Abstract
Connection domain mutations (CDMs) in HIV-1 reverse transcriptase (RT) alter susceptibility to some nucleoside/nonnucleoside RT inhibitors (NRTIs/NNRTIs). Their effects on susceptibility and virologic responses to etravirine were analyzed. Seventeen CDMs were evaluated: L283I, E312Q, G333D, G333E, G335C, G335D, N348I, A360I, A360T, A360V, V365I, T369I, A371V, A376S, I393L, E399D, and E399G. CDM prevalence and effects on virologic responses were analyzed retrospectively using clinical data. The effects on etravirine susceptibility were assessed in clinical samples and confirmed using site-directed mutants. The most prevalent CDMs (>10%) were A371V, E399D, A376S, N348I, A360T, G333E, and L283I. CDM presence was positively correlated with thymidine analogue-associated mutations, but not with NNRTI resistance-associated mutations (RAMs). The presence or number of CDMs did not significantly reduce etravirine susceptibility, although small reductions were seen in samples with G333D, N348I, A360V, T369I, and A376S. N348I, E399G, and N348I/T369I were associated with reduced etravirine susceptibility when present with K103N, L100I, or Y181C. N348I or T369I was associated with reduced etravirine susceptibility when present with K101P or K103R/V179D. Virologic responses to an etravirine-containing regimen were slightly diminished when G333D, G335D, or A376S was present, but this was not confirmed in subgroups with higher baseline resistance or without etravirine RAMs. CDMs alone do not confer substantial reductions in etravirine susceptibility but can further reduce etravirine susceptibility in combination with certain NNRTI mutations. Since virologic responses to etravirine were not affected by CDMs, the clinical impacts of these mutations on etravirine susceptibility appear to be minimal.
INTRODUCTION
Human immunodeficiency type 1 (HIV-1) reverse transcriptase (RT) is a heterodimer consisting of two subunits, p66 and p51 (18, 36). The p66 subunit is composed of three domains. The polymerase domain (amino acids 1 to 318) and active site are situated in the N-terminal region and contain the binding sites for both nucleoside and nonnucleoside RT inhibitors (NRTIs and NNRTIs). The C-terminal region of RT includes the connection domain (amino acids 319 to 426) and the RNase H domain (amino acids 427 to 560) (18, 22). The p51 subunit contains identical N-terminal sequences but lacks the C-terminal RNase H domain.
Because most known NRTI and NNRTI resistance mutations are clustered around the polymerase active site and the NNRTI binding pocket (17, 22, 32), routine genotypic analysis of mutations in RT covers the first 300 to 400 N-terminal amino acids. However, recent data have shown that mutations in or near the connection domain of RT are also selected, along with thymidine analogue-associated mutations (TAMs), and can affect both NRTI and NNRTI resistance (11, 12). The Y318F mutation, located next to the connection domain, is associated with resistance to delavirdine (DLV), efavirenz (EFV), and nevirapine (NVP) (15) and is part of the NNRTI binding pocket. N348I induces decreased susceptibility to nevirapine and delavirdine (12). Recently, T369I was shown to confer NNRTI resistance and impair replication capacity, especially when combined with N348I (11).
Etravirine (ETR) (TMC125) is a more recently introduced NNRTI with potent in vitro activity against wild-type and NNRTI-resistant HIV-1 and exhibits a higher genetic barrier to resistance than the first-generation NNRTIs, which may be explained by its molecular flexibility (1, 6, 34). As such, etravirine provides an opportunity to suppress HIV-1 replication in patients with NNRTI resistance. The efficacy and safety of etravirine at 48 weeks in treatment-experienced HIV-1-infected patients was demonstrated in the DUET-1 (use of etravirine to demonstrate undetectable viral load in patients experienced with ARV therapy) and DUET-2 phase III clinical studies (20). These studies were randomized, double-blind, placebo-controlled, 48-week clinical trials that enrolled treatment-experienced patients with HIV-1 infection to assess the efficacy, tolerability, and safety of etravirine compared with placebo when given with a background antiretroviral (ARV) regimen. In both studies, the primary endpoint was the proportion of patients with a confirmed plasma HIV-1 RNA level of <50 copies/ml (intent to treat, time to loss of virologic response [ITT-TLOVR]) at week 24 (23, 24). Analyses of baseline resistance data from the DUET studies has resulted in the identification of 17 etravirine resistance-associated mutations (RAMs)—V90I, A98G, L100I, K101E/K101H/K101P, V106I, E138A, V179D/V179F/V179T, Y181C/Y181I/Y181V, G190A/G190S, and M230L—based on their association with decreased virologic response and/or increased etravirine fold change (FC) in the 50% effective concentration (35). Other genotypic algorithms have been developed in parallel (14).
Recently, it was shown that mutations in the RNase H domain also could confer resistance to NNRTIs by reducing RNase H cleavage and providing more time for the NNRTI to dissociate from the RT (26). Furthermore, the effect on NNRTI resistance was shown to be dependent upon the affinity of each NNRTI for the RT, with Q475A, Y501A, and D549N enhancing resistance to NVP and DLV, but not to EFV or etravirine (26).
No clinical data are currently available on whether connection domain mutations (CDMs) affect resistance to etravirine. Therefore, the present study was undertaken to evaluate the effects of CDMs on phenotypic susceptibility and virologic responses to etravirine by constructing and testing site-directed mutants (SDMs) and by interrogating clinical data derived from the DUET studies, respectively.
