Abstract
Our objective was to investigate neonatal emtricitabine (FTC) plasma and intracellular pharmacokinetics. The study was designed as a phase I/II prospective trial in two sequential steps evaluating the combination of tenofovir disoproxil fumarate (TDF) and FTC for the prevention of mother-to-child-transmission (PMTCT) of HIV. HIV-1-infected pregnant women received two tablets of TDF (300 mg) and FTC (200 mg) at onset of labor and then one tablet daily for 7 days postpartum. Based on the data obtained in the first part of the Tenofovir/Emtricitabine in Africa and Asia (TEmAA) Study, single doses of 2 mg/kg of FTC and 13 mg/kg of TDF were given to the neonates within 12 h after birth. A total of 540 FTC plasma concentrations and 44 active intracellular phosphorylated metabolite FTC-TP concentrations were taken from the 36 enrolled women and their neonates. Concentrations were measured by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method and analyzed by a population approach. The proposed dose obtained by simulations based on plasma drug concentrations was confirmed. However, median FTC-TP exposures were, respectively, 5.9 and 6.8 times higher in the fetus and the neonate than in the adult. High FTC-TP concentrations were observed in the four children who had serious adverse events (SAEs), but the link between FTC-TP concentrations and SAEs in children was not formally identified. The exposure to the active form of FTC was high in neonates despite plasma drug concentrations equivalent to those in adults. Our results are similar to those obtained with zidovudine or lamivudine.
INTRODUCTION
The tenofovir-emtricitabine (FTC) combination was proposed during the perinatal period for prevention of mother-to-child transmission (PMTCT) of HIV and/or to reduce viral resistance to nevirapine, thanks to administration to pregnant women at the start of labor (2, 5). In a previous study using a population pharmacokinetic approach, we proposed the following doses and schemes for the use of FTC during the perinatal period: for the mother, two tablets of tenofovir disoproxil fumarate (TDF) (300 mg)-FTC (200 mg) were given at the onset of labor, and if she did not deliver within the 12 h from the first administration, another two tablets of TDF-FTC, followed by one tablet per day were administered for 7 days postpartum. For the neonate, a 2-mg/kg FTC single dose within the 12 h after birth was proposed; this recommendation was based on pharmacokinetics data obtained from neonates from the transplacental transfer of FTC, after administration of FTC to the pregnant women before delivery (9). It was coadministered with a single dose of 13 mg/kg of TDF.
The first objective of the following study was to evaluate our selected dose of FTC in pregnant women and their neonates. The second objective was to measure the active intracellular metabolites of FTC in neonates, as previously high intracellular phosphorylated metabolites for lamivudine triphosphate (3TC-TP) and zidovudine triphosphate (AZT-TP) during the first 2 weeks of life have been detected (7). Few data have been reported so far on FTC-TP in adults (14, 17), and there are, to our knowledge, no data from neonates. In the present study, FTC-TP concentrations were measured in peripheral blood mononuclear cells (PBMCs) extracted from the cord and from neonatal plasma and compared to adult concentrations.
MATERIALS AND METHODS
Patients.
Patients eligible for the study were pregnant women at between 28 and 38 weeks of gestation, older than 18 years, infected by HIV-1 and/or HIV-2, and naïve to all antiretroviral treatment, who had an indication for antiretroviral prophylaxis for prevention of mother-to-child-transmission (PMTCT) during pregnancy (in line with the following international or national recommendations: WHO's clinical stage 1 or 2 and a CD4 count of ≥200/mm3 or stage 3 and a CD4 count of ≥350/mm3). Neonates with a gestational age greater than 37 weeks and a birth weight greater than 2,000 g were eligible. The study protocol was approved by the national ethics committees of Ivory Coast and Cambodia and by the University of the Witwatersrand Health Research Ethics Committee in South Africa and by each country's health or medicine regulatory authorities. The mother and, where possible, the father of the child to be born provided signed informed consent forms.
Treatments.
Mothers were administered ZDV (300 mg twice a day) from enrollment in the study (between the 28 and 36 weeks of amenorrhea) to delivery, with one tablet of NVP (200 mg) and two tablets of TDF (300 mg)-FTC (200 mg) given at the onset of labor. An additional dose of two tablets of TDF-FTC was administered to the mothers who did not deliver within the 12 h after the first administration. In postpartum, mothers received one tablet of TDF (300 mg)-FTC (200 mg) per day for 7 days. Neonates received on the first day of life a single dose of NVP syrup (2 mg/kg of body weight), within the 12 h after birth a single dose of TDF oral solution (13 mg/kg) and a single dose of FTC oral solution (2 mg/kg), and for 7 days ZDV syrup (4 mg/kg every 12 h).
Sampling.
