Abstract
Paradoxical growth of Candida in vitro at echinocandin concentrations exceeding the MIC is well described, but the clinical relevance is unknown. We assessed echinocandin paradoxical effects against Candida bloodstream isolates (BSI) in the presence or absence of human serum and investigated regulatory mechanisms. As determined by broth microdilution, a paradoxical effect was evident for 60% (18/30), 23% (7/30), and 13% (4/30) of Candida albicans BSI exposed to caspofungin, anidulafungin, and micafungin, respectively, at achievable human serum concentrations (≤8 μg/ml). A paradoxical effect was not evident among 34 C. glabrata BSI and was observed only for caspofungin against C. parapsilosis (4%, 1/23). As determined in time-kill studies, a caspofungin paradoxical effect was demonstrated by C. albicans (2/3), C. glabrata (1/3), and C. parapsilosis (1/3), including BSI that were determined to be negative by microdilution. In 50% human serum, a paradoxical effect was eliminated at caspofungin concentrations up to 64 μg/ml for 100% (8/8) of the C. albicans BSI. A caspofungin paradoxical effect was also eliminated by chitin synthase inhibitor nikkomycin Z and at achievable concentrations of calcineurin pathway inhibitors, tacrolimus and cyclosporine. Moreover, these agents were synergistic with caspofungin against 100, 100, and 88% (7/8) of C. albicans, respectively, and exerted their own paradoxical effects. Finally, paradoxical growth was eliminated in C. albicans irs4- and inp51-null mutants, which lack phosphatidylinositol-(4,5)-bisphosphate 5′-phosphatase. Our findings suggest that the paradoxical effect is unlikely to be important in vivo but remains an important tool to study cell wall stress responses. We implicate the Irs4-Inp51 phosphatidylinositol-(4,5)-bisphosphate 5′-phosphatase as a novel regulator of paradoxical growth.
INTRODUCTION
The echinocandins have emerged as frontline agents for the treatment of invasive candidiasis (20). These agents exert concentration-dependent fungicidal effects through noncompetitive inhibition of β-1-3-glucan synthase, an enzyme that produces a major constituent of the fungal cell wall. Echinocandins are well tolerated in humans at up to three times the standard doses (4), a circumstance that has created interest in exploring the use of high-dose regimens to maximize clinical efficacy. Along these lines, concerns have been expressed about the possible therapeutic implications of an in vitro paradoxical effect, in which certain Candida isolates exhibit increased growth in the presence of echinocandin concentrations above the minimum inhibitory concentration (MIC). Although the paradoxical effect is typically apparent in vitro at concentrations that are achievable in human serum with conventional dosing strategies, the clinical relevance of the phenomenon in the treatment of patients with candidiasis is unproven.
To date, paradoxical effects have been demonstrated against Candida albicans, C. parapsilosis, C. krusei, C. tropicalis, and C. dubliniensis isolates (3, 5, 10, 16, 23, 25, 28). Moreover, they have been described with all three commercially available echinocandins (5), although rates differ among these agents. The precise mechanisms accounting for paradoxical effects are unknown but do not involve point mutations in hot spot regions of FKS1, the gene encoding glucan synthase, or upregulation of β-1-3-glucan synthesis (25). Rather, paradoxical growth likely depends on several interacting mechanisms, including activation of the high-osmolarity glycerol and calcineurin stress pathways, and increases in the synthesis of chitin, another component of the cell wall (24, 28).
The objectives of the present study were to assess the possible clinical relevance of paradoxical effects and identify mechanisms by which paradoxical effects are mediated. Toward these ends, we first assessed echinocandin paradoxical effects among a large number of Candida bloodstream isolates (BSI) recovered from patients at our institution, including the most commonly isolated species C. albicans, C. glabrata, and C. parapsilosis. In order to assess the possible clinical relevance of paradoxical effects, we next investigated the phenomenon in the presence of increasing concentrations of human serum. Finally, we studied mechanisms by which paradoxical effects were mediated. We investigated the impact of calcineurin pathway and chitin synthesis inhibitors and characterized potentially novel mechanisms such as the activation of hyphal regulatory pathways and the regulation of phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] levels by the 5′-phosphatase Inp51 and its interacting protein Irs4. We anticipated that the study would be useful for several reasons. Elimination of paradoxical effects in the presence of serum would indicate that they are not likely to impact the treatment of patients with candidemia. Nevertheless, paradoxical effect experiments would remain important tools for the study of Candida cell wall stress responses. Furthermore, understanding the cellular and molecular mechanisms by which paradoxical effects are mediated may identify novel targets for the development of antifungal therapies.