(Data in this paper were presented at the 18th International HIV Drug Resistance Workshop, Florida, 9 to 13 June 2009.)
MATERIALS AND METHODS
Lists of mutations.
The following 17 CDMs were identified from scientific literature searches and analyzed in the present study: L283I (5, 13), E312Q (25), G333D (9, 21), G333E (9, 21), G335C (13, 25), G335D (13, 25), N348I (9, 10, 12, 25, 37), A360I (25), A360T (13, 25), A360V (9, 25), V365I (25), T369I (10, 38), A371V (4), A376S (25, 27), I393L (13), E399D (10, 31), and E399G (8).
The prevalence of the CDMs was analyzed using a large phase III clinical trial data set (DUET) containing baseline resistance data from 1,184 samples, as well as, for comparison, in a pooled data set containing baseline resistance data from 2,190 treatment-naïve subjects enrolled in other Tibotec clinical trials.
In addition, a genotypic data set comprising RH/IN and PR/RT nucleotide sequences from 246 unique patient viruses submitted to Monogram Biosciences for commercial HIV genotypic resistance testing were utilized to calculate the prevalence of CDMs; 166 were subtype B and 80 were non-subtype B samples. Thirty-seven of the 166 subtype B samples lacked NRTI and NNRTI mutations (as defined by International AIDS Society-USA) (19), whereas 129 contained mutations in RT. Similarly, 12 of the 80 non-subtype B samples lacked RT mutations, whereas 68 contained RT mutations.
Covariation between CDMs, etravirine RAMs, and TAMs in the DUET data set was assessed using phi correlation coefficients, which calculate values in the range from −1 to 1, with a phi of −1 meaning that mutations were negatively associated, a phi of 0 indicating no association, and a phi of +1 meaning that mutations were positively correlated or clustered. The P values were adjusted for multiplicity using the Benjamini-Hochberg method (3). The list of etravirine RAMs comprises the following mutations: V90I, A98G, L100I, K101E/K101H/K101P, V106I, E138A, V179D/V179F/V179T, Y181C/Y181I/Y181V, G190A/G190S, and M230L (35). The list of TAMs contained M41L, D67N, K70R, L210W, T215F/T215Y, and K219E/K219Q (19).
Patient cohorts.
This analysis reports pooled results from the DUET-1 and DUET-2 studies for treatment-experienced patients who initiated treatment with etravirine in combination with a background regimen. To reduce bias in the resistance analyses, patients who discontinued the study for reasons other than virologic failure (VF) before week 24 were excluded (the non-VF excluded population) (35). De novo enfuvirtide users were also excluded in order to avoid the confounding effect of the activity contributed to the regimen by a new ARV class. This non-VF excluded, not de novo enfuvirtide population included 406 etravirine-treated patients, 403 of whom had both genotypic and phenotypic data available for analysis (35).
In a separate analysis, 123 patient viruses submitted for routine HIV resistance testing at Monogram Biosciences were utilized to generate RNase H/integrase (RH/IN) resistance test vectors (RTVs) containing the C terminus of RT (beginning at codon 317 and including the RNase H domain) and IN. Traditional protease (PR)/RT RTVs (29) from the same patient viruses were also generated, and both sets of RTVs were tested for etravirine susceptibility.
Analysis of virologic response.
The effect on virologic response (<50 copies/ml at week 24) was studied in etravirine-treated DUET patients not using enfuvirtide de novo and excluding those who discontinued for reasons other than VF (n = 406) (35). Reduced virologic response was defined as a virologic response of <51.9%, i.e., <75% of the response observed in a subgroup of patients without baseline NNRTI RAMs (for more details, see reference 35).
Receiver-operator characteristic (ROC) curves were constructed to compare the performances of the etravirine weighted genotypic score based on the 17 etravirine RAMs supplemented or not with specific CDMs. ROC curves depict the tradeoff between the true-positive rate (sensitivity) and the false-positive rate (1 − specificity) of each genotypic interpretation system, and a corresponding area under the curve (AUC) is used to assess the predictive value of each score (an AUC of 1 denotes a perfect test, and an AUC of 0.5 denotes a test without discriminatory power).
Construction of resistance test vectors and SDMs.
RTVs containing patient-derived HIV-1 pol sequences encoding PR and the first 305 amino acids of RT, collectively referred to as PR/RT, were constructed as previously described (29). A modification of this method was used to construct RTVs containing pol sequences encoding the C terminus of RT (codons 305 to 426; codons 306 to 316 span the 5′ primer and are identical to the NL4-3 sequence; codons 317 to 426 are derived from the patient virus), RNase H and IN, collectively referred to as RH/IN. RH/IN RTVs were generated by inserting RH/IN amplification products that are flanked by 5′ PinAI and 3′ XhoI restriction sites into an HIV-1 (NL4-3) genomic vector adapted with PinAI and XhoI RH/IN sequence acceptor sites and containing a luciferase reporter cassette that partially replaces the env gene.