Blood samples were collected for pharmacokinetic analysis: at delivery; 1, 2, 3, 5, 8, 12 and 24 h after the administration of 400 mg FTC at the onset of labor; and 24 h after the 1st, 2nd or 3rd, and 7th administrations of 200 mg FTC. Two cord blood samples were obtained at delivery: the first to measure plasma drug concentrations and the second to measure FTC-TP concentration in PBMCs. The neonate had 4 plasma samples collected, in the windows 0.5 to 1.5 h, 6 to 10 h, and 24 to 36 h after the administration of FTC syrup. Three samples, one in each time window, were collected to measure plasma FTC concentrations, and one sample was collected between 24 and 36 h to measure FTC-TP in PBMCs. Blood samples (4 ml for cord blood and 2 ml for newborn blood) for PBMCs were obtained by direct venipuncture or through an indwelling catheter. Concentrations in PBMC samples could be measured only when a Ficoll gradient centrifugation was performed within the 6 h after the sampling time. This was not possible for all samples.
Analytical method.
FTC and FTC-TP were from Moravek Biochemicals (Brea, CA). For determination of the plasma FTC concentration, a liquid-liquid extraction procedure was performed using 300 μl of methanol-dichloromethane (10/16 [vol/vol]) containing 0.1% hydrochloric acid added to 100 μl of plasma samples. After vortex and centrifugation at 20,000 × g at +4°C during 20 min, the upper phase was withdrawn and evaporated under nitrogen to dryness. The sample was then reconstituted with 100 μl of the mobile phase, and 40 μl of the extract was injected in the analytical system. FTC concentrations were measured by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method previously described (11) and adapted for FTC. The limit of quantification (LOQ) was 10 ng/ml.
PBMCs were collected from treated patients according to a previously reported method, including a specific red blood cell lysis step (6). PBMCs were then kept frozen at −80°C, transferred on dry ice to the analytical laboratory. For the determination of FTC-TP levels, a previously reported LC-MS/MS assay method (12) was used, slightly modified for FTC-TP mass spectrometry detection. The limit of quantification of FTC-TP was 0.77 pmol/sample (i.e., 0.077 pmol/106 cells for a sample pellet containing 10 million cells), and the limit of quantification used for the determination of the FTC level in PBMCs was 40 pg/sample.
Accuracy was within ±20%, and the percentage coefficient of variation (CV) did not exceed 20% for the lower limit of quantification (LLOQ). Within-assay reproducibility ranged from 94.8 to 112.4% and from 0.6 to 3.2% for accuracy and precision, respectively. Between-assay reproducibility, including LLOQ level, ranged from 93.8 to 114.6% and from 1.6 to 4% for accuracy and precision, respectively.
Population pharmacokinetic model.
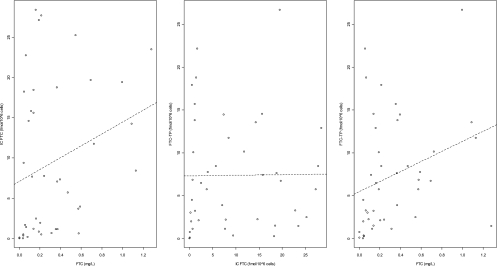
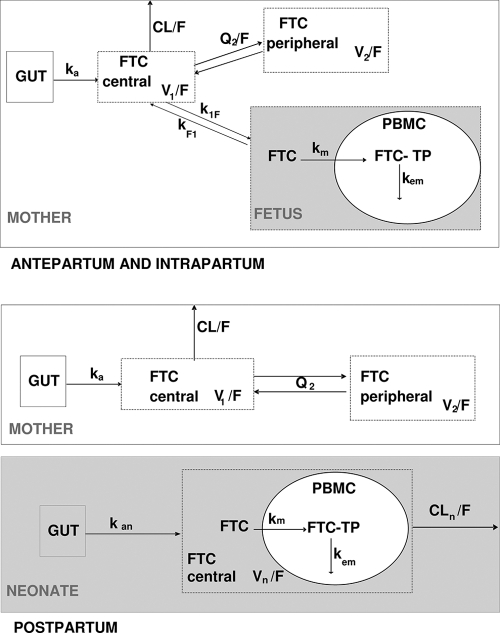
The link between FTC concentrations in plasma, FTC concentrations in PBMCs, and FTC-TP concentrations in PBMCs was evaluated in the fetus/neonate by using Spearman correlation tests. Fetal and neonatal FTC-TP concentrations were significantly correlated to FTC concentrations in plasma (r = 0.45, P = 0.003) but not to FTC concentrations in PBMCs (Fig. 1). Data were analyzed by using the MONOLIX software (version 3.1s; http://wfn.software.monolix.org) (10), and the SAEM algorithm was used. A two-compartment model with first-order absorption and elimination best described the maternal data. Plasma cord concentrations were described with an effect compartment model linked to the maternal circulation. After delivery, this fetal compartment was disconnected, and neonate was described by a one-compartment model with first-order absorption. An effect compartment linked to fetal and then neonatal circulation represented intracellular phosphorylated concentrations (FTC-TP) (Fig. 2). The parameters of the model were the maternal absorption rate constant (ka), maternal apparent elimination clearance from the central compartment (CL/F), maternal apparent volume of the central maternal compartment (V1/F), maternal apparent intercompartmental clearance (Q2/F), maternal apparent volume of the peripheral maternal compartment (V2/F), maternal-to-fetal rate constant (k1F), fetal-to-maternal rate constant (kF1), neonatal absorption (kan), apparent neonatal volume of distribution (Vn/F), apparent neonatal elimination clearance (CLn/F), neonatal FTC to intracellular FTC-TP metabolism constant rate (km), and the neonatal FTC-TP elimination constant rate (kem). When plasma FTC concentrations were below the LOQ, we set them to half of the LOQ. Goodness of fit was evaluated thanks to diagnostic plots. The model used to describe plasma FTC and intracellular FTC-TP concentrations was validated by using a visual predictive check, as previously described (9).