MATERIALS AND METHODS
Isolates.
C. albicans, C. glabrata, and C. parapsilosis BSI were collected at the University of Pittsburgh Medical Center (UPMC) from August 2007 to December 2009. Isolates were identified to the species level by the standard mycological methods of germ tube formation in serum, carbohydrate assimilation tests using the API 20C kit (bioMérieux, Inc., Hazelwood, MO) (29), and morphology on cornmeal agar. C. albicans SC5314 and C. parapsilosis ATCC 22019 were used as controls throughout (13). In addition, we analyzed well-characterized mutant C. albicans isolates created by the Ura-Blaster and SAT-flipper methods (1, 2, 14), along with their respective parental strains C. albicans SC5314 and CAI-12. Mutant isolates included irs4-, inp51-, cph1-, efg1-, and cph1/efg1-null mutants and the corresponding gene reinsertion strains (1, 2, 14) (Table 1). All experiments described below were performed in duplicate on at least two separate occasions to assure reproducibility.
Table 1.
Paradoxical growth of Candida albicans mutant strains
| Straina | Description | Caspofungin MIC (μg/ml) | Occurrence of paradoxical growthb | Reference |
|---|---|---|---|---|
| SC5314 | Wild type | 0.125 | Yes (2-8) | 13 |
| CAI-12 | URA3/ura3Δ | 0.25 | Yes (1-4) | 11 |
| irs4 mutant 1* | URA3/ura3Δ irs4Δ::hisG/irs4Δ::hisG | 0.25 | No | 1 |
| irs4 mutant 2† | irs4Δ/irs4Δ | 0.25 | No | 2 |
| inp51 mutant† | inp51Δ/inp51Δ | 0.25 | No | 2 |
| efg1 mutant* | ura3Δ/ura3Δ efg1Δ::hisG/efg1Δ::hisG-URA3-hisG | 0.25 | Yes (1-4) | 16 |
| efg1/cph1 mutant* | ura3Δ/ura3Δ cph1Δ::hisG/cph1Δ::hisG efg1Δ::hisG/efg1Δ::hisG-URA3-hisG | 0.25 | Yes (1-4) | 16 |
*, Mutants created by using the Ura-Blaster method (the comparator strain is C. albicans CAI-12); †, mutants created by using the SAT-flipper method (the comparator strain is C. albicans SC5314).
If yes, the range (in μg/ml) is indicated in parentheses. The range was defined by the lowest and highest concentrations associated with a paradoxical effect for a given isolate.
In vitro susceptibility testing.
Caspofungin powder was acquired from the UPMC pharmacy as a product for clinical use; anidulafungin and micafungin pure powders were provided by Pfizer, Inc. (Groton, CT), and Astellas Pharma, Inc. (Tokyo, Japan), respectively. Broth microdilution in standard RPMI 1640 (buffered to a pH of 7.0 with morpholinepropanesulfonic acid) utilizing a 50% turbidity endpoint at 24 h was used to determine the MIC for each isolate in duplicate (8). The range of echinocandin concentrations tested was 0.06 to 64 μg/ml. Differences in MIC between the echinocandins were compared by using a Student t test. Significance was defined as a P value of ≤0.05.
Identification of paradoxical growth.
A paradoxical effect was defined as growth in consecutive wells that was ≥2 drug dilutions above the MIC after 48 h (5). Identification of a paradoxical effect was determined by visual interpretation, aided by a magnified reading mirror. The visual results were further corroborated by using a spectrophotometer measuring the optical density at 530 nm after 48 h of incubation. In all experiments, visual inspection and spectrophotometric methods yielded the same results. The paradoxical effect also was determined by broth microdilution for caspofungin, as the representative echinocandin, in the presence or absence of 10 and 50% human serum. Sera were collected from patients not receiving antifungal therapy under an Institutional Review Board-approved protocol at the University of Pittsburgh. Paradoxical growth was further measured by a time-kill assay according to standard methods (6). In the present study, C. albicans, C. glabrata, and C. parapsilosis BSI (n = 3 each) were tested against clinically relevant caspofungin concentrations of 2 and 8 μg/ml, which correspond to typical serum troughs and peaks (26). A paradoxical effect was defined as greater growth (≥1 log CFU/ml) in the presence of 8 μg/ml of caspofungin than in the presence of 2 μg/ml of caspofungin after 24 h of incubation.