Single or double mutations were introduced into the HIV-1 genomic vector described above, using the megaprimer method of site-directed mutagenesis (33). Specifically, mutations in the connection domain of RT (amino acid 333, 348, 369, or 399) were introduced into the RH/IN vector, while mutations in the polymerase domain of RT (K103N, Y181C, G190A, G190S, or L100I) were introduced into the PR/RT vector. The RH/IN fragments were then transferred into the PR/RT RTV as PinAI/XhoI fragments (11). Similarly, RTVs containing chimeric pol sequences comprised of PR/RT fragments containing well-characterized NRTI and NNRTI mutations, together with RH/IN fragments containing 3′ RT mutations at amino acid 333, 348, 369, or 399, were constructed by replacing, at the clonal level, the “wild-type” PR/RT regions of RH/IN RTVs with the “NRTI and NNRTI drug-resistant” PR/RT regions of PR/RT RTVs that were initially derived from patient viruses or by site-directed mutagenesis (11).
Phenotypic drug susceptibility testing.
NNRTI susceptibility was measured in single- or multicycle replication assays as previously described (16, 28, 29).
In the Monogram single-cycle assays (29), pseudotyped viruses were produced by cotransfecting 293 (human embryonic kidney) cell cultures with RTV plasmid DNA, plus an expression plasmid encoding the Env protein of amphotropic murine leukemia virus. Following inoculation of target cells, infectivity was assessed by measuring luciferase activity in the presence or absence of drug.
In the Antivirogram multicycle assay (16, 28), HIV-1 pol sequences encoding PR and the first 400 amino acids of RT were amplified from patient-derived viral RNA and transfected with a HIV-1 genomic vector with PR/RT deleted. Virus replication was assessed by measuring enhanced green fluorescent protein (EGFP) production to assess viral replication in HIV-1-infected MT4 long terminal repeat (LTR) EGFP cells.
RESULTS
Prevalence of CDMs and covariation with NRTI and NNRTI RAMs.
Based on the DUET baseline samples (n = 1,184, with matched genotypic and phenotypic resistance data), the most prevalent CDMs (defined as >10%) were A371V (27.2%), E399D (21.9%), A376S (16.0%), N348I (14.3%), A360T (13.5%), G333E (12.3%), and L283I (11.4%) (Table 1). Among the same 1,184 DUET samples, 233 (19.7%), 435 (36.7%), 334 (28.2%), and 182 (15.4%) harbored zero, one, two, and three or more CDMs, respectively. Furthermore, for 11 of 17 CDMs, a similar prevalence was observed in the genotypic data set comprising 246 samples submitted to Monogram Biosciences for routine drug resistance testing (Table 1). For 5 of the 6 other CDMs (L283I, A360V, V365I, A376S, and E399D), higher prevalence was observed in the DUET than in the Monogram data set, which is consistent with the higher level of treatment experience and resistance in DUET. The relatively low prevalence of G335D in DUET compared to the other data sets could be related to the HIV-1 subtype (G335D is mainly found in the non-B subtype). The prevalence data in the DUET and Monogram data sets were also compared with a large data set containing resistance data from treatment-naïve subjects (Table 1). These results confirmed that the prevalence of some CDMs increased with increasing levels of treatment experience (e.g., N348I and E399D).
Table 1.
Overview of CDMs
| CDM | Associated with resistance to: |
Prevalence (%) in: |
Reference(s) | |||
|---|---|---|---|---|---|---|
| NRTIs | NNRTIsa | Pooled DUET studies (n = 406) (%)b | Monogram data set (n = 246) | Treatment-naïve subjects (n = 2,190) (%)c | ||
| L283I | +d | + | 11.4 | 0.4 | 7.4 | 5, 13 |
| E312Q | + | 1.3 | 0.0 | 1.0 | 25 | |
| G333D | + | 1.2 | 0.8 | 1.0 | 9, 21 | |
| G333E | + | 12.3 | 7.7 | 9.4 | 9, 21 | |
| G335C | + | 1.2 | 0.0 | 0.6 | 13, 25 | |
| G335D | + | 5.5 | 32.9 | 32.1 | 13, 25 | |
| N348I | + | + | 14.3 | 10.2 | 0.3 | 9, 10, 12, 25, 37 |
| A360I | + | 1.5 | 0.8 | 0.1 | 25 | |
| A360T | + | 13.5 | 13.4 | 9.5 | 13, 25 | |
| A360V | + | 4.9 | 2.0 | 1.4 | 9, 25 | |
| V365I | + | 9.5 | 5.7 | 6.0 | 25 | |
| T369I | + | + | 0.2 | 0.8 | 0.1 | 10, 38 |
| A371V | + | 27.2 | 29.7 | 21.0 | 4 | |
| A376S | + | + | 16.0 | 8.9 | 9.1 | 25, 27 |
| I393L | + | 0.1 | 0.4 | 0.0 | 13 | |
| E399D | + | + | 21.9 | 17.9 | 10.5 | 10, 31 |
| E399G | + | + | 1.5 | 2.4 | 0.5 | 8 |
Efavirenz and nevirapine.
Non-VF excluded not de novo enfuvirtide population.
Treatment-naïve samples were collected from other Tibotec clinical trials, as described in Materials and Methods.
+, associated with resistance to NRTIs or NNRTIs.
