Abstract
A novel extended-spectrum β-lactamase (ESBL) was identified in a Pseudomonas aeruginosa clinical isolate obtained from a patient admitted to a hospital in Pennsylvania in 2008. The patient had a prolonged hospitalization in a hospital in Dubai, United Arab Emirates, before being transferred to the United States. The novel ESBL, designated PME-1 (Pseudomonas aeruginosa ESBL 1), is a molecular class A, Bush-Jacoby-Medeiros group 2be enzyme and shared 50, 43, and 41% amino acid identity with the L2 β-lactamase of Stenotrophomonas maltophilia, CTX-M-9, and KPC-2, respectively. PME-1 conferred clinically relevant resistance to ceftazidime, cefotaxime, cefepime, and aztreonam in P. aeruginosa PAO1 but not to carbapenems. Purified PME-1 showed good hydrolytic activity against ceftazidime, cefotaxime, and aztreonam, while activity against carbapenems and cefepime could not be measured. PME-1 was inhibited well by β-lactamase inhibitors, including clavulanic acid, sulbactam, and tazobactam. The blaPME-1 gene was carried by an approximately 9-kb plasmid and flanked by tandem ISCR24 elements.
INTRODUCTION
Pseudomonas aeruginosa is a major nosocomial pathogen which is characterized by its capacity to develop resistance to multiple classes of antimicrobials through both intrinsic mechanisms (e.g., constitutive expression of β-lactamases and various efflux pumps, combined with low permeability of the outer membrane) and acquisition of transferable resistance determinants (e.g., genes encoding β-lactamases or enzymes inactivating aminoglycosides or modifying their targets), decreased expression of porins, or mutations in fluoroquinolone targets (6). An important mechanism of resistance to β-lactams in P. aeruginosa is the production of chromosomal AmpC β-lactamase, which can be induced or derepressed to confer high-level penicillin and cephalosporin resistance. On the other hand, production of the Ambler class A extended-spectrum β-lactamase (ESBL), which is the most common cause of cephalosporin resistance in other Gram-negative pathogens, such as Escherichia coli and Klebsiella pneumoniae, is relatively rare in P. aeruginosa. ESBL types that have been identified in P. aeruginosa include VEB, GES, PER, BEL, SHV, and TEM, which have been found in a limited number of geographical areas (6). Here we report the identification and characterization of PME-1 (Pseudomonas aeruginosa ESBL 1), a novel class A ESBL, from a P. aeruginosa clinical isolate collected at our hospital.
(Part of this work was presented at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2010.)
MATERIALS AND METHODS
Susceptibility testing.
The antimicrobial susceptibility of P. aeruginosa GB771 to β-lactams, fluoroquinolones, and aminoglycosides were tested using the standard disk diffusion method on Mueller-Hinton (MH) agar plates (BD Microbiology Systems, Sparks, MD) and using the breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) (2). MICs of ampicillin, aztreonam, ceftazidime, ceftazidime-clavulanic acid, cefotaxime, cefotaxime-clavulanic acid, cefepime, imipenem, meropenem, and doripenem for P. aeruginosa GB771, the P. aeruginosa transformant, and the E. coli isogenic clone were tested using the dilution method with MH agar plates, and the breakpoints were defined by the CLSI (2). Phenotypic confirmation of ESBL production was conducted with ceftazidime and ceftazidime-clavulanic acid combination disks, using the criteria endorsed for Enterobacteriaceae by the CLSI (2).
Identification of β-lactamase genes.
PCR analyses were performed to identify β-lactamase genes in P. aeruginosa GB771. The genes investigated included the common ESBL genes blaTEM, blaSHV, and blaCTX-M (including the blaCTX-M-1, blaCTX-M-2, and blaCTX-M-9 groups) (4).
Cloning and sequencing.