Synergy between caspofungin and inhibitors of the calcineurin pathway and chitin synthase.
The presence or absence of paradoxical growth was also determined when caspofungin was combined with tacrolimus, cyclosporine, and nikkomycin Z by checkerboard synergy testing. Synergy with caspofungin was defined as a fractional inhibitory concentration (FIC) index of ≤0.5. The FIC index is calculated as the ΣFIC = FIC A + FIC B, where FIC A is the MIC of drug A as part of the combination divided by the MIC of drug A alone and FIC B is the MIC of drug B as part of the combination divided by the MIC of drug B alone.
RESULTS
Echinocandin paradoxical effects among C. albicans, C. glabrata, and C. parapsilosis bloodstream isolates.
The results of liquid broth microdilution assays of caspofungin, anidulafungin, and micafungin against 30 C. albicans, 34 C. glabrata, and 23 C. parapsilosis BSI are shown in Table 2. The geometric mean MICs of caspofungin against C. albicans and C. glabrata were significantly higher than those of anidulafungin or micafungin (P < 0.001 for both). The geometric mean MICs of caspofungin and micafungin against C. parapsilosis BSI were significantly higher than that of anidulafungin (P < 0.05 and < 0.001, respectively). A paradoxical effect by the broth microdilution method was most common against C. albicans, occurring in 60% (18/30), 23% (7/30), and 13% (4/30) of BSI exposed to caspofungin, anidulafungin, and micafungin, respectively. No paradoxical effects were evident against C. glabrata BSI within the range of concentrations tested, and only caspofungin exhibited a paradoxical effect against C. parapsilosis (4% of BSI [1/23]). Geometric mean MICs of caspofungin, anidulafungin, and micafungin were similar against C. albicans BSI that demonstrated paradoxical effects and those that did not (0.29 versus 0.33 μg/ml, 0.06 versus 0.07 μg/ml, and 0.06 versus 0.07 μg/ml, respectively). The range of concentrations associated with paradoxical growth was similar for the three agents and was consistent with concentrations that can be achieved in human serum (typical serum troughs and peaks of 2 and 8 μg/ml, respectively) (Table 2). The paradoxical effect was quadriphasic, a phenomenon that was more evident with longer incubation times. Specifically, a dramatic decrease in growth was identified at the MIC, which was followed by persistent inhibition at supra-MIC concentrations. The third phase, representing paradoxical growth, typically began at concentrations 8 to 32 times the MIC. Finally, a phase of complete killing was noted at concentrations beyond the range of the paradoxical growth.
Table 2.
Characteristics of Candida BSI
| Candida characteristicsa | Anidulafungin | Caspofungin | Micafungin |
|---|---|---|---|
| C. albicans (n = 30) | |||
| Geometric mean MIC (μg/ml) | 0.06 | 0.31 | 0.07 |
| MIC50 (μg/ml) | 0.06 | 0.25 | 0.06 |
| MIC range (μg/ml) | 0.06–0.5 | 0.125–2 | 0.06–2 |
| % Exhibiting paradoxical effect (n) | 23 (7) | 60 (18) | 13 (4) |
| Paradoxical range | 1–8 | 2–8 | 0.5–8 |
| C. glabrata (n = 34) | |||
| Geometric mean MIC (μg/ml) | 0.08 | 0.48 | 0.07 |
| MIC50 (μg/ml) | 0.06 | 0.25 | 0.06 |
| MIC range (μg/ml) | 0.06–2 | 0.125–16 | 0.06–0.5 |
| % Exhibiting paradoxical effect (n) | 0 | 0 | 0 |
| Paradoxical range | |||
| C. parapsilosis (n = 23) | |||
| Geometric mean MIC (μg/ml) | 0.55 | 1.03 | 1.39 |
| MIC50 (μg/ml) | 0.5 | 2 | 2 |
| MIC range (μg/ml) | 0.06–1 | 0.06–2 | 0.06–4 |
| % Exhibiting paradoxical effect (n) | 0 | 4 (1) | 0 |
| Paradoxical range | 8–32 |
The paradoxical range is defined by the lowest and highest concentrations (in μg/ml) associated with a paradoxical effect for a given BSI. The range given in the table is the median range for a given drug against all isolates tested.
