Abstract
The resistance of biofilms to conventional antibiotics complicates the treatment of chronic cystic fibrosis (CF). We investigated the effects of peptoids, peptides, and conventional antibiotics on the biomass and cell viability within Pseudomonas aeruginosa biofilms. At their MICs, peptoids 1 and 1-C134mer caused maximum reductions in biomass and cell viability, respectively. These results suggest that peptoids of this class could be worth exploring for the treatment of pulmonary infections occurring in CF patients.
TEXT
Pseudomonas aeruginosa is one of most fatal known pathogens, causing 200,000 hospital-acquired infections annually in the United States (17). More than 80% of cystic fibrosis (CF) patients are infected by P. aeruginosa (13, 26), and chronic CF infections result from the formation of P. aeruginosa biofilms in the lung (14, 27). The current standard of care for the treatment of P. aeruginosa infections is long-term use of antibiotics in combination (12). The frequent use of antibiotics has led to the widespread emergence of multidrug-resistant Pseudomonas strains, exacerbating the overall problem (4, 6). The high prevalence of antibiotic resistance has created a pressing need to discover pharmaceutical candidates that could replace or complement current therapies.
Antimicrobial peptides (AMPs) are integral components of the innate host defense mechanism in many organisms (11). Unlike conventional antibiotics, AMPs seem to function through a nonspecific mechanism (3), and the failure of bacteria to evolve resistance to AMPs is believed to be attributable to this generalized mechanism of action (11, 23). Although AMPs seem promising as therapeutics to treat bacterial infections, many peptides have low bioavailability (11). Efforts to ameliorate these biostability disadvantages have prompted the design, synthesis, and use of nonnatural mimics of AMPs (2, 3, 5, 24, 25, 28, 29). Oligo-N-substituted glycines (peptoids) are isomers of peptides, with side chains attached to the backbone nitrogen rather than the α-carbon (22, 32). This structural difference makes them highly resistant to protease activity and reduces the likelihood of immunogenicity (15, 20). In this study, we investigated the activities of seven different peptoids and two AMPs, relative to those of three conventional antibiotics, against planktonic cells and biofilms of P. aeruginosa (strain PA 14).
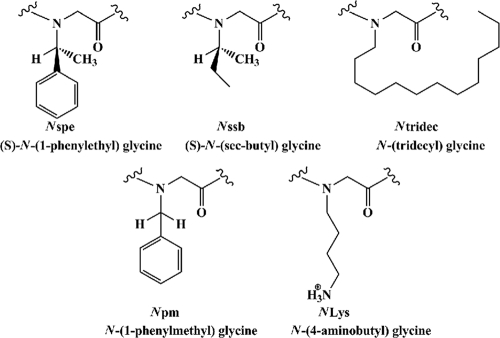
The selected peptoids (Table 1; Fig. 1) are analogues of our lead compound and positive control, an amphipathic and cationic dodecamer peptoid, peptoid 1, known to have good activity against planktonic bacteria; in contrast, peptoid 1-(S)-N-(sec-butyl) glycine (Nssb) is almost inactive and acts as a negative control (5, 22; A. M. Czyzewski, R. Kapoor, N. P. Chongsiriwatana, M. T. Dohm, S. Vakulenko, S. Mobashery, and A. E. Barron, submitted for publication). Peptoids were synthesized by a solid-phase, submonomer protocol (32); the monomer structures are shown in Fig. 1. All results are presented as averages of results from three independent replicates in three parallel trials. Error bars represent the means ± standard deviations. Statistical differences from the control (without an added antimicrobial) were determined by one-way analysis of variance (ANOVA) with post hoc testing by the Tukey-Kramer method. Differences were considered statistically significant at a P of <0.001. For detailed materials and methods, see the supplemental material.
Table 1.