The genomic DNA of P. aeruginosa GB771 was extracted, digested with ApaI (New England Biolabs, Ipswich, MA), and ligated with vector pBK-CMV (Stratagene, La Jolla, CA), which had been digested with the same restriction enzyme. E. coli DH10B was transformed with the ligated product by electroporation. Clones with the β-lactamase gene were selected on Luria-Bertani (LB) agar plates containing ampicillin (50 μg/ml) and kanamycin (30 μg/ml). The cloned DNA fragment was then sequenced in full using several sequencing primers.
The detection of blaPME-1 utilized primers PME1-622-F (5′-GAGATGCTCGTACCCGAAGA-3′) and PME1-848-R (5′-TGGGCATCGGATTCGTAATA-3′), yielding a 227-bp product. The run conditions used for this reaction were 94°C for 30 s, 56°C for 30s, and 72°C for 40s for 30 cycles.
Transfer of the blaPME-1 group gene.
Plasmid DNA was extracted from P. aeruginosa GB771 by using the standard alkaline lysis method. Electrocompetent cells of P. aeruginosa PAO1 and E. coli DH10B were then prepared and transformed with the plasmid DNA. Transformants were selected on LB agar plates containing ceftazidime (2 μg/ml).
PCR cloning of blaPME-1.
The structural gene corresponding to the enzyme was amplified with primers blaPME-1-F-XbaI (5′-GCGTCTAGAATGTTCCTTTACTTCACA-3′; the XbaI site is underlined) and blaPME-1-R-HindIII (5′-GCGAAGCTTTCAATCGCCCGCCCAG-3′; the HindIII site is underlined). The PCR product and pBC-SK(−) (Stratagene) were double digested with XbaI and HindIII (New England Biolabs), ligated, and used to transform E. coli DH10B by electroporation. Transformants harboring the blaPME-1 gene were selected on LB agar plates containing chloramphenicol (30 μg/ml) and ampicillin (50 μg/ml). The DNA fragment inserted on the vector was sequenced on both strands to confirm the identity as blaPME-1.
Purification of PME-1.
The above-mentioned E. coli DH10B clone harboring PCR-generated blaPME-1 was grown in 2 liters of LB broth containing 50 μg/ml of ampicillin overnight at 37°C. The bacteria were collected by centrifugation and resuspended twice with Tris-HCl (50 mM). The pellet was then frozen and thawed five times. Ultracentrifugation was performed at 24,200 × g for 60 min at 4°C, and the supernatant was used for the subsequent purification steps. PME-1 was purified by gel filtration chromatography (HiLoad 16/60 Superdex 75; GE Healthcare, Waukesha, WI). Fractions with β-lactamase activity were then subjected to ion-exchange chromatography (HiTrap Q HP; GE Healthcare). The total protein concentration was measured by the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Rockford, IL). The purity of the PME-1 enzyme was estimated to be over 90% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (data not shown). Finally, the purified enzyme was dialyzed overnight against 20 mM phosphate buffer (pH 7.0) and used for kinetic measurements.
Kinetic properties of PME-1.
Purified β-lactamase was used for determination of kinetic parameters (kcat and Km; Ki in the case of meropenem) in a reaction buffer made of 200 mM phosphate buffer (pH 7.0). The initial rates of hydrolysis of β-lactams were determined with a UV spectrophotometer (DU800; Beckman Coulter, Brea, CA). The 50% inhibitory concentration (IC50) was determined as the clavulanate, tazobactam, or sulbactam concentration that reduced the hydrolysis rate of 100 μM ampicillin by 50% under conditions in which the enzyme was preincubated with various concentrations of inhibitor for 5 min before the addition of the substrate. The kinetic constants were determined three times.
aIEF.
Analytical isoelectric focusing (aIEF) was carried out with a polyacrylamide gel (Criterion IEF gel, pH 3 to 10; Bio-Rad, Hercules, CA). β-Lactamases of known isoelectric points (pIs) (TEM-1, DHA-1, and CTX-M-15) were used as standards.
Nucleotide sequence accession number.
The nucleotide sequence reported in this paper was deposited in the GenBank database under accession no. HQ541434.