Fig. 1.
Correlations between fetal/neonatal plasma FTC, intracellular FTC, and intracellular FTC-TP concentrations.
Fig. 2.
Population pharmacokinetic model for the simultaneous prediction of plasma FTC concentrations in the mother, the cord and the neonate, and intracellular FTC-TP in the fetus and the neonate during intrapartum and postpartum. A two-compartment model with first order absorption and elimination best described the maternal data. Plasma cord concentrations were described with an effect compartment model linked to the maternal circulation. After delivery, this fetal compartment was disconnected, and the neonate was then described by a one-compartment model with first-order absorption. An effect compartment linked to fetal and then neonatal circulation represented intracellular phosphorylated concentrations (FTC-TP). Parameters of the model were the maternal absorption rate constant (ka), maternal apparent elimination clearance from the central compartment (CL/F), F for bioavailability, maternal apparent volume of the central maternal compartment (V1/F), maternal apparent intercompartmental clearance (Q2/F), maternal apparent volume of the peripheral maternal compartment (V2/F), maternal-to-fetal rate constant (k1F), fetal-to-maternal rate constant (kF1), neonatal absorption (kan), apparent neonatal volume of distribution (Vn/F) and apparent neonatal elimination clearance(CLn/F), neonatal FTC to intracellular FTC-TP metabolism constant rate (km), and the neonatal FTC-TP elimination constant rate (kem).
Proposed dose evaluation.
After the administration of FTC syrup to each neonate, the area under the concentration-time curve (AUC) and minimal (24 h postdose) plasma FTC concentrations (Cmin) were derived from the estimated individual pharmacokinetic parameters. Umbilical and neonatal FTC-TP profiles were drawn, and the corresponding exposures were compared to those previously published for adults.
RESULTS
Demographic data.
Data from the 36 enrolled women and their neonates were available for FTC pharmacokinetic evaluation. Table 1 summarizes the patients' characteristics. Two mothers delivered within the 30 min of their TDF-FTC administration. Two mothers did not deliver within the 12 h after the first administration and thus received another two-tablet dose of TDF-FTC. All of the children received FTC less than 15 h after birth, except one child who received it at 48.5 h of life.
Table 1.
Characteristics of the 36 HIV-infected pregnant women enrolled in the pharmacokinetic study of the TEmAA ANRS 12109 Trial, Step 2
| Covariate | Median value (range) |
|---|---|
| Maternal body wt at delivery (kg) | 67.5 (48–95.9) |
| Gestational age (wk) | 38 (37–44) |
| Delivery: vaginal, caesarian section (n) | 16, 20 |
| Neonatal body wt at birth (kg) | 3 (2.4–3.7) |
| Delay (h) between: | |
| Last maternal FTC administration and delivery | 6 (0.4–12) |
| Birth and neonatal FTC administration | 9.2 (1.5–48.6) |
| Neonatal FTC administration and sampling | 25.5 (10–45.5) |
Population pharmacokinetics.