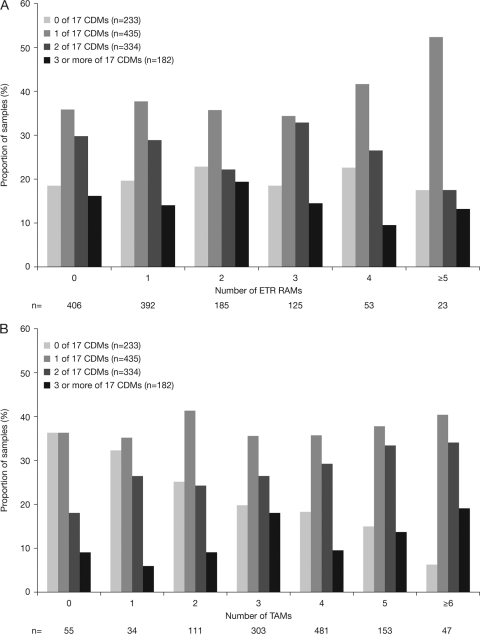
The overall distributions of viruses by number of etravirine RAMs in DUET were similar with or without CDMs (Fig. 1A) (P = 0.640 for the correlation between ETR RAMs and CDMs, determined with a chi-square test). More specifically, the proportions of viruses with zero, one, or more CDMs were similar whether or not the viruses also had zero, one, or more etravirine RAMs. In contrast, CDMs occurred more frequently in viruses with more TAMs, i.e., the proportion of samples with higher numbers of CDMs gradually increased when the number of TAMs increased in the same isolates, while the proportion of viruses without CDMs was lowest among those with the highest number of TAMs (Fig. 1B) (P = 0.013 for the correlation between CDMs and TAMs, determined with a chi-square test).
Fig. 1.
(A) Samples with different numbers of CDMs are similarly distributed irrespective of the number of etravirine RAMs (baseline data from DUET). (B) The number of CDMs increases with the number of TAMs (baseline data from DUET).
In an analysis of the covariance (phi correlation factor), none of the CDMs appeared to occur more frequently with specific etravirine RAMs or NRTI RAMs, whereas known positive or negative associations were confirmed among certain etravirine RAMs (e.g., Y181C and G190A) or NRTI RAMs (e.g., the TAM-1 mutation M41L cooccurs with other TAM-1 mutations, L210W or T215Y, but not with the TAM-2 mutations K70R and K219Q) (see the supplemental material).
Effect of CDMs on virologic response to etravirine in DUET.
When the response rates in the clinical samples from DUET were compared according to the presence or absence of each of the CDMs at baseline, the data showed that the virologic responses were unaffected by most CDMs, except for G333D, G335D, and A376S. Although the differences were not statistically significant, the virologic response in the presence of any of these three CDMs was close to the predefined threshold (Table 2). Since the overall response rate in the etravirine arms of the DUET studies was relatively high for these types of highly treatment-experienced patients (61.8% achieved an undetectable viral load at week 24), a subgroup analysis was performed in patients with less active ARVs in their regimens (defined as those patients with an etravirine FC of >3 and a darunavir FC of >10 at baseline) to more precisely assess the potential impacts of these CDMs on etravirine susceptibility. The results presented in Table 3 demonstrate that, for those CDMs occurring in at least 5 patients, the reduction in virologic response was not significantly different in this subgroup.
Table 2.
Response rates in the clinical samples from DUET according to the presence or absence of each of the CDMs at baseline
| CDM | Proportion of responders (HIV-1 RNA < 50 copies/ml at week 24) in clinical samples from the pooled DUET studiesa |
||
|---|---|---|---|
| Samples without CDM at baseline [n/total (%)] | Samples with CDM at baseline [n/total (%)] | Pc | |
| L283I | 218/356 (61.2) | 33/50 (66.0) | 0.539 |
| E312Q | 249/404 (61.6) | 2/2 (100.0) | ND |
| G333D | 249/401 (62.1) | 2/5 (40.0b) | ND |
| G333E | 218/353 (61.8) | 33/53 (62.3) | 1.000 |
| G335C | 246/400 (61.5) | 5/6 (83.3) | ND |
| G335D | 241/386 (62.4) | 10/20 (50.0b) | 0.345 |
| N348I | 216/346 (62.4) | 35/60 (58.3) | 0.567 |
| A360I | 246/397 (62.0) | 5/9 (55.6) | ND |
| A360T | 211/351 (60.1) | 40/55 (72.7) | 0.076 |
| A360V | 236/381 (61.9) | 15/25 (60.0) | 0.835 |
| V365I | 232/371 (62.5) | 19/35 (54.3) | 0.366 |
| T369I | 251/405 (62.0) | 0/1 (0.0b) | ND |
| A371V | 182/296 (61.5) | 69/110 (62.7) | 0.909 |
| A376S | 222/349 (63.6) | 29/57 (50.9b) | 0.078 |
| I393L | 251/406 (61.8) | 0/0 (NAd) | ND |
| E399D | 201/319 (63.0) | 50/87 (57.5) | 0.384 |
| E399G | 247/400 (61.8) | 4/6 (66.7) | ND |
Overall, 251/406 (61.8%).
Below predefined threshold of 51.9%.
P values were determined using the Fisher exact test; ND, not determined due to small sample size (n < 10).
NA, not applicable.
Table 3.