RESULTS AND DISCUSSION
Clinical isolate.
Pseudomonas aeruginosa GB771 was isolated from the sputum from an inpatient who was admitted for transplant evaluation at the University of Pittsburgh Medical Center in December 2008. The patient had just been transferred from a hospital in Dubai, United Arab Emirates (UAE), where she had undergone a 6-month hospitalization after complications arising from abdominal hernia repair. P. aeruginosa with the same susceptibility profile also grew later from blood, surgical wound, and urine specimens at our hospital.
Susceptibility testing.
P. aeruginosa GB771 was resistant to all β-lactams, all aminoglycosides except amikacin, and all fluoroquinolones by disk diffusion testing. The isolate showed an ESBL phenotype by the double-disk method. With the agar dilution method, P. aeruginosa GB771 was highly resistant to ticarcillin, cefotaxime, and aztreonam (MIC, >256 μg/ml), cefepime and ceftazidime (MIC, 64 μg/ml), and imipenem, meropenem, and doripenem (MICs, 16 to 32 μg/ml) (Table 1).
Table 1.
MICs of PME-1-producing P. aeruginosa and its transformant and the E. coli clone
| Antimicrobial | MIC (μg/ml) |
||||
|---|---|---|---|---|---|
|
P. aeruginosa |
E. coli |
||||
| GB771 | PAO1 (pGB771)a | PAO1 | DH10B (pPME-1)b | DH10B [pBC-SK(−)] | |
| Ampicillin | >256 | >256 | >256 | >256 | 2 |
| Cefoxitin | >256 | >256 | >256 | 4 | 2 |
| Ticarcillin | >256 | 256 | 32 | >256 | 2 |
| Aztreonam | >256 | 16 | 4 | >256 | 0.125 |
| Ceftazidime | 64 | 64 | 2 | 256 | 0.125 |
| Ceftazidime-clavulanic acid | 16 | 32 | 2 | 0.25 | 0.125 |
| Cefotaxime | >256 | >256 | 16 | 4 | 0.063 |
| Cefotaxime-clavulanic acid | >256 | >256 | 16 | 0.032 | 0.032 |
| Cefepime | 64 | 8 | 1 | 2 | 0.063 |
| Imipenem | 32 | 1 | 1 | 0.25 | 0.125 |
| Meropenem | 32 | 0.5 | 0.5 | 0.063 | 0.016 |
| Doripenem | 16 | 1 | 0.5 | 0.032 | 0.016 |
Transformant harboring a PME-1-encoding plasmid from P. aeruginosa GB771.
Transformant harboring a PCR-generated recombinant plasmid encoding PME-1.
Identification of PME-1.
PCR for blaTEM, blaSHV, and blaCTX-M carried by P. aeruginosa GB771 was negative. However, a clone with β-lactamase activity was obtained by genomic cloning. The clone had a 6.6-kb insert, on which a novel β-lactamase gene with a GC content of 69% was identified by sequencing. The β-lactamase, now designated PME-1 (Pseudomonas aeruginosa ESBL 1), consisted of 309 amino acids, possessed the class A conserved motifs, and shared 50, 43, and 41% amino acid identity with the L2 β-lactamase of Stenotrophomonas maltophilia, CTX-M-9 of E. coli, and KPC-2 of Klebsiella pneumoniae, respectively (Fig. 1).
Fig. 1.
Alignment of the deduced amino acid sequence of PME-1, along with those of L2 β-lactamase (GenBank accession no. EU032534), CTX-M-9 (GenBank accession no. AJ416345), and KPC-2 (GenBank accession no. DQ523564). The conserved sequences that are typical of class A serine β-lactamases are boxed.
β-Lactam resistance phenotype of blaPME-1.
