis using stored plasma screening samples from MOTIVATE-1, abstr. WELBA101. Abstr. 5th Int. AIDS Soc. Conf., Cape Town, South Africa, 19 to 22 July 2009 [Google Scholar]
- 7. Hunt P., et al. 2007. Longitudinal evaluation of viral co-receptor tropism switches among HIV-infected patients with drug resistant viremia, poster 619. Abstr. 14th Conf. Retrovir. Opportunistic Infect., Los Angeles, CA, 25 to 28 February 2007 [Google Scholar]
- 8. Jubb R., et al. 2010. CCR5-tropic resistance to maraviroc is uncommon even among patients on functional MVC monotherapy or with ongoing low-level replication, poster 639. Abstr. 17th Conf. Retrovir. Opportunistic Infect [Google Scholar]
- 9. Lalezari J., et al. Safety, efficacy, and pharmacokinetics of TBR-652, a CCR5/CCR2 antagonist, in HIV-1-infected, treatment-experienced, CCR5 antagonist-naïve subjects. J. Acquir. Immune Defic. Syndr., in press [DOI] [PubMed] [Google Scholar]
- 10. McGovern R. A., et al. 2010. Population-based sequencing of the V3-loop is comparable to the enhanced sensitivity Trofile assay (ESTA) in predicting virologic response to maraviroc of treatment-naïve patients in the MERIT trial, abstr. 92. Abstr. 17th Conf. Retrovir. Opportunistic Infect, San Francisco, CA, 16 to 19 February 2010 [Google Scholar]
- 11. Moore J. P., Kuritzkes D. R. 2009. A pièce de resistance: how HIV-1 escapes small molecule CCR5 inhibitors. Curr. Opin. HIV AIDS 4:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palleja S., et al. 2009. The multiple-dose safety, tolerability, and pharmacokinetics of TBR-652, a chemokine receptor 5 (CCR5) antagonist, in healthy volunteers, abstr. A1-1308. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009 [Google Scholar]
- 13. Rosario M. C., Poland B., Sullivan J., Westby M., van der Ryst E. 2006. A pharmacokinetic-pharmacodynamic model to optimize the phase IIa development program of maraviroc. J. Acquir. Immune Defic. Syndr. 42:183–191 [DOI] [PubMed] [Google Scholar]
- 14. Schlecht H. P., Schellhorn S., Dezube B. J., Jacobson J. M. 2008. New approaches in the treatment of HIV/AIDS—focus on maraviroc and other CCR5 antagonists. Ther. Clin. Risk Manag. 4:473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shafer R. W., Shapiro J. M. 2008. HIV-1 drug resistance mutations: an updated framework for the second decade of HAART. AIDS Rev. 10:67–84 [PMC free article] [PubMed] [Google Scholar]
- 16. Shepherd J. C., et al. 2008. Emergence and persistence of CXCR4-tropic HIV in a population of men from the Multicenter AIDS Cohort Study. J. Infect. Dis. 198:1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Suleiman J., et al. 2010. Vicriviroc in combination therapy with an optimized background regimen for treatment-experienced subjects: 48-week results of the VICTOR-E1 phase 2 trial. J. Infect. Dis. 201:590–599 [DOI] [PubMed] [Google Scholar]
- 18. Trkola A., et al. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. U. S. A. 99:395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waters L., et al. 2008. The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to a first antiretroviral therapy regimen. Clin. Infect. Dis. 46:1617–1623 [DOI] [PubMed] [Google Scholar]
- 20. Westby M., van der Ryst E. 2005. CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection. Antivir. Chem. Chemother. 16:339–354 [DOI] [PubMed] [Google Scholar]
- 21. Westby M., et al. 2006. Emergence of CXCR4-using human immunodeficiency virus type 1 (HIV-1) variants in a minority of HIV 1-infected patients following treatment with the CCR5 antagonist maraviroc is from a pretreatment CXCR4-using virus reservoir. J. Virol. 80:4909–4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whitcomb J. M., et al. 2007. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob. Agents Chemother. 51:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]