Response rates and association between individual CDMs and etravirine susceptibility in a subgroup of patients with less active ARVsa in their regimen
| CDM | Presence of CDM | No. of samples | Median etravirine FC | Median etravirine score | Virologic response rate (%) | P valuec for response |
|---|---|---|---|---|---|---|
| Allb | 47 | 10.3 | 4.00 | 21.3 | ||
| N384I | With | 9 | 13.4 | 3.50 | 22.2 | 1.000 |
| Without | 38 | 8.4 | 4.25 | 21.1 | ||
| A371V | With | 10 | 9.0 | 3.75 | 10.0 | 0.665 |
| Without | 37 | 13.0 | 4.00 | 24.3 | ||
| A376S | With | 8 | 5.3 | 2.75 | 25.0 | 1.000 |
| Without | 39 | 13.4 | 4.50 | 20.5 | ||
| E399D | With | 18 | 6.3 | 3.75 | 27.8 | 0.473 |
| Without | 29 | 13.4 | 4.50 | 17.2 |
Defined as an etravirine FC of >3 and a darunavir FC of >10 at baseline.
R333D, R335D, and T369I were present in fewer than 5 patients.
P values were determined using the Fisher exact test.
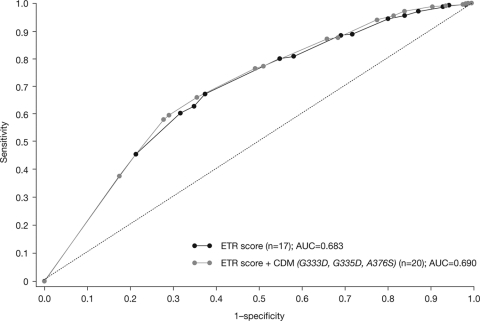
A multivariate analysis taking into account the baseline viral load and the darunavir FC demonstrated that addition of the three CDMs to the etravirine weighted genotypic score, containing the 17 previously identified etravirine RAMs, did not significantly improve the association with virologic response to etravirine. The AUC values of the ROC curves were 0.68 and 0.69 in the absence and presence of the CDMs, respectively (Fig. 2).
Fig. 2.
Addition of CDMs to the etravirine weighted genotypic score did not improve the association with response to etravirine.
Effects of CDMs on phenotypic susceptibility to etravirine using clinical samples from DUET.
Using the baseline phenotypic and genotypic data from DUET, the number of CDMs (i.e., the total count of all CDMs present in a viral isolate) was shown not to affect etravirine susceptibility. In the groups of isolates with zero, one, two, and three or more CDMs, 63.9%, 66.4%, 66.2%, and 62.8% had an etravirine FC of ≤3, respectively, and 15.9%, 12.0%, 12.9%, and 19.2% had an etravirine FC of >13, respectively. The median and interquartile range (Q1 and Q3) were calculated for the samples with or without each CDM, using the same baseline data from DUET. The median etravirine FC values were comparable among samples with or without the CDMs, except for T369I, for which the sample size was very small (n = 2) (Fig. 3). Higher Q3 values for the etravirine FC were observed for G333D, N348I, A360V, T369I, and A376S (Fig. 3).
Fig. 3.
Individual CDMs were associated with a modest reduction in etravirine susceptibility (baseline data from DUET).
Effects of CDMs on etravirine susceptibility in clinical samples submitted for routine HIV drug resistance testing.
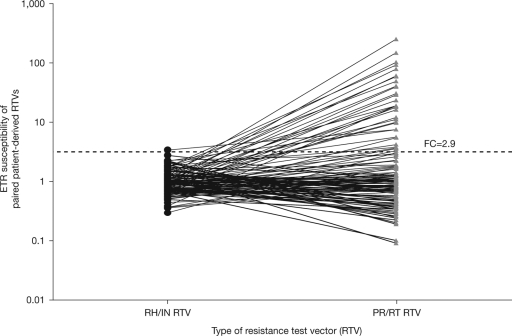
Using a different approach and an independent set of clinical samples (n = 123), the effects of CDMs on etravirine susceptibility were studied using two PhenoSense (Monogram Biosciences) assay methods. The first method involved the construction of conventional RTVs containing the PR/RT region, comprised of the entire protease open reading frame and the first 305 amino acids of RT (29). The second method involved the construction of RTVs containing the RH/IN region comprised of the connection and RNase H domains of RT and the entire IN coding region. The latter approach provided the opportunity to study the impacts of CDMs in the absence of NRTI and NNRTI mutations within the N terminus of RT. Etravirine susceptibility in the majority of isolates (122 out of 123; 99.2%) was equivalent to the NL4-3 reference RH/IN RTV (50% inhibitory concentration [IC50] FC, <2.9) (Fig. 4). In contrast, 33 out of 123 (26.8%) patient isolates exhibited reduced etravirine susceptibility using conventional PR/RT RTVs. The only patient-derived RH/IN RTV that exhibited reduced etravirine susceptibility (FC = 3.4) contained mutations N348I and E399G in the RT connection domain.
Fig. 4.
Comparison of etravirine susceptibilities in matching RH/IN and PR/RT RTVs from 123 patient samples.
Effects of specific CDMs on etravirine susceptibility based on site-directed mutagenesis.