An isogenic E. coli clone harboring blaPME-1 was constructed to define the resistance phenotype conferred by this gene. In E. coli DH10B, blaPME-1 conferred resistance to ceftazidime, aztreonam (MIC, >256 μg/ml), and cefotaxime (MIC, 4 μg/ml) (Table 1). While it did not confer resistance to cefepime, the MICs were elevated by 30-fold in the presence of blaPME-1, suggesting that PME-1 could hydrolyze cefepime as well. On the other hand, the MICs of carbapenems were elevated only minimally by 2- to 4-fold in the presence of blaPME-1 (Table 1). The presence of clavulanic acid at 4 μg/ml reduced the MICs of cefotaxime and ceftazidime by 128- and 1,024-fold, respectively, a characteristic trait of an ESBL. These functional properties define PME-1 as a group 2be enzyme in the revised functional classification (1).
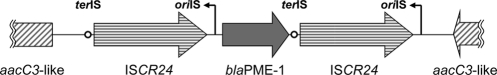
Genetic environment of blaPME-1.
The genetic environment of blaPME-1 is shown in Fig. 2. blaPME-1 was flanked on both ends by tandem and identical ISCR3/5-like elements. Both ISCR3 and ISCR5 have been reported in P. aeruginosa and likely encode a transposase (3, 5, 7). The ISCR3/5-like element found adjacent to blaPME-1 had a deduced amino acid sequence which was chimeric of ISCR3 and ISCR5, sharing 97 and 95% amino acid identity with the respective sequences. This new element is now designated ISCR24 (M. Toleman, personal communication). Putative terIS and oriIS elements were identified at the ends of ISCR24 (Fig. 2). These findings suggested that ISCR24 may function as a composite transposon and facilitate the acquisition of blaPME-1 by P. aeruginosa. Furthermore, partial aacC3-like genes were present in tandem upstream and downstream of blaPME-1. aacC3, which has been found only in P. aeruginosa, encodes aminoglycoside-(3)-N-acetyltransferase III, conferring resistance to gentamicin (8). However, the P. aeruginosa PAO1 transformant harboring this plasmid was not resistant to gentamicin. It has been reported that expression of aacC3 in P. aeruginosa requires the presence of cysC encoding adenosine 5′-phosphosulfate kinase upstream of the gene (8), which may account for the apparent lack of expression in our transformant.
Fig. 2.
Schematic presentation of the genetic environment of blaPME-1.
Transfer of the blaPME-1 gene.
P. aeruginosa GB771 yielded transformants which were positive for blaPME-1, with P. aeruginosa PAO1 as the recipient, whereas transformants could not be obtained with E. coli DH10B despite repeated attempts. The P. aeruginosa transformant demonstrated resistance to cefotaxime (MIC, >256 μg/ml) and ceftazidime (MIC, 64 μg/ml) and intermediate resistance to aztreonam (MIC, 16 μg/ml), which reflected a 4- to >16-fold increase over the corresponding MICs of the recipient strain (Table 1). The MIC of cefepime increased from 1 μg/ml to 8 μg/ml, which was still in the susceptible range. Overall, the degree of MIC differences for ticarcillin, cephalosporins, and aztreonam was less pronounced between the P. aeruginosa transformant and its control than between the E. coli clone and its control. blaPME-1 is expressed by a native promoter on the natural plasmid originating from P. aeruginosa GB771 in the P. aeruginosa PAO1 transformant, whereas it is expressed by the lacZ promoter on a high-copy-number vector in the E. coli clone. These factors may have affected the observed fold differences of MICs in the presence and absence of blaPME-1 in P. aeruginosa and E. coli.
DNA hybridization of the purified plasmid using the blaPME-1-specific probe yielded a single 9-kb band when digested with BamHI, which cuts within blaPME-1 (results not shown). These results suggested that blaPME-1 is carried by an approximately 9-kb plasmid and contributed to various levels of cephalosporin and monobactam resistance in P. aeruginosa GB771.
Kinetic properties of PME-1.