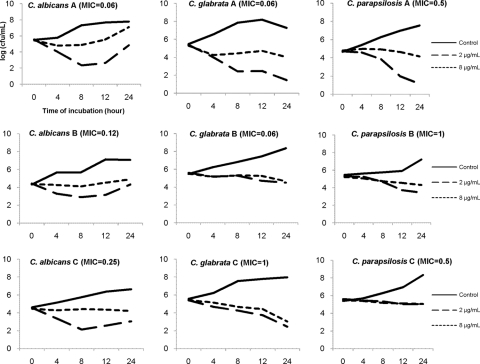
To further assess paradoxical effects, we performed caspofungin time-kill assays against selected C. albicans, C. glabrata, and C. parapsilosis BSI (n = 3 each). Time-kill assays corroborated the paradoxical effects of caspofungin against one C. albicans BSI (C. albicans A, MIC = 0.06 μg/ml) and identified a paradoxical effect that was not evident by standard broth microdilution methods against a second isolate (C. albicans C, MIC = 0.25 μg/ml) (Fig. 1). In addition, reproducible paradoxical effects were demonstrated against 1 of 3 C. glabrata and C. parapsilosis BSI, despite not being apparent by broth microdilution (Fig. 1). Surviving Candida organisms at 24 h were subjected to susceptibility testing, and the caspofungin MICs remained unchanged from the baseline.
Fig. 1.
Paradoxical effect of caspofungin as demonstrated by a time-kill assay. Time-kill curves for each BSI were determined at caspofungin concentrations of 2 and 8 μg/ml (the typical trough and peak concentrations in humans) in duplicate. The effects were consistent in all experiments, and the results of representative experiments for each isolate are displayed. Paradoxical effects were evident against two C. albicans BSI, including one isolate that did not demonstrate paradoxical effects by broth microdilution (isolate C; MIC = 0.25 μg/ml). None of the C. glabrata or C. parapsilosis BSI demonstrated paradoxical effects by broth microdilution, but paradoxical effects were evident for one isolate of each by time-kill (C. glabrata and C. parapsilosis isolates A).
Effects of human serum on the paradoxical effect of caspofungin.
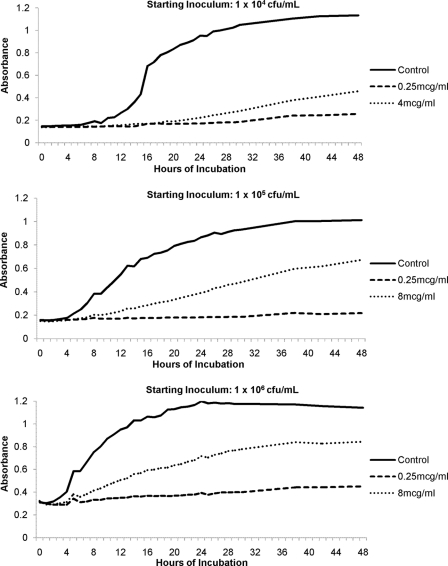
To characterize paradoxical growth in greater detail, we studied caspofungin against 8 C. albicans BSI, which were chosen from among those demonstrating reproducible paradoxical effects by broth microdilution. For each isolate, the time to paradoxical growth was dependent upon the starting inoculum (Fig. 2). The median times to onset (defined as the earliest time point that differentiates the growth of the isolates incubated in caspofungin concentrations at the MIC from isolates incubated in supra-MIC concentrations using an optical density difference of 0.1) were shorter for inocula of 106 (8 h) and 105 (17 h) than for inocula of 104 CFU/ml (40 h; P < 0.0001 [analysis of variance]). In order to assess the possible clinical relevance of echinocandin paradoxical effects, we next tested each BSI in increasing concentrations of human serum (Table 3). In 10% human serum (diluted in RPMI 1640), caspofungin MICs were not affected, and paradoxical effects were still evident. The median concentration at which a paradoxical effect was first noted, however, was increased from 2 to 32 μg/ml. In 50% human serum, caspofungin MICs were increased 2-fold (median, 0.5 μg/ml; range, 0.5 to 1 μg/ml), and paradoxical effects were eliminated for all isolates up to the maximum tested concentration of 64 μg/ml.
Fig. 2.
Onset of paradoxical growth based on starting inoculum of a representative C. albicans BSI, as measured by hourly spectrophotometric readings in the presence of caspofungin. By increasing the inoculum, the onset of paradoxical growth is evident as early as 7 h for a representative C. albicans isolate.
Table 3.