SDMs were constructed to directly assess the effects of the CDMs N348I, T369I, G333E, and E399D on etravirine susceptibility, either alone or in combination with specific NNRTI mutations (data on G333D, A376S, and E399G were not available). The single SDMs N348I, T369I, G333E, and E399D and the double SDM N348I/T369I did not alter etravirine susceptibility in the absence of well-characterized NNRTI mutations (Table 4). E399D and G333E did not cause a reduction in etravirine susceptibility in combination with any of the tested NNRTI mutations. Furthermore, N348I or T369I, in combination with either K103N, G190A, G190S, or V179D, did not reduce etravirine susceptibility. The double SDM N348I/T369I exhibited modest reductions in etravirine susceptibility when present in combination with K103N (FC = 3.5), but not in the presence of G190A or G190S. While either T369I or N348I alone, combined with L100I, did not reduce etravirine susceptibility, the double SDM N348I/T369I, combined with L100I, conferred a 10-fold reduction in etravirine susceptibility (FC = 10.2). The addition of N348I, T369I, or N348I/T369I mutations to Y181C conferred larger reductions in etravirine susceptibility (FC = 8.2, 12.2, and 51.2, respectively) than Y181C alone (FC = 5.1). Similarly, the addition of N348I or T369I to K103R/V179D conferred larger reductions in etravirine susceptibility (FC = 6.7 and 5.2, respectively) than K103R/V179D alone (FC = 3.1). Finally, the addition of either N348I or T369I to K101P conferred larger reductions in etravirine susceptibility (FC = 9.5 and 6.6, respectively) than K101P alone (FC = 4.4).
Table 4.
Impacts of CDMs N348I, T369I, G333E, and E399D on the etravirine susceptibility of viruses with or without NNRTI mutations
| Mutation(s) in RT | Etravirine EC50a FC |
|---|---|
| T369I | 1.5 |
| N348I | 1.4 |
| T369I/N348I | 1.7 |
| E399D | 1.2 |
| G333E | 0.9 |
| K103N | 1.28 |
| K103N/T369I | 1.77 |
| K103N/N348I | 1.63 |
| K103N/T369I/N348I | 3.54 |
| K103N/E399D | 1.35 |
| K103N/G333E | 1.29 |
| G190S | 0.42 |
| G190S/T369I | 0.45 |
| G190S/N348I | 0.43 |
| G190S/T369I/N348I | 2.54 |
| G190S/E399D | 0.5 |
| G190S/G333E | 0.5 |
| K103R/V179D | 3.09 |
| K103R/V179D/T369I | 6.7 |
| K103R/V179D/N348I | 5.2 |
| K103R/V179D/T369I/N348I | NA |
| K103R/V179D/E399D | 4.31 |
| K103R/V179D/G333E | 3.55 |
| V179D | 1.02 |
| V179D/T369I | 1.65 |
| V179D/N348I | 1.93 |
| V179D/T369I/N348I | NA |
| V179D/E399D | 1.31 |
| V179D/G333E | 1.11 |
| K101P | 4.36 |
| K101P/T369I | 6.61 |
| K101P/N348I | 9.54 |
| K101P/T369I/N348I | NA |
| K101P/E399D | 5.49 |
| K101P/G333E | 3.78 |
| Y181C | 5.1 |
| Y181C/T369I | 8.18 |
| Y181C/N348I | 12.21 |
| Y181C/T369I/N348I | 51.24 |
| Y181C/E399D | NA |
| Y181C/G333E | NA |
| G190A | 0.82 |
| G190A/T369I | 1.09 |
| G190A/N348I | 1.58 |
| G190A/T369I/N348I | 1.88 |
| G190A/E399D | NA |
| G190A/G333E | NA |
| L100I | 1.42 |
| L100I/T369I | 1.88 |
| L100I/N348I | 2.89 |
| L100I/T369I/N348I | 10.18 |
| L100I/E399D | NA |
| L100I/G333E | NA |
Changes in phenotypic susceptibility are expressed as the FC in the 50% effective concentration (EC50) based on the EC50 of a drug-susceptible reference virus (EC50 of sample/EC50 of reference). NA, not applicable.
DISCUSSION
Mutations in the connection domain of HIV-1 RT (CDMs) have been associated with resistance to first-generation NNRTIs, including efavirenz and nevirapine. Based on data from clinical samples and supportive in vitro data presented here, we conclude that CDMs do not confer clinically significant reductions in etravirine susceptibility. Furthermore, SDMs harboring CDMs were susceptible to etravirine when present alone, and only in combination with specific NNRTI RAMs was decreased susceptibility to etravirine detected. No obvious impact on the virologic response to etravirine was observed for viruses that contained CDMs alone.
The prevalence of CDMs was evaluated in subjects enrolled in the DUET studies. The data showed that the presence of CDMs was not linked to the presence of etravirine RAMs, but they were more prevalent in samples with more TAMs. These observations confirmed previous reports that indicated that some of the CDMs can increase resistance to NRTIs, at least in the presence of TAMs (4, 7, 21, 25). On the other hand, previous studies have demonstrated phenotypic hypersusceptibility to etravirine observed among HIV-1 isolates carrying NAMs, M184V, or both (2, 30). Since CDMs appear to be coselected with NRTI resistance mutations and NRTI mutations confer hypersusceptibility to NNRTIs, the net effect of CDMs on NNRTI resistance may be offset by the opposing effects of CDMs and NRTI mutations on NNRTI susceptibility.