Sequences of peptoids, AMPs, and conventional antibiotics and their antimicrobial activities against planktonic PA 14
| Peptoid, AMP, or antimicrobial | Sequence (amino to carboxyamide)a | MICb for P. aeruginosa (PA 14) in: |
|
|---|---|---|---|
| μM | mg/liter | ||
| 1 | H-(NLys-Nspe-Nspe)4-NH2 | 12.5 | 22.7 |
| 1-11mer | H-(NLys-Nspe-Nspe)3-NLys-Nspe-NH2 | 12.5–25 | 20.7–41.4 |
| 1-Pro9 | H-(NLys-Nspe-Nspe)2-NLys-Nspe-l-Pro-NLys-Nspe-Nspe-NH2 | 25–50 | 43.8–87.6 |
| 1-achiral | H-(NLys-Npm-Npm)4-NH2 | 25 | 42.6 |
| 1-C134mer | H-Ntridec-NLys-Nspe-Nspe-NLys-NH2 | 12.5–25 | 10.4–20.8 |
| 14mer | H-NLys-Nspe-Nspe-NLys-NH2 | >100 | >59.5 |
| 1-Nssb | H-(NLys-Nssb-Nssb)4-NH2 | >100 | >143.4 |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 25–50 | 112.3–224.6 |
| Pexiganan | GIGKFLKKAKKFGKAFVKILKK | 12.5–25 | 30.9–61.8 |
| Ciprofloxacin | 0.4 | 0.1 | |
| Tobramycin | 1.6 | 0.7 | |
| Kanamycin | >100 | >48.4 | |
Fig. 1.
Peptoid submonomer chemical structures.
Activities of peptoids against planktonic cells.
MICs were determined in accordance with CLSI M7-A6 protocols (Table 1) (5, 7). Ciprofloxacin and tobramycin were active in the low micromolar range, while PA 14 showed a high tolerance to kanamycin (Table 1). Pexiganan and LL-37 were active in the range of 12.5 to 50 μM. Peptoid 1 was active at 12.5 μM, whereas peptoid 1-Nssb was completely inactive. Alkylated peptoid 1-C134mer was active in the range of 12.5 to 25 μM, while unalkylated peptoid 14mer was ineffective (Table 1). Other variants displayed lower antimicrobial activity than peptoid 1.
Biofilm formation assay.
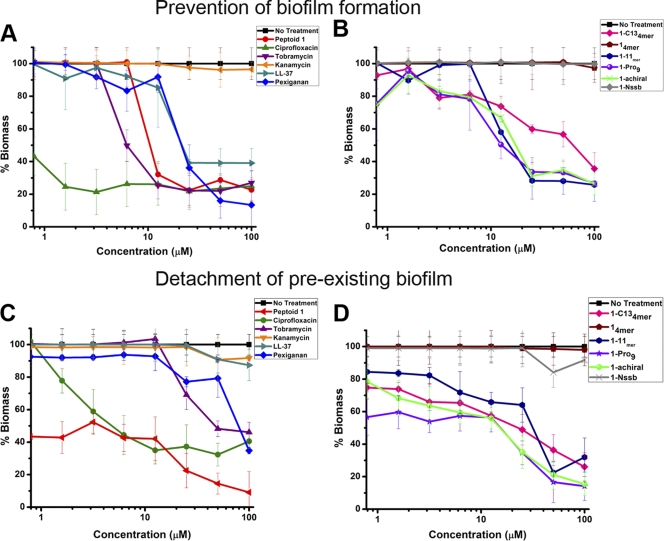
To determine if peptoids could be used prophylactically to prevent biofilm formation, we spectrophotometrically tested whether peptoids eliminated P. aeruginosa prior to biofilm formation using a crystal violet (CV) staining assay (Fig. 2A and B) (30). Since the MICs of most of the peptoids were in the range of 12.5 to 25 μM, we selected the lower value (12.5 μM) as the threshold of antimicrobial activity. Ciprofloxacin and tobramycin caused an ∼70% reduction in biofilm formation at 4× and 8× their MICs, respectively, and were distinct from kanamycin, which had no effect (Fig. 2A). AMPs showed only an ∼10% reduction in biomass, compared to peptoids 1-Nssb and 14mer (Fig. 2B). Peptoids 1 and 1-C134mer provided significant reductions in biomass (∼70% and ∼40%, respectively) (Fig. 2A and B). The other peptoids showed activities similar to that of peptoid 1-C134mer.