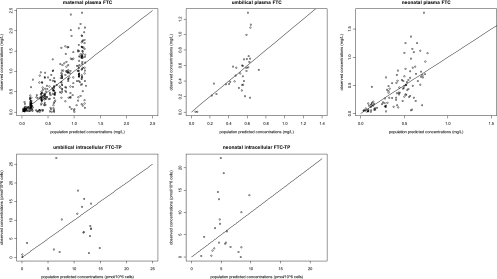
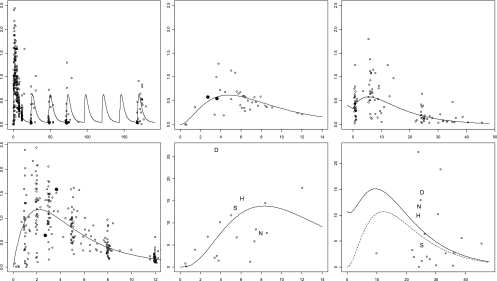
A total of 584 concentrations (398 from maternal plasma, 36 from cord plasma, 108 from neonatal plasma, 20 from cord PBMCs, and 22 from neonatal PBMCs) were available for pharmacokinetic analysis. Seven concentrations were below the LOQ (2 maternal plasma, 2 cord plasma, 1 cord cell, and 2 neonatal cell), so they were set to half of the LOQ (reported to 106 cells for FTC-TP). For FTC in maternal, fetal, and neonatal plasma and for FTC-TP in fetal and neonatal PBMCs, observed versus population predicted concentrations are displayed in Fig. 3 and observed versus predicted concentrations versus time are presented in Fig. 4. Table 2 summarizes the final population pharmacokinetic estimates. The available data were not sufficient to estimate intersubject variability (ISV) on V1/F, k1F, Vn/F, V2/F, kF1, km, and kan and fixing the variance of these random effects to zero had no influence on the OFVs. All residual variabilities were best described by a proportional error model. The type of delivery (vaginal versus caesarian) had a limited but significant effect on Q/F (P = 0.002; decrease in ISV from 27% to 13%) i.e., with caesarian modifying maternal intercompartmental flows, and on CLn/F (P = 1.3.10−5, decrease in ISV from 12% to 6%), i.e., caesarian decreasing by 30% the neonatal elimination clearance. None of the other covariates (maternal body weight, site of study, neonatal body weight, postnatal age of administration, gestational age, sex, and type of alimentation) had a significant effect on maternal or neonatal pharmacokinetics. As at postpartum, only residual concentrations were collected, thus the impact of pregnancy (labor versus postpartum) on CL/F could not be estimated precisely in our model. The model was validated thanks to a satisfactory diagnostic plot and visual predictive check (Fig. 5). The posterior predictive checks are broad for intercellular concentrations due to the low number of observed points and high variability in intracellular FTC-TP metabolism constant rate. However, with deletion of this variability, the neonatal FTC to intracellular FTC-TP metabolism constant rate had a poor estimation and the residual variability for cord TFV-DP increased from 51 to 80%.
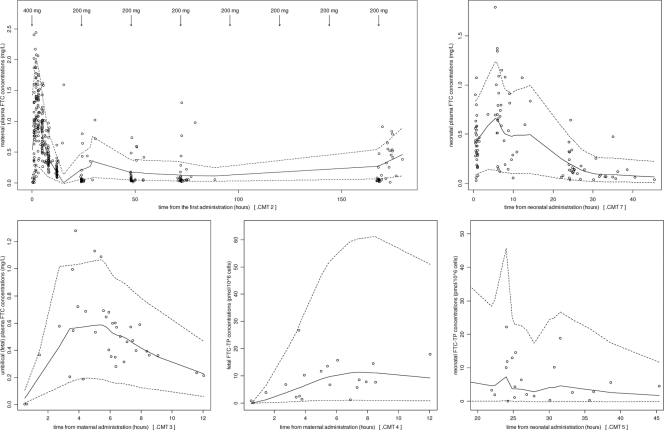
Fig. 3.
Observed versus population-predicted concentrations for maternal plasma FTC (top left panel), for cord plasma FTC (top middle panel) and cord PBMC FTC-TP (bottom left panel), for neonatal plasma FTC (top right panel), and for neonatal PBMC FTC-TP (bottom middle panel). The solid line represents the identity line.
Fig. 4.
Observed (symbols and letters) and population-predicted (line) concentrations versus time for maternal plasma FTC (all treatment long, top left; during the first 12 h, bottom left), for cord plasma FTC (top middle), and cord PBMC FTC-TP (bottom middle), for neonatal plasma FTC (top right) and for neonatal PBMC FTC-TP (bottom right). The dashed line represents the FTC-TP profile resulting only from neonatal administration. Solid symbols represent the 2 mother-fetus pairs for which the mother was readministered FTC because she delivered more than 12 h after the first intake. In the FTC-TP graph, concentrations in children with serious adverse events are represented by letters: D for death, H for hyperbilirubinemia, N for neutropenia, and S for serious brain injury.
Table 2.