Further analysis of the DUET data set demonstrated that virologic responses were comparable in the presence or absence of the CDMs, with the exception of G333D, G335D, and A376S, for which virologic responses were modestly affected. However, this effect was not evident in a subgroup of patients with less active ARVs in their regimens or who lacked etravirine RAMs, where this effect would be expected to be more pronounced.
Overall, the number of CDMs did not affect etravirine susceptibility, although modest reductions in etravirine susceptibility were observed in samples with G333D, N348I, A360V, T369I, and A376S. In vitro SDM data demonstrated that some of these CDMs, including N348I and T369I, in combination with some of the etravirine RAMs, such as L100I or Y181C, conferred resistance to etravirine, but this effect was not observed in the absence of the etravirine RAMs. Furthermore, the prevalence of CDMs, including G333D and T369I, was very low (<1%) in the 2 different data sets analyzed, making any conclusions less robust.
The A376S mutation was the only CDM that appeared to confer a reduction in etravirine susceptibility in clinical samples, as well as in vitro (SDM data were not available to confirm this finding). It is conceivable that some of the CDMs occurred more frequently in samples with more resistance to NRTIs and/or NNRTIs and were therefore indirectly associated with decreased response. These results suggested that these mutations were not essential but only enhanced the effect of other “classic” NNRTI RAMs. This was confirmed by the multivariate analysis, which accounted for the baseline viral load and darunavir FC and which demonstrated that addition of these CDMs to the etravirine weighted genotypic score did not improve the association with virologic response to etravirine in DUET. Since the proportion of ETR resistance that cannot be fully explained by the presence of ETR RAMs is very low, we conclude that the inclusion of CDMs in genotypic interpretation tools for etravirine will not significantly improve their predictive power.
Supplementary Material
ACKNOWLEDGMENTS
Editorial support was provided by Gardiner-Caldwell Communications, Macclesfield, United Kingdom. This support was funded by Tibotec.
Potential conflicts of interest: J.S. has served as a consultant, advisor, or speaker for Abbott Laboratories, GlaxoSmithKline, ViiV, Merck & Co, Inc., Pfizer Inc., Roche Pharmaceuticals, Tibotec-Janssen Cilag, Quest, and Virology Education and has received research support from Boehringer Ingelheim, GlaxoSmithKline, Pfizer, ViiV, Quest, and Tibotec. B.C. has been a consultant on advisory boards, has participated in speakers' bureaus, or has conducted clinical trials with Roche, Boehringer-Ingelheim, Abbott, BMS, GSK, Gilead, Tibotec, Janssen, Merck, Pfizer, Siemens, Monogram Biosciences, and Panacos. R.P. has received consulting fees from Pfizer and grant support from Pfizer, Siemens, Merck, and Boehringer Ingelheim. J.V., L.T., H.A., S.N., and G.P. are full-time employees of the DUET trial sponsor, Tibotec. S.F., A.F., E.C., C.J.P., and W.H. are full-time employees of Monogram Biosciences Inc. S.G. was a full-time employee of Monogram Biosciences at the time the research was carried out and is now a full-time employee of Abbott Virology, Arlington, VA.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org.
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Andries K., et al. 2004. TMC125, a novel next-generation nonnucleoside reverse transcriptase inhibitor active against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 48:4680–4686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benhamida J., Coakley E., Parkin N. T., Chappey C. 2008. Increased phenotypic susceptibility to etravirine in HIV-1 with NRTI resistance. Antivir. Ther. 13(Suppl. 3):A24 (Abstract.) [Google Scholar]
- 3. Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Stat. Methodol. 57:289–300 [Google Scholar]
- 4. Brehm J. H., et al. 2007. Selection of mutations in the connection and RNase H domains of human immunodeficiency virus type 1 reverse transcriptase that increase resistance to 3′-azido-3′-dideoxythymidine. J. Virol. 81:7852–7859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown A. J., et al. 2000. Reduced susceptibility of human immunodeficiency virus type 1 (HIV-1) from patients with primary HIV infection to nonnucleoside reverse transcriptase inhibitors is associated with variation at novel amino acid sites. J. Virol. 74:10269–10273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Das K., et al. 2004. Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (Etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants. J. Med. Chem. 47:2550–2560 [DOI] [PubMed] [Google Scholar]
- 7. Delviks-Frankenberry K. A., Nikolenko G. N., Barr R., Pathak V. K. 2007. Mutations in human immunodeficiency virus type 1 RNase H primer grip enhance 3′-azido-3′-deoxythymidine resistance. J. Virol. 81:6837–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Figueiredo A., et al. 2007. The E399G mutation in the connection domain of the HIV-1 reverse transcriptase potentiates resistance to efavirenz, abstr. MOPEA060. Abstr. 4th IAS Conf. HIV Pathol., Treat. Prev. [Google Scholar]
- 9. Götte M. 2007. Should we include connection domain mutations of HIV-1 reverse transcriptase in HIV resistance testing. PLoS Med. 4:1858–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta S., et al. 2007. Novel electrophoretic-tag-based in vitro assay to quantify dimerization of P66 and P51 subunits of HIV-1 reverse transcriptase (RT). Antivir. Ther. 12:S156 (Abstract.) [Google Scholar]