Fig. 2.
Efficacies of antimicrobial peptoids, AMPs, and conventional antibiotics against biofilm formation (A and B) and for the detachment of preexisting P. aeruginosa biofilms (C and D) for two different groups of antimicrobial compounds tested. Prevention of biofilm formation and detachment of existing biofilms are plotted as percent biomass, as analyzed by a crystal violet (CV) staining assay.
Biofilm detachment assay.
Peptoids were evaluated as potential therapeutics for CF by testing their efficacy against preexisting biofilms. The detachment assay was performed by pregrowing the biofilms without the presence of an antibiotic and measuring the biofilm reduction by the CV staining process (Fig. 2C and D) (30). Ciprofloxacin and tobramycin caused reductions of ∼60% and ∼30%, respectively, but AMPs were unable to reduce the biomass of established biofilms (Fig. 2C). Peptoids 1 and 1-C134mer impaired existing biofilms by 60% and 40%, respectively (Fig. 2C and D). Other peptoids had activities equivalent to that of peptoid 1-C134mer.
Cell viability.
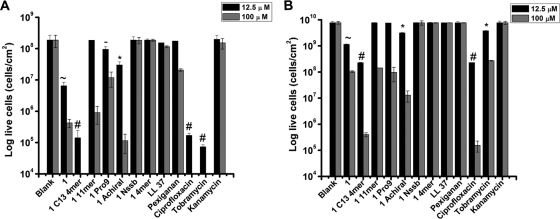
CV staining assays measure total biofilm biomass but provide no information about cell viability (9). To determine whether peptoids were effective in killing the bacterial cells within biofilms, cell viability was measured by bacterial plating (9) at a concentration close to the MIC (12.5 μM) and at a completely lethal concentration (100 μM) (Fig. 3). At 12.5 μM, ciprofloxacin and tobramycin prevented the formation of biofilms by reducing cell viability by ∼3 logs, and kanamycin showed no killing (Fig. 3A). In contrast, LL-37 and pexiganan reduced viability by ∼14% and ∼7%, respectively. Peptoids 1-Nssb and 14mer were inactive, whereas peptoids 1 and 1-C134mer were active to a significant degree, reducing cell viability by ∼1.5 and ∼3 logs, respectively. Peptoids 1-11mer, 1-Pro9, and 1-achiral were less active, yielding an ∼0.5-log reduction in viable cells (Fig. 3A).
Fig. 3.
Effects of antimicrobials (tested at 12.5 and 100 μM) on cell viability in preventing the formation of biofilms (A) and in reducing the established P. aeruginosa (PA 14) biofilms (B). All symbols (∼, #, -, and *) indicate that values for antimicrobials at 12.5 μM are statistically significantly different (P < 0.001) from the value with no antimicrobial (blank). Different symbols indicate a statistically significant difference (P < 0.001) between compounds with two different symbols (e.g., peptoids 1 and 1-C134mer) but not between compounds with the same symbol (e.g., ciprofloxacin and tobramycin in panel A). The absence of a symbol indicates no statistically significant difference from the value with no antimicrobial. Statistical significance at 100 μM is not shown.
Like conventional antibiotics, peptoids were less effective in killing bacteria in established biofilms than in killing planktonic bacteria prior to the formation of biofilms (Fig. 3). Ciprofloxacin and tobramycin caused significant ∼2- and ∼1-log decreases in viable cells, respectively, while AMPs had no effect (Fig. 3B). Significant reductions were caused by peptoids 1 (∼1-log decrease) and 1-C134mer (∼2-log decrease), whereas peptoids 1-11mer and 1-Pro9 were ineffective in killing the cells in established biofilms; finally, peptoid 1-achiral caused ∼0.5-log killing.