Population pharmacokinetic parameters of emtricitabine from the final model for 36 HIV-infected pregnant women after receiving 400 mg of emtricitabine at the start of the labor and for their 36 neonates enrolled in the TEmAA ANRS 12109 Trial, Step 2a
| Structural model parameter | Estimate by structural model (% RSE) | Statistical model |
|
|---|---|---|---|
| Parameter | Estimate (% RSE) | ||
| ka | 0.709 h−1 (16) | ωka | 45% (39) |
| CL/F | 39.9 liters/h (4) | ωCL/F | 21% (32) |
| V1/F | 187 liters (9) | ωQ/F | 35% (65) |
| Q/F | 7.51 liters/h (19) | ωkm | 88% (39) |
| Q/F,θTDE | 2.03 (32) | ωCLn/F | 24% (44) |
| V2/F | 364 liters (19) | σmother plasma | 45% (4) |
| k1F | 0.292 h−1 (19) | σcord plasma | 42% (13) |
| kF1 | 0.398 h−1 (21) | σneonate plasma | 48% (8) |
| km | 4.92 h−1 (29) | σcord cells | 51% (39) |
| kem | 0.257 h−1 (24) | σneonate cells | 62% (29) |
| kan | 0.185 h−1 (30) | ||
| Vn/F | 7.62 liters (28) | ||
| CLn/F | 0.869 h−1 (13) | ||
| CLn/F,θTDE | 0.625 (24) | ||
RSE, relative standard error (standard error of estimate/estimate · 100); ka, absorption rate constant; CL/F maternal apparent elimination clearance from the central compartment; V1/F, apparent volume of distribution of the central maternal compartment; Q/F, apparent maternal intercompartmental clearance; V2/F, apparent volume of distribution of the peripheral maternal compartment; k1F, maternal-to-fetal rate constant; kF1, fetal-to-maternal rate constant; kan, neonatal absorption; Vn/F, apparent neonatal volume of distribution; CLn/F, apparent neonatal elimination clearance, km, FTC to intracellular FTC-TP metabolism constant rate; kem, FTC-TP elimination constant rate; σ, residual variability estimates (CV of residual variability [%]); ω, interindividual variability estimates (CV of intersubject variability [%]). Q/F, θTDE and CLn/F, θTDE, respectively, are the influential factors for the type of delivery (vaginal versus caesarian) on apparent intercompartmental clearance and neonatal apparent clearance.
Fig. 5.
Evaluation of the final model: comparison between the 5th (dashed line), 50th (full line) and 95th (dashed line) percentiles obtained from 1,000 simulations and the observed data (points) for plasma FTC concentrations in mother (top left panel), in cord (bottom left panel), and in neonates (top right panel) and for intracellular concentrations in PBMC FTC-TP in the fetus (bottom middle panel) and FTC-TP in the neonate (bottom right panel).
As the infant absorption rate constant was lower than expected, the sampling time windows used (0.5 to 1.5 h for the first sample and 6 to 10 h for the second) did not allow estimation of precisely the peak, which could have grossly affected the Vn/F estimate. Maternal FTC pharmacokinetics in plasma was described using a two-compartment model, whereas a one-compartment model was used to describe neonatal plasma FTC pharmacokinetics. In order to compare infant half-life and mother half-life, a one-compartment model was fitted to maternal data and compared to a one-compartment model for neonatal data. The half-life value was 9.7 h for a neonate born after a vaginal delivery, longer than the maternal half-life. In the first step of the TEmAA Study, the FTC median neonatal half-life was 10.6 h; it was 12.5 h in neonates from birth to 21 days in the study by Blum et al. (presented at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, 5 to 8 February 2006).
Proposed dose evaluation.
Using the proposed schedule, drug exposure to FTC was similar in pregnant women to that in nonpregnant adults (4, 13, 18). We had recommended readministration of FTC after 12 h if the mother had not yet delivered (9). As shown in Fig. 4, the two mothers and fetuses for whom FTC was readministered because labor continued for more than 12 h after the initial dose had comparable FTC concentrations to those of mothers who had a single administration at the start of labor.
We also proposed to give 2 mg/kg within the 12 h after birth as a first neonatal dose in order to obtain plasma exposure similar to that seen in adults. The median delay between birth and administration of FTC to the neonate was 9.2 h (range, 1.5 to 48.5 h). All of the neonates had plasma drug concentrations above the adult minimal concentrations before their administration of FTC syrup, except five: two who were born less than 30 min after the maternal drug intake and three more who received FTC syrup more than 12 h after their birth. The median area under the curve from 0 to 24 h after neonatal administration (AUC0–24) was 8.10 mg/liter · h (from 2.7 to 15.2). For 24 h, children had concentrations higher than or equal to the mean adult minimal concentrations of 0.077 mg/liter reported from three previous studies of adults after administration of 200 mg FTC once a day (QD): 0.071 mg/liter for Zhong et al. (18), 0.075 mg/liter for Blum et al. (presented at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, 5 to 8 February 2006), and 0.085 mg/liter for Ramanathan et al. (13).