- 11. Gupta S., et al. 2010. Combinations of mutations in the connection domain of human immunodeficiency virus type 1 reverse transcriptase: assessing the impact on nucleoside and nonnucleoside reverse transcriptase inhibitor resistance. Antimicrob. Agents Chemother. 54:1973–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hachiya A., et al. 2008. Amino acid mutation N348I in the connection subdomain of human immunodeficiency virus type 1 reverse transcriptase confers multiclass resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors. J. Virol. 82:3261–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hachiya A., et al. 2009. Clinical relevance of substitutions in the connection subdomain and RNase H domain of HIV-1 reverse transcriptase from a cohort of antiretroviral treatment-naïve patients. Antiviral Res. 82:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haddad M., Stawiski E., Benhamida J., Coakley E. 2010. Improved genotypic algorithm for predicting etravirine susceptibility: comprehensive list of mutations identified through correlation with matched phenotype, abstr 574. Abstr. 17th Conf. Retrovir. Opportunistic Infect [Google Scholar]
- 15. Harrigan P. R., et al. 2002. A mutation in the 3′ region of the human immunodeficiency virus type 1 reverse transcriptase (Y318F) associated with nonnucleoside reverse transcriptase inhibitor resistance. J. Virol. 76:6836–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hertogs K., et al. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang H., Chopra R., Verdine G. L., Harrison S. C. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669–1675 [DOI] [PubMed] [Google Scholar]
- 18. Jacobo-Molina A., et al. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc. Natl. Acad. Sci. U. S. A. 90:6320–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson V. A., et al. 2009. Update of the drug resistance mutations in HIV-1: December 2009. Top. HIV Med. 17:138–145 [PubMed] [Google Scholar]
- 20. Katlama C., et al. 2009. Efficacy and safety of etravirine in treatment-experienced, HIV-1 patients: pooled 48 week analysis of two randomized, controlled trials. AIDS 23:2289–2300 [DOI] [PubMed] [Google Scholar]
- 21. Kemp S. D., et al. 1998. A novel polymorphism at codon 333 of human immunodeficiency virus type 1 reverse transcriptase can facilitate dual resistance to zidovudine and L-2′,3′-dideoxy-3′-thiacytidine. J. Virol. 72:5093–5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. 1992. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256:1783–1790 [DOI] [PubMed] [Google Scholar]
- 23. Lazzarin A., et al. 2007. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 370:39–48 [DOI] [PubMed] [Google Scholar]
- 24. Madruga J. V., et al. 2007. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet 370:29–38 [DOI] [PubMed] [Google Scholar]
- 25. Nikolenko G. N., et al. 2007. Mutations in the connection domain of HIV-1 reverse transcriptase increase 3′-azido-3′-deoxythymidine resistance. Proc. Natl. Acad. Sci. U. S. A. 104:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nikolenko G. N., Delviks-Frankenberry K. A., Pathak V. K. 2010. A novel molecular mechanism of dual resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors. J. Virol. 84:5238–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paredes R., et al. 2009. Mutation A376S in the RT connection domain is associated with an increased risk of VF to nevirapine-based therapy in NNRTI-naïve HIV-infected subjects: the EuroSIDA study, abstr 464. Abstr. 16th Conf. Retrovir. Opportunistic Infect [Google Scholar]
- 28. Pauwels R., et al. 1998. Comprehensive HIV drug resistance monitoring using rapid, high throughput phenotypic and genotypic assays with correlative data analysis. Antivir. Ther. 3(Suppl. 1):35–36 (Abstract.)10723507 [Google Scholar]
- 29. Petropoulos C. J., et al. 2000. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Picchio G., Vingerhoets J., Parkin N., Azijn H., de Béthune M. P. 2008. Nucleoside-associated mutations cause hypersusceptibility to etravirine (ETR). Antivir. Ther. 13(Suppl. 3):A25 (Abstract.) [Google Scholar]
- 31. Poveda E., et al. 2008. Phenotypic impact of resistance mutations on etravirine susceptibility in HIV patients with prior failure to nonnucleoside analogues. AIDS 22:2395–2398 [DOI] [PubMed] [Google Scholar]
- 32. Sarafianos S. G., et al. 1999. Touching the heart of HIV-1 drug resistance: the fingers close down on the dNTP at the polymerase active site. Chem. Biol. 6:R137–R146 [DOI] [PubMed] [Google Scholar]
- 33. Sarkar G., Sommer S. S. 1990. The “megaprimer” method of site-directed mutagenesis. Biotechniques 8:404–407 [PubMed] [Google Scholar]
- 34. Vingerhoets J., et al. 2005. TMC125 displays a high genetic barrier to the development of resistance: evidence from in vitro experiments. J. Virol. 79:12773–12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vingerhoets J., et al. 2010. Resistance profile of etravirine: combined analysis of baseline genotypic and phenotypic data from the randomized, controlled Phase III clinical studies AIDS 24:503–514 [DOI] [PubMed] [Google Scholar]
- 36. Wang J., Smerdon S. J., Jäger J., Kohlstaedt L. A., Rice P. A., Friedman J. M., Steitz T. A. 1994. Structural basis of asymmetry in the human immunodeficiency virus type 1 reverse transcriptase heterodimer. Proc. Natl. Acad. Sci. U. S. A. 91:7242–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yap S. H., et al. 2007. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 4:1887–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Z., et al. 2007. A novel nonnucleoside analogue that inhibits human immunodeficiency virus type 1 isolates resistant to current nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 51:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.