The molecular mass of the mature enzyme was calculated to be 30.4 kDa, and its isoelectric point (pI) was determined to be 5.0 by aIEF. The kinetic parameters of PME-1 are shown in Table 2. PME-1 efficiently hydrolyzed cephalothin and aztreonam. It also showed good hydrolytic activity against ampicillin and ticarcillin, for which the relatively low turnover rates for a class A enzyme were compensated by low Km values. Ceftazidime and cefotaxime were also good substrates due primarily to high turnover rates. However, no hydrolysis was observed with cefepime and carbapenems under the experimental conditions employed. For meropenem, the Ki was determined to be 0.37 mM, which indicated that meropenem was a weak competitive inhibitor of PME-1. PME-1 was inhibited well by β-lactamase inhibitors, clavulanic acid, tazobactam, and sulbactam, with an IC50 of <1 nM when using 100 μM ampicillin as the reporter substrate.
Table 2.
Kinetic parameters of PME-1
| Substrate | Km (μM)a | kcat (s−1) | kcat/Km (μM−1 · s−1) |
|---|---|---|---|
| Ampicillin | 3.2 ± 1.3 | 8.1 ± 0.04 | 2.5 |
| Ticarcillin | 7.2 ± 0.8 | 8.6 ± 0.04 | 1.2 |
| Cephalothin | 16.7 ± 4.6 | 110.1 ± 3.9 | 6.6 |
| Ceftazidime | 61.8 ± 2.4 | 13.7 ± 0.2 | 0.22 |
| Cefotaxime | 31.4 ± 2.8 | 23.7 ± 3.5 | 0.75 |
| Aztreonam | 8.7 ± 0.4 | 34.6 ± 0.1 | 4.0 |
Km values are the means ± standard deviations from three different measurements.
Conclusion.
We identified a novel ESBL, PME-1, in a Pseudomonas aeruginosa isolate obtained from a patient who was transferred from the UAE, where the isolate apparently originated. PME-1 possessed the conserved class A β-lactamase motifs and hydrolyzed oxyimino-cephalosporins as well as aztreonam, conferring resistance to these agents. blaPME-1 was bounded by ISCR24, which likely formed a composite transposon and facilitated its acquisition of P. aeruginosa. Whether or not this novel ESBL is common in the geographic region remains to be seen.
ACKNOWLEDGMENTS
We thank Yoshikazu Ishii for his technical advice.
This work was supported by the National Institutes of Health (grant 1K22AI080584). G.-B.T. was supported in part by the China Scholarship Council (CSC). Y.D. was supported in part by the National Institutes of Health (grant 1R03AI079296) and the Pennsylvania Department of Health (grant 4100047864).
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Bush K., Jacoby G. A. 2010. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 54:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Coyne S., Courvalin P., Galimand M. 2010. Acquisition of multidrug resistance transposon Tn6061 and IS6100-mediated large chromosomal inversions in Pseudomonas aeruginosa clinical isolates. Microbiology 156:1448–1458 [DOI] [PubMed] [Google Scholar]
- 4. de Oliveira Garcia D., et al. 2008. Multiclonal outbreak of Klebsiella pneumoniae producing extended-spectrum β-lactamase CTX-M-2 and novel variant CTX-M-59 in a neonatal intensive care unit in Brazil. Antimicrob. Agents Chemother. 52:1790–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H., Walsh T. R., Toleman M. A. 2009. Molecular analysis of the sequences surrounding blaOXA-45 reveals acquisition of this gene by Pseudomonas aeruginosa via a novel ISCR element, ISCR5. Antimicrob. Agents Chemother. 53:1248–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mesaros N., et al. 2007. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578 [DOI] [PubMed] [Google Scholar]
- 7. Toleman M. A., Bennett P. M., Walsh T. R. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vliegenthart J. S., Ketelaar-van Gaalen P. A., van de Klundert J. A. 1991. Nucleotide sequence of the aacC3 gene, a gentamicin resistance determinant encoding aminoglycoside-(3)-N-acetyltransferase III expressed in Pseudomonas aeruginosa but not in Escherichia coli. Antimicrob. Agents Chemother. 35:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]