Intracellular FTC-TP concentrations.
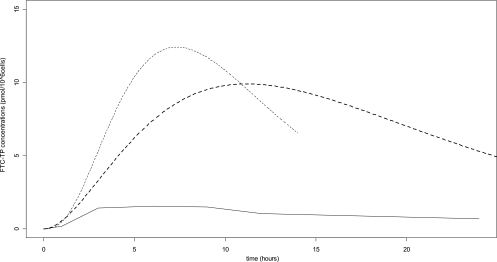
Although a very high intersubject variability was observed for neonatal FTC-TP concentrations, most of the children had very high FTC-TP concentrations compared to adults: from 1.5 to 26.7 pmol/106 cells in cord PBMCs 4 h after maternal administration and from 0.4 to 22 pmol/106 cells in neonatal PBMCs around 30 h after neonatal administration (after 22 to 38 h for 20 children and after 10 and 45 h for 2 other children). This neonatal sample was not at steady state. In this study, maternal FTC-TP concentrations in PBMCs were not measured, so an adult study with a single 200-mg FTC administration was used as a reference (17). In adults, the observed concentrations were 1.07 pmol/106 cells (interquartile range [IQR], 0.47 to 1.90), whereas in the cord, median FTC-TP concentrations were 7.65 pmol/106 cells (IQR, 3.19 to 12.66), and in the neonate, approximately 24 h after neonatal administration, concentrations were much higher: 5.14 pmol/106 cells (IQR, 2.23 to 10.77). Thanks to the population approach, the intracellular FTC-TP concentration-time profile could be drawn for cord (400-mg dose administered to the mother), for the neonate (6-mg dose administered to a median neonate with a body weight of 3 kg), and for the adult (single 200-mg administration), using data published by Wang et al. (17) (Fig. 6). Although the neonatal half-life (median of 10 h) was lower than that of an adult (around 20 h), median FTC-TP exposures were, respectively, 5.9- and 6.8-fold higher in the fetus and the neonate than in the adult.
Fig. 6.
FTC-TP concentrations as a function of time. Shown are simulated concentrations in cord (dashed thin line) for a 400-mg dose administered to the mothers, in neonates with a median body weight of 3 kg (dashed bold line) for a 6-mg dose administered to the neonates, and in nonpregnant adults for a single 200-mg administration (data from Wang et al. [17]).
The active FTC-TP concentrations in cord and neonatal PBMCs, represented differently in Fig. 4, seemed higher in the four children with serious adverse events (SAEs) (a transient grade 3 neutropenia in one South African child at day 28 visit, a transient grade 3/4 hyperbilirubinemia in two South African children at the day 7 visit, a serious infectious event in one Ivorian child leading to death and a serious brain injury in one Ivorian child at birth) (15) than in the other children without SAEs. One infant tested at 3 days and at 4 weeks of life had detectable HIV-1 RNA plasma viral load, suggesting in utero HIV infection (2.9%, 95% confidence interval [CI], 0.0 to 15.4), with no viral resistance at day 28. This neonate was born less than 30 min after maternal drug intake, and thus plasma FTC and intracellular FTC-TP concentrations in cord were lower than the LOQ.
DISCUSSION
The model used adequately described FTC concentrations in mothers and FTC/FTC-TP concentrations in fetuses and neonates. The population approach used was particularly adapted to pregnant women and neonates because only a few samples were required to describe their pharmacokinetics (if the number of patients is sufficient). Moreover this approach was necessary to analyze intracellular drug concentrations in neonates and their link with plasma drug concentrations (3). Indeed in neonates, due to the low blood volume available, only a single blood sample was collected to measure both plasma and intracellular drug concentrations. Since plasma and intracellular half-lives could be very different (i.e., different plasma and intracellular slopes of elimination), a simple ratio (whatever the time is) could not be used, so the population approach was thus the most appropriate method.