Effect of human serum on the paradoxical growth of eight C. albicans BSI in the presence of caspofungin
| BSI | Caspofungin concn (μg/ml) rangea |
|
|---|---|---|
| No serum | 10% Serumb | |
| 1 | 2–8 | 8–32 |
| 2 | 2–16 | 32–64 |
| 3 | 2–16 | 32–64 |
| 4 | 1–8 | 8–32 |
| 5 | 2–4 | 16–32 |
| 6 | 2–8 | 32–64 |
| 7 | 2–8 | 32–64 |
| 8 | 1–8 | 32–64 |
Paradoxical growth was observed for all isolates. The caspofungin range tested was 0.06 to 64 μg/ml.
No paradoxical growth was observed in 50% serum for any of the BSI at the caspofungin concentrations tested.
Contribution of calcineurin pathway and chitin synthesis to paradoxical growth.
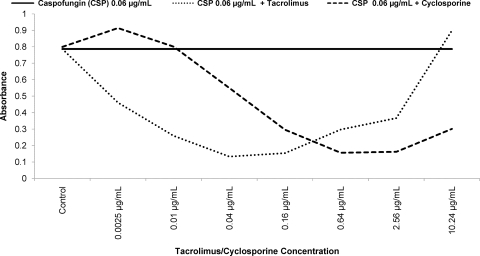
Paradoxical growth has been shown to be mediated, among other mechanisms, by activation of the calcineurin pathway and chitin synthesis. We tested the eight C. albicans BSI against caspofungin in combination with tacrolimus or cyclosporine, calcineurin inhibitors that are widely used as immunosuppressive agents in humans, and nikkomycin Z, a chitin synthase inhibitor. For each isolate, the paradoxical effect was eliminated in the presence of tacrolimus (range of concentrations tested, 0.0025 to 10.25 μg/ml), cyclosporine (range, 0.0025 to 10.25 μg/ml), or nikkomycin Z (range, 2 to 128 μg/ml) (Table 4). Moreover, tacrolimus, cyclosporine, and nikkomycin Z were synergistic in combination with caspofungin against 100, 100, and 88% of isolates, respectively (Table 4). Caspofungin MICs against all eight isolates were reduced to ≤0.06 μg/ml, the lowest concentration tested. Finally, increasing concentrations of both tacrolimus and cyclosporine were associated with their own paradoxical effects against 100% (8/8) and 63% (5/8) of the isolates, respectively, when tested in combination with the lowest caspofungin concentration (0.06 μg/ml) (Fig. 3). Tacrolimus and cyclosporine paradoxical effects were initiated at median concentrations of 2.56 μg/ml, which significantly exceeds levels that are typically achieved in vivo (0.005 to 0.02 μg/ml and 0.150 to 0.600 μg/ml, respectively) (15).
Table 4.
Effects of tacrolimus, cyclosporine, and nikkomycin Z on the paradoxical growth of eight C. albicans BSI in the presence of caspofungina
| Drug B | Median MIC (μg/ml) aloneb |
Median MIC (μg/ml) in combination |
Median FIC | No. of isolates (%) exhibiting: |
||||
|---|---|---|---|---|---|---|---|---|
| Caspofungin | Drug B | Caspofungin | Drug B | Synergy | Caspofungin PE | Secondary PE | ||
| Cyclosporine | 0.1875 (0.125–0.5) | >10.25 | ≤0.06 | 0.64 (0.16–2.56) | 0.486 (0.13–0.5) | 8 (100) | 0 (0) | 5 (63) |
| Nikkomycin | 0.1875 (0.125–0.5) | 96 (32–>128) | ≤0.06 | 2 (2–8) | 0.482 (0.12–0.56) | 7 (88)c | 0 (0) | 0 (0) |
| Tacrolimus | 0.1875 (0.125–0.5) | >10.25 | ≤0.06 | 0.04 | 0.364 (0.12–0.48) | 8 (100) | 0 (0) | 8 (100) |
Abbreviations: FIC, fractional inhibitory concentration; PE, paradoxical effect. Ranges are given parenthetically for concentrations where applicable. No concentration range was included when all eight isolates produced the same result.
The ranges of drug concentrations tested were as follows: caspofungin (0.06 to 64 μg/ml), cyclosporine (0.0025 to 10.25 μg/ml), nikkomycin Z (2 to 128 μg/ml), and tacrolimus (0.0025 to 10.25 μg/ml).
One isolate was indifferent (FIC = 0.56).
Fig. 3.