It is clear that peptoids prevent biofilm formation by killing planktonic cells that could otherwise contribute to biofilm formation, and peptoid 1 is the most promising agent. Bacterial detachment from the surface by peptoids resulted in biomass reduction. Cationic AMPs and peptoids are known to bind DNA (10, 16, 21), and this binding may facilitate detachment or disruption of otherwise-stable biofilm structures. It has previously been reported that extracellular DNA (eDNA) is involved in cell-cell attachment (1); the development of P. aeruginosa biofilms is disrupted by DNase I (31). Effective biofilm detachment by peptoid 1 relative to that of other peptoids may be due to the inherent oligomerization of peptoid 1 (19; Yoriel Marcano and Annelise Barron, unpublished data). Oligomerization via interactions of aromatic side chains, which is disrupted upon an encounter with an anionic bacterium, would increase the localized concentration of peptoid near the cell membrane, increasing its activity and perhaps contributing to biofilm detachment.
In contrast, peptoid 1-C134mer was most efficient in reducing cell viability relative to other peptoids. The hydrophobic tail gives peptoid 1-C134mer a surfactant-like nature which may interact strongly with and disrupt the hydrophobic exopolysaccharide matrix, facilitating deeper peptoid penetration into the matrix than that of peptoid 14mer, which lacks this tridecyl surfactant tail. Alkylated peptides and peptoids have the potential for micelle formation at the MIC (18), which again, as for peptoid 1, may increase their local concentration when they reach the anionic bacterial cell membrane, if we presume that the cationic micelles spontaneously dissemble after adsorption to the anionic membrane surface.
In this study, we have shown that antimicrobial peptoids reduce the viability of P. aeruginosa (PA 14) biofilms at their MICs, whereas antibiotics showed comparable results only at concentrations 8 to 30 times higher than their MICs (Fig. 3). To the best of our knowledge, this is the first study demonstrating the effects of antimicrobial peptoids against P. aeruginosa biofilms. Peptoids such as those we have tested in this study may prove to be interesting as a novel class of antibiotics, as they act rapidly and seem to be able to overcome the resistance that makes conventional antibiotics less effective against chronic infections.
Supplementary Material
Acknowledgments
We thank Man-Wah Tan (Department of Genetics, Stanford University) for kindly gifting the P. aeruginosa (PA 14) strain, Christopher Contag (Department of Pediatrics, Stanford University) and Henk Haagsman (Department of Infectious Diseases and Immunology, Utrecht University) for reviewing the manuscript, and Janus Haagensen for insightful discussion and valuable advice.
This work was supported by NIH grant 5 R01 AI072666-04.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Allesen-Holm M., et al. 2006. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 59:1114–1128 [DOI] [PubMed] [Google Scholar]
- 2. Arnt L., Tew G. N. 2002. New poly(phenyleneethynylene)s with cationic, facially amphiphilic structures. J. Am. Chem. Soc. 124:7664–7665 [DOI] [PubMed] [Google Scholar]
- 3. Bessalle R., Kapitkovsky A., Gorea A., Shalit I., Fridkin M. 1990. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 274:151–155 [DOI] [PubMed] [Google Scholar]
- 4. Boucher H. W., et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1–12 [DOI] [PubMed] [Google Scholar]
- 5. Chongsiriwatana N. P., et al. 2008. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 105:2794–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chopra I., et al. 2008. Treatment of health-care-associated infections caused by Gram-negative bacteria: a consensus statement. Lancet Infect. Dis. 8:133–139 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2006. Performance standards for antimicrobial susceptibility texting; sixteenth informational supplement. CLSI document M100-S16. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Reference deleted.