FTC undergoes intracellular phosphorylation by various cellular kinases to an active triphosphate, FTC-TP. In this second step of the study, FTC-TP exposure was shown to be 5.9 times higher in the fetus and 6.8 times higher in neonates than in adults (17). Unfortunately maternal intracellular concentrations were not measured in our study; thus, adult exposure was reported from the study by Wang et al. (17). To verify the variation of the intracellular assays between the two laboratories, we compared FTC-TP concentrations at steady state that we have in the two laboratories. In our lab, 14 patients (23 samples) were taking one tablet of truvada daily; at steady state, the median FTC-TP concentrations in PBMCs were 6.9 pmol/106 cells (IQR, 6.3 to 8.6). In the study by Wang et al. (17), an apparent plateau median level of approximately 4 pmol/106 cells was reported for FTC-TP in PBMCs. In the same study, Wang et al. reported FTC-TP concentrations of 1.04 pmol/106 cells (IQR, 0.47 to 1.90) 12 h after a single administration of FTC. We could thus suppose that after a single administration in adults, the FTC-TP concentrations would have been between 1 and 2 pmol/106 cells in our lab and thus much lower than umbilical and neonatal FTC-TP concentrations measured in our study. Intracellular FTC-TP competes with the endogenous dCTP (which was not measured in our study) as a substrate for HIV reverse transcriptase. Thus, FTC-TP activity is a function of not only its concentrations but also the competing dCTP; an FTC-TP/dCTP ratio would have been more relevant than an absolute measure of FTC-TP to describe emtricitabine activity in PBMCs. This absolute increase in FTC-TP exposure is in agreement with a previous study showing that newborns are likely to be overexposed to intracellular phosphorylated 3TC-TP and AZT-TP during the first 2 weeks of life (7). This could be related to differences in the PBMC activation states between adults and neonates, with FTC like 3TC being more efficiently phosphorylated in activated cells (8). This high activation of FTC in neonates could be an advantage for the efficacy of this drug to prevent perinatal transmission of HIV. However, the potential toxicity of this drug in the neonates needs to be assessed even if the mitochondrial toxicity of this drug seems less important than the toxicity of zidovudine or lamuvidine (16). Indeed, in this study, four serious adverse events were observed in children who had relatively high FTC-TP concentrations; it seems unlikely that an SAE is related to a high concentration of FTC-TP. However, our study could not draw a definitive conclusion, and additional studies are needed to better study the relationship between high FTC-TP concentrations and the occurrence of severe adverse events in neonates.
Our result confirms the recommendation made in the first step of the study for the use of FTC during the perinatal period. However, these recommendations were based on the comparison of plasma drug concentrations between neonates and nonpregnant adults (5). Obtaining the same drug exposure in children's plasma as that in adults' plasma is a currently accepted method to determine the dose to administer to the children, but this method has its limits. As reported here for FTC and previously described for AZT and 3TC (7), the exposure to the intracellular concentrations of the drug (active and/or potentially toxic compound) could be much higher in neonates/children than in adults, and this cannot be detected with plasma samples only. Moreover, this overexposure to intracellular phosphorylated AZT-TP, 3TC-TP, and FTC-TP during the first 2 weeks of life does not seem to be systematic for all nucleoside reverse transcriptase inhibitors (NRTIs), as can be suggested by the high activation state in the PBMCs of the neonate (8). Indeed, no correlation was found between intracellular FTC-TP concentrations and the intracellular form of the tenofovir disoproxil fumarate (TFV-DP), the drug coadministered to the neonate with FTC (joint data). Different enzymes are implicated in the phosphorylation of emtricitabine and that of tenofovir (1).
In conclusion, the exposure to the active form of FTC was high in neonates, despite plasma drug concentrations equivalent to those in adults, as previously shown for AZT or 3TC.
ACKNOWLEDGMENTS
We acknowledge the French Agence Nationale de Recherches sur le VIH/SIDA et les hépatites virales (ANRS) for sponsoring the trial, as well as the European and Developing Country Clinical Trials Partnership (EDCTP) and the French charity Sidaction for additional financial support.
We greatly thank the local investigators and their staff in the Formations Sanitaires Urbaines de Youpougon-Attié and Abobo-Avocatier, the Centre Hospitalier Universitaire de Yopougon and the Centre de Diagnostic et de Recherches sur le SIDA in Abidjan, in the Calmette Hospital, and Pasteur Institute in Phnom Penh, and in the Perinatal HIV Research Unit, Lesedi Clinic in Soweto, and BARC SA Clinical Trials in Johannesburg. We also thank the women who agreed to participate in the trial and their infants. We acknowledge Gilead Sciences for providing the study drugs.
The TEmAA Study Group is constituted as follows. The primary investigators are François Dabis (INSERM U897, ISPED, Bordeaux, France) and Didier Koumavi Ekouévi (PACCI, Abidjan, Ivory Coast). The coinvestigators are Christine Rouzioux, Stéphane Blanche, Jean-Marc Treluyer, Marie-Laure Chaix, and Elisabeth Rey (Paris, France); N′Dri-Yoman (Abidjan, Ivory Coast); Leang Sim Kruy and Eric Nerrienet (Phnom Penh, Cambodia); and Glenda Gray and James McIntyre (Soweto, South Africa). The trial coordinator is Elise Arrivé (Bordeaux, France).