Paradoxical growth of a representative C. albicans BSI in the presence of cyclosporine or tacrolimus. A solid line represents the optical density of C. albicans in the presence of caspofungin at 0.06 μg/ml alone. Broken lines represent the change in optical density of C. albicans when caspofungin at 0.06 μg/ml is combined with increasing concentrations of cyclosporine (dots) or tacrolimus (dashes).
Contribution of the Inp51-Irs4 PI(4,5)P2 regulatory complex and the Cek1-MAPK and Ras-cAMP hyphal regulatory pathways to paradoxical growth.
Activation of the PKC-Mkc1 cell wall integrity signaling pathway also has been shown to contribute to paradoxical growth (17, 27, 28), at least in part by coordinately regulating chitin synthesis in conjunction with the calcineurin pathway. In previous studies, we demonstrated that independent disruptions of the PI(4,5)P2 5′-phosphatase Inp51 and its interacting EH-domain protein Irs4p resulted in elevated PI(4,5)P2 levels, overactivation of the PKC-Mkc1 pathway in response to cell wall stress, and hypersusceptibility to caspofungin (1, 2). Similar to previous reports concerning mkc1-null mutant strains (28), we demonstrated here that paradoxical effects were eliminated in inp51- and irs4-null mutant strains. Paradoxical effects were reestablished in INP51 and IRS4 reinsertion strains, demonstrating that disruptions of the respective genes were responsible for the results rather than unrecognized events elsewhere in the genome (data not shown). In the past, we also showed that dysregulation of PI(4,5)P2 in inp51- and irs4-null mutants is associated with attenuated hyphal formation during contact with agar and within mouse kidneys (1, 2). To determine whether pathways regulating hyphal formation play roles in paradoxical growth, we tested an efg1-null mutant strain and a cph1/efg1 double mutant, in which the downstream transcription factors of the Ras-cAMP and Cph1-MAPK hyphal regulatory pathways, respectively, are disrupted. Paradoxical effects remained evident for these mutants (Table 1).
DISCUSSION
Paradoxical growth of Candida isolates at echinocandin concentrations that exceed the MIC is well recognized in vitro (23, 25). In the present study, we characterized echinocandin paradoxical effects against a large number of C. albicans, C. glabrata, and C. parapsilosis BSI. Using the standard broth microdilution methods, we demonstrated paradoxical effects for each of the echinocandins against C. albicans BSI at concentrations that are achievable in human serum, at rates of 10, 23, and 60% (micafungin, anidulafungin, and caspofungin, respectively). In fact, time-kill assays performed at typical serum trough and peak concentrations of caspofungin (2 and 8 μg/ml, respectively) revealed paradoxical effects against C. albicans, C. glabrata, and C. parapsilosis BSI that were not evident by broth microdilution. To our knowledge, this is the first report of paradoxical growth by C. glabrata. Our 4% rate of C. parapsilosis paradoxical growth was lower than in earlier studies (5), and the unmasking of the phenomenon by time-kill speaks to the relative insensitivity of broth microdilution methods. As such, our data demonstrate that definitive assessments of the presence or absence of paradoxical effects should be made using the more sensitive time-kill methods. Of note, our results were verified as paradoxical growth and not tolerance, since caspofungin MICs before and after antifungal exposure did not change. Taken together, therefore, the data imply that paradoxical growth is more common than currently recognized and stems from compensatory responses to the inhibition of β-1,3-glucan synthesis that are shared by diverse Candida species.
At the same time, we observed that paradoxical effects against C. albicans were shifted to higher caspofungin concentrations in the presence of 10% human serum and eliminated in 50% human serum (at least at concentrations up to 64 μg/ml). These results suggest that protein binding or some other serum factor tempers paradoxical growth at clinically relevant drug concentrations. As such, our data strongly imply that paradoxical effects are unlikely to have a major impact on outcomes among patients treated with echinocandin agents for candidemia. In fact, the clinical significance of paradoxical growth during echinocandin therapy of candidiasis is unproven. In two clinical trials, there were no differences in responses to echinocandins among patients with candidiasis who received high- or low-dose regimens (150 versus 100 mg of micafungin/day and 150 mg/day versus a 70-mg load, followed by 50 mg of caspofungin/day) (4, 21). In a mouse model of systemic candidiasis, only one of four C. albicans isolates that demonstrated a paradoxical effect in vitro showed a similar pattern in vivo with a higher fungal burden recovered from mice treated with high-dose caspofungin (20 mg/kg). The apparent paradoxical growth was not reproducible in subsequent experiments (7). To date, the latter study remains the only one to suggest paradoxical growth of Candida in vivo, since other mouse models of C. tropicalis and C. dubliniensis infections have yielded negative results (3, 16). Of further note, our findings were consistent with earlier reports demonstrating an increase in MICs in the presence of serum (11, 12, 18, 19). The issue of incorporating serum in routine susceptibility testing has been discussed extensively in other studies, and our study design does not allow us to address the impact of this practice. However, before this method can be applied to clinical practice, validation of its value using a large number of isolates associated with clinical failure of the echinocandins is necessary; in addition, the methods must be standardized, and intra- and interlaboratory reproducibility must be demonstrated.