- 9. Di Bonaventura G., Spedicato I., D'Antonio D., Robuffo I., Piccolomini R. 2004. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrob. Agents Chemother. 48:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hale J. D., Hancock R. E. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 5:951–959 [DOI] [PubMed] [Google Scholar]
- 11. Hancock R. E., Sahl H. G. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24:1551–1557 [DOI] [PubMed] [Google Scholar]
- 12. Hancock R. E., Speert D. P. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Updat. 3:247–255 [DOI] [PubMed] [Google Scholar]
- 13. Heijerman H. 2005. Infection and inflammation in cystic fibrosis: a short review. J. Cyst. Fibros. 4(Suppl. 2):3–5 [DOI] [PubMed] [Google Scholar]
- 14. Hoiby N., et al. 2001. Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3:23–35 [DOI] [PubMed] [Google Scholar]
- 15. Kirshenbaum K., et al. 1998. Sequence-specific polypeptoids: a diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. U. S. A. 95:4303–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lobo B. A., Vetro J. A., Suich D. M., Zuckermann R. N., Middaugh C. R. 2003. Structure/function analysis of peptoid/lipitoid:DNA complexes. J. Pharm. Sci. 92:1905–1918 [DOI] [PubMed] [Google Scholar]
- 17. Lyczak J. B., Cannon C. L., Pier G. B. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2:1051–1060 [DOI] [PubMed] [Google Scholar]
- 18. Makovitzki A., Avrahami D., Shai Y. 2006. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci U. S. A. 103:15997–16002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcano Y. 2008. Structural and biophysical studies of biomimetic poly-N-substituted glycine oligomers: intermolecular and intracellular peptoid interactions in solutions. Ph.D. dissertation Northwestern University, Evanston, IL [Google Scholar]
- 20. Miller S. M., Ng S. R. S., Zuckermann R. N., Kerr J. M., Moos W. H. 1995. Comparison of the proteolytic susceptibilities of homologous l-amino acid, d-amino acid, and N-substituted glycine peptide and peptoid oligomers. Drug Dev. Res. 35:20–32 [Google Scholar]
- 21. Otvos L., Jr 2005. Antibacterial peptides and proteins with multiple cellular targets. J. Pept. Sci. 11:697–706 [DOI] [PubMed] [Google Scholar]
- 22. Patch J. A., Barron A. E. 2002. Mimicry of bioactive peptides via non-natural, sequence-specific peptidomimetic oligomers. Curr. Opin. Chem. Biol. 6:872–877 [DOI] [PubMed] [Google Scholar]
- 23. Peschel A., Sahl H. G. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529–536 [DOI] [PubMed] [Google Scholar]
- 24. Porter E. A., Wang X., Lee H. S., Weisblum B., Gellman S. H. 2000. Non-haemolytic beta-amino-acid oligomers. Nature 404:565. [DOI] [PubMed] [Google Scholar]
- 25. Porter E. A., Weisblum B., Gellman S. H. 2002. Mimicry of host-defense peptides by unnatural oligomers: antimicrobial beta-peptides. J. Am. Chem. Soc. 124:7324–7330 [DOI] [PubMed] [Google Scholar]
- 26. Rajan S., Saiman L. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47–56 [DOI] [PubMed] [Google Scholar]
- 27. Romling U., et al. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 170:1616–1621 [DOI] [PubMed] [Google Scholar]
- 28. Sanborn T. J., Wu C. W., Zuckermann R. N., Barron A. E. 2002. Extreme stability of helices formed by water-soluble poly-N-substituted glycines (polypeptoids) with alpha-chiral side chains. Biopolymers 63:12–20 [DOI] [PubMed] [Google Scholar]
- 29. Tew G. N., et al. 2002. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. U. S. A. 99:5110–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thormann K. M., Saville R. M., Shukla S., Pelletier D. A., Spormann A. M. 2004. Initial phases of biofilm formation in Shewanella oneidensis MR-1. J. Bacteriol. 186:8096–8104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitchurch C. B., Tolker-Nielsen T., Ragas P. C., Mattick J. S. 2002. Extracellular DNA required for bacterial biofilm formation. Science 295:1487. [DOI] [PubMed] [Google Scholar]
- 32. Zuckermann R. N., Kerr J. M., Kent S. B. H., Moos W. H. 1992. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 114:10646–10647 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.