The other members of the TEmAA ANRS 12109 Study Group are as follows: from Paris, Déborah Hirt, Saik Urien, and Alain Pruvost; from Abidjan, Gérard Allou, Divine Avit, Clarisse Amani-Bosse, Kouakou Brou, Patrice Coffie, Patrice Fian, Eulalie Kanga, Broulaye Kone, Suzanne Kouadio, Jeanne Eliam Kouakou, Sidonie Ngatchou, Touré Pety, Zenica Seoue, and Mamourou Kone; from Phnom Penh, Laurence Borand, Pinn Chou, Kearena Chhim, Meng Ly Ek, Leakhena Say, Seng Hout, Sethikar Im, Saroeum Keo, Vannith Lim, Sopheak Ngin, Vara Ouk, Vibol Ung, and the Magna and Maryknoll Associations; and from Soweto, Promise Duma, Portia Duma, Sarita Lalsab, Shini Legote, Joseph Makhura, Modise Maphutha, Selvan Naidoo, Mandisa Nyati, and Ravindre Panchia.
The Scientific Board comprises Bernard Koffi Ngoran (Abidjan, Ivory Coast), Koum Kanal (Phnom Penh, Cambodia), Lynn Morris (Johannesburg, South Africa), Séverine Blesson (ANRS, Paris, France), Camille Aubron-Olivier (Gilead Sciences, Paris, France), Gilles Peytavin (Paris, France), Koen Van Rompay (Davis, CA), and Valériane Leroy (Bordeaux, France).
The Independent Committee comprises John Sullivan (Worcester, MA), Philippe Lepage (Brussels, Belgium), Laurent Mandelbrot (Paris, France), Marie-Louise Newell (London, United Kingdom), and Anne-Marie Taburet (Paris, France).
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Anderson P. L., Kakuda T. N., Lichtenstein K. A. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 38:743–753 [DOI] [PubMed] [Google Scholar]
- 2. Arrive E., et al. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36:1009–1021 [DOI] [PubMed] [Google Scholar]
- 3. Bazzoli C., et al. 2010. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin. Pharmacokinet. 49:17–45 [DOI] [PubMed] [Google Scholar]
- 4. Blum M. R., Chittick G. E., Begley J. A., Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47:751–759 [DOI] [PubMed] [Google Scholar]
- 5. Chi B. H., et al. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698–1705 [DOI] [PubMed] [Google Scholar]
- 6. Durand-Gasselin L., Da Silva D., Benech H., Pruvost A., Grassi J. 2007. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 51:2105–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durand-Gasselin L., et al. 2008. High levels of zidovudine (AZT) and its intracellular phosphate metabolites in AZT- and AZT-lamivudine-treated newborns of human immunodeficiency virus-infected mothers. Antimicrob. Agents Chemother. 52:2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao W. Y., Agbaria R., Driscoll J. S., Mitsuya H. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633–12638 [PubMed] [Google Scholar]
- 9. Hirt D., et al. 2009. Population pharmacokinetics of emtricitabine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Antimicrob. Agents Chemother. 53:1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuhn E., Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 49:1020–1038 [Google Scholar]
- 11. Pruvost A., et al. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob. Agents Chemother. 53:1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pruvost A., Theodoro F., Agrofoglio L., Negredo E., Benech H. 2008. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. J. Mass Spectrom. 43:224–233 [DOI] [PubMed] [Google Scholar]
- 13. Ramanathan S., Shen G., Cheng A., Kearney B. P. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J. Acquir. Immune Defic. Syndr. 45:274–279 [DOI] [PubMed] [Google Scholar]
- 14. Rousseau F. S., et al. 2001. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J. Antimicrob. Chemother. 48:507–513 [DOI] [PubMed] [Google Scholar]
- 15. TEmAA ANRS 12109 Study Group 2010. Maternal and nenonatal nevirapine, tenofovir and emtricitabine to prevent vertical transmission of HIV-1: tolerance and resistance. AIDS 24:2481–2488 [DOI] [PubMed] [Google Scholar]
- 16. Venhoff N., Setzer B., Melkaoui K., Walker U. A. 2007. Mitochondrial toxicity of tenofovir, emtricitabine and abacavir alone and in combination with additional nucleoside reverse transcriptase inhibitors. Antivir. Ther. 12:1075–1085 [PubMed] [Google Scholar]
- 17. Wang L. H., et al. 2004. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res. Hum. Retroviruses 20:1173–1182 [DOI] [PubMed] [Google Scholar]
- 18. Zong J., et al. 2007. Pharmacokinetic evaluation of emtricitabine in combination with other nucleoside antivirals in healthy volunteers. J. Clin. Pharmacol. 47:877–889 [DOI] [PubMed] [Google Scholar]