Even if echinocandin paradoxical effects against Candida spp. are not relevant in the treatment of candidiasis, paradoxical growth remains a useful model for studying cell wall stress responses. At present, the precise mechanisms of paradoxical growth are incompletely understood. Clearly, the attenuation of echinocandin activity at supra-MICs is not attributable to FKS gene mutations or the upregulation of glucan synthase (25). Rather, activation of Hog1 (high-osmolarity glycerol response) and calcineurin stress response pathways and increases in chitin synthesis have been implicated (24, 28). We demonstrated that compensatory responses to β-1,3-glucan synthesis inhibition are mediated within hours of echinocandin exposure through diverse molecular and cellular mechanisms, including both well-described and previously unrecognized responses. Consistent with previous studies, we showed that blockade of the calcineurin pathway with tacrolimus or cyclosporine and inhibition of chitin synthesis with nikkomycin Z eliminated the caspofungin-induced paradoxical growth of all eight C. albicans BSI tested (at least at concentrations up to 64 μg of caspofungin/ml) (28). Moreover, we made the novel observation that the effects of tacrolimus and cyclosporine on paradoxical growth were evident at concentrations of each agent that are achievable in human serum (0.01 and 0.64 μg/ml, respectively). Further evidence that the interaction between caspofungin and tacrolimus or cyclosporine may be clinically relevant was provided by the demonstration of synergy against 100% of C. albicans BSI, at concentrations of both cyclosporine and tacrolimus that were clinically achievable. Similar results have been documented against Aspergillus fumigatus (22), suggesting that further studies of the utility of combination therapy in vivo are warranted. Since many patients who develop candidiasis are receiving immunosuppressive treatment with tacrolimus or cyclosporine, synergy between these agents and echinocandins might be clinically useful. At the same time, the results suggest that the identification of nonimmunosuppressive agents that impact the calcineurin or other compensatory pathways may reveal novel antifungal strategies.
To our knowledge, this is the first study to demonstrate that tacrolimus and cyclosporine were associated with their own paradoxical effects when they were tested in the presence of low caspofungin concentrations. These results suggest that paradoxical effects represent generalized compensatory responses to a range of cell wall insults, rather than specific responses to inhibition of β-1,3-glucan synthesis. Our demonstration that the Inp51-Irs4 PI(4,5)P2 5′-phosphatase complex played a role in mediating paradoxical growth was also novel. PI(4,5)P2 likely contributes to paradoxical growth through regulation of the Mkc1-cell wall integrity pathway, which targets chitin synthase and other proteins involved in cell wall biogenesis and stress responses (1, 2). In C. albicans irs4- and inp51-null mutants, PI(4,5)P2 levels are elevated and the PKC-Mkc1 cell wall integrity pathway is overactivated in response to cell wall stress (1, 2). In previous studies, paradoxical growth was eliminated in an mkc1-null mutant strain, thereby implicating activation of the cell wall integrity pathway (28). Our finding that paradoxical effects were also eliminated in irs4- and inp51-null mutants thus shows that proper regulation of the cell wall integrity pathway is crucial, rather than mere activation of the pathway.
Finally, it is unclear whether the differences in the rates of paradoxical effects that we noted between the echinocandins bear any significance. Despite sharing a common target, echinocandins manifest differences in pharmacokinetics, drug-drug interactions, and adverse effects (9). Our finding that paradoxical effects were most common for caspofungin was consistent with previous reports (5, 10). The reasons for disparities between agents are unclear but may stem from structural differences. Nevertheless, differences in clinical efficacy between the echinocandins have not been demonstrated, and the current recommendations are that the agents be considered therapeutically equivalent (20).
ACKNOWLEDGMENTS
Experiments were conducted in the laboratories of C. J. Clancy and M. H. Nguyen at the University of Pittsburgh. This project was funded in part by an investigator-initiated research grant from Merck to C. J. Clancy.
Footnotes
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Badrane H., et al. 2005. Candida albicans IRS4 contributes to hyphal formation and virulence after the initial stages of disseminated candidiasis. Microbiology 151:2923–2931 [DOI] [PubMed] [Google Scholar]
- 2. Badrane H., et al. 2008. The Candida albicans phosphatase Inp51p interacts with the EH domain protein Irs4p, regulates phosphatidylinositol-4,5-bisphosphate levels and influences hyphal formation, the cell integrity pathway and virulence. Microbiology 154:3296–3308 [DOI] [PubMed] [Google Scholar]
- 3. Bayegan S., et al. 2010. In vivo studies with a Candida tropicalis isolate exhibiting paradoxical growth in vitro in the presence of high concentration of caspofungin. J. Microbiol. 48:170–173 [DOI] [PubMed] [Google Scholar]
- 4. Betts R. F., et al. 2009. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin. Infect. Dis. 48:1676–1684 [DOI] [PubMed] [Google Scholar]
- 5. Chamilos G., Lewis R. E., Albert N., Kontoyiannis D. P. 2007. Paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clancy C. J., Huang H., Cheng S., Derendorf H., Nguyen M. H. 2006. Characterizing the effects of caspofungin on Candida albicans, Candida parapsilosis, and Candida glabrata isolates by simultaneous time-kill and postantifungal-effect experiments. Antimicrob. Agents Chemother. 50:2569–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clemons K. V., Espiritu M., Parmar R., Stevens D. A. 2006. Assessment of the paradoxical effect of caspofungin in therapy of candidiasis. Antimicrob. Agents Chemother. 50:1293–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clinical Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27–A3, 3rd ed Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Eschenauer G., Depestel D. D., Carver P. L. 2007. Comparison of echinocandin antifungals. Ther. Clin. Risk Manag. 3:71–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleischhacker M., Radecke C., Schulz B., Ruhnke M. 2008. Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur. J. Clin. Microbiol. Infect. Dis. 27:127–131 [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Effron G., Lee S., Park S., Cleary J. D., Perlin D. S. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-d-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia-Effron G., Park S., Perlin D. S. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillum A. M., Tsay E. Y., Kirsch D. R. 1984. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of Saccharomyces cerevisiae ura3 and Escherichia coli pyrF mutations. Mol. Gen. Genet. 198:179–182 [DOI] [PubMed] [Google Scholar]
- 14. Hao B., et al. 2009. Candida albicans RFX2 encodes a DNA binding protein involved in DNA damage responses, morphogenesis, and virulence. Eukaryot. Cell 8:627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahan B. D., Keown P., Levy G. A., Johnston A. 2002. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin. Ther. 24:330–350 [DOI] [PubMed] [Google Scholar]
- 16. Marine M., et al. 2009. Paradoxical growth of Candida dubliniensis does not preclude in vivo response to echinocandin therapy. Antimicrob. Agents Chemother. 53:5297–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munro C. A., et al. 2007. The PKC, HOG, and Ca2+ signaling pathways coordinately regulate chitin synthesis in Candida albicans. Mol. Microbiol. 63:1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odabasi Z., Paetznick V., Rex J. H., Ostrosky-Zeichner L. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paderu P., et al. 2007. Serum differently alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappas P. G., et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pappas P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883–893 [DOI] [PubMed] [Google Scholar]
- 22. Steinbach W. J., et al. 2004. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob. Agents Chemother. 48:4922–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevens D. A., Espiritu M., Parmar R. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stevens D. A., Ichinomiya M., Koshi Y., Horiuchi H. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stevens D. A., White T. C., Perlin D. S., Selitrennikoff C. P. 2005. Studies of the paradoxical effect of caspofungin at high drug concentrations. Diagn. Microbiol. Infect. Dis. 51:173–178 [DOI] [PubMed] [Google Scholar]
- 26. Stone J. A., et al. 2002. Single- and Multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walker L. A., et al. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 4:e1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiederhold N. P., Kontoyiannis D. P., Prince R. A., Lewis R. E. 2005. Attenuation of the activity of caspofungin at high concentrations against candida albicans: possible role of cell wall integrity and calcineurin pathways. Antimicrob. Agents Chemother. 49:5146–5148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J., et al. 2002. Comparison of API20C with molecular identification of Candida spp. isolated from bloodstream infections. J. Clin. Pathol. 55:774–777 [DOI] [PMC free article] [PubMed] [Google Scholar]