Abstract
The antimalarial activity of the human immunodeficiency virus protease inhibitors indinavir and saquinavir was evaluated in rhesus macaques for the first time. Indinavir effectively suppressed the growth of Plasmodium cynomolgi and Plasmodium knowlesi in vivo after a 7- or 3-day treatment, respectively, with clinically relevant doses, whereas saquinavir showed only weak activity against P. cynomolgi.
TEXT
Malaria is one of the most prevalent infectious diseases worldwide, affecting approximately 225 million people, of whom 781,000 died in 2009 (24). Malaria is endemic mainly in sub-Saharan Africa, Southeast Asia, and South America, where human immunodeficiency virus (HIV) infection and AIDS are also prevalent. Some HIV protease inhibitors (HIV PIs), a class of highly active antiretroviral therapy (HAART) drugs, inhibited the proliferation of some laboratory clones and clinical isolates of Plasmodium falciparum and Plasmodium vivax in vitro and ex vivo (1, 15–17, 20, 21) and also showed inhibitory activity against Plasmodium chabaudi and Plasmodium yoelii in a murine model (1, 13). However, the antimalarial effect of HIV PIs in humans is still unknown.
Plasmodium cynomolgi and Plasmodium knowlesi are two species of monkey malaria parasites and are closely related to P. vivax (5, 23). The pathology and clinical symptoms of a P. cynomolgi infection in monkeys are similar to those of a P. vivax infection in humans (23); P. knowlesi causes a lethal infection in macaques and severe quotidian malaria in humans (3, 9, 14). Based on the previous studies and our unpublished data, indinavir (15a), ritonavir, and saquinavir behaved synergistically with chloroquine and mefloquine against malaria in vitro and in a rodent model (10–12, 20). In the current study, we used P. cynomolgi or P. knowlesi infection in rhesus macaques to evaluate the antimalarial activities of two of the HIV PIs: indinavir (Crixivan) and saquinavir (Invirase). The monkeys were free of malaria parasites, simian immunodeficiency virus, B virus, D-type simian retrovirus, simian T-lymphotropic virus type-1, and the tubercle bacillus and were housed at the Non-human Primate Animal Center of the Guangzhou Institutes of Biomedicine and Health (GIBH). Experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals. Animal protocols were approved by the GIBH Institutional Animal Care and Use Committee.
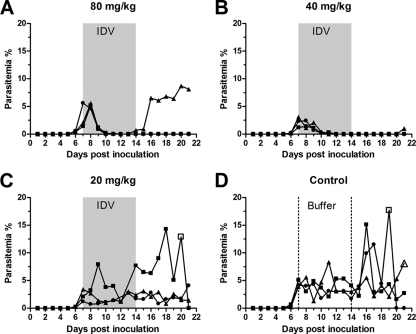
First, we evaluated the antimalarial activity of indinavir at different dosages in P. cynomolgi-infected monkeys. Twelve 3- to 4-year-old Chinese-origin rhesus macaques (Macaca mulatta) were each inoculated intravenously with 1 × 107 P. cynomolgi-parasitized erythrocytes thawed after liquid nitrogen storage. Body temperature measurements were taken daily, and parasitemia was recorded daily by examining Giemsa-stained thick and thin blood smears. All monkeys had fever when the parasitemia reached 0.73% to 5.6% (parasite density, presented as the percentage of infected erythrocytes among the total red blood cell [RBC] count) 5 to 7 days after inoculation. Beginning on day 8, three doses of indinavir (20, 40, and 80 mg/kg of body weight dissolved in saline buffer) and the control saline buffer were administered (three monkeys per group) intragastrically three times daily (TID) for seven consecutive days. All the monkeys administered 40 mg/kg or 80 mg/kg indinavir experienced a rapid normalization of body temperature (from high fever to normal temperature, data not shown) and a significant decrease in parasitemia (to less than 0.5%) (Fig. 1A and B) during the initial 3-day treatment. The parasite densities in five of the six monkeys decreased to undetectable levels after a 7-day treatment, and these levels were maintained for 3 to 7 days after termination of the therapy, at which point there was a recrudescence of infection. The monkeys treated with the 20-mg/kg dose underwent some decrease in parasitemia after a 5-day treatment (Fig. 1C) but without resolution of fever during the treatment. The control monkeys treated with the saline buffer experienced high levels of parasitemia (Fig. 1D) and the typical pattern of periodic high fever (data not shown). One monkey in the 20-mg/kg-dose group and two monkeys in the control group required treatment with artesunate to prevent death due to high parasitemia (Fig. 1C and D). At the endpoint of the experiment (day 25), chloroquine was administered (25 mg/kg of body weight per day for 3 days) to terminate the infection (data not shown).
Fig. 1.
Efficacy of indinavir against P. cynomolgi in rhesus macaques. Monkeys infected with 1 × 107 P. cynomolgi-parasitized RBCs were treated intragastrically, three times daily, for seven consecutive days with different doses of indinavir (IDV) or the control as follows: 80 mg/kg (A), 40 mg/kg (B), 20 mg/kg (C), or control saline buffer (D). The parasitemia was recorded daily by examining Giemsa-stained thick and thin blood smears. The gray areas and the area between dotted lines indicate the duration of indinavir and buffer treatment, respectively. An open symbol indicates oral treatment of the monkey with artesunate to control the parasitemia. The significance of differences in comparison with the control group during treatment (days 8 to 14) was analyzed with the nonparametric Kruskal-Wallis test with Dunn's posttest (80 mg/kg, P < 0.01; 40 mg/kg, P < 0.01; and 20 mg/kg, P > 0.05).
Next, we compared the antimalarial activities of indinavir and saquinavir. Nine monkeys were inoculated with P. cynomolgi as described above and divided into three groups (three animals per group) as follows: indinavir (30 mg/kg TID), saquinavir (22.5 mg/kg TID), and saline buffer controls. Both doses were converted from the recommended human doses (indinavir, 800 mg TID [4], and saquinavir, 600 mg TID [18]) to monkey-equivalent doses based on body weight (80 kg) as follows: human dose divided by body weight times 3 (7). A 7-day treatment of three times per day was initiated on day 11 (parasitemia of >1%). The monkeys treated with indinavir experienced lower levels of parasitemia (<2.5%) at peak levels than the control animals or the saquinavir-treated animals, and the parasitemia was suppressed to undetectable levels 4 days after initiation of the treatment (Fig. 2A), whereas saquinavir only mildly controlled the parasitemia (Fig. 2B). All the monkeys received a 3-day treatment of chloroquine (25 mg/kg once per day) to terminate the malaria infection because none of the animals had been cured by either of the two HIV PIs.
Fig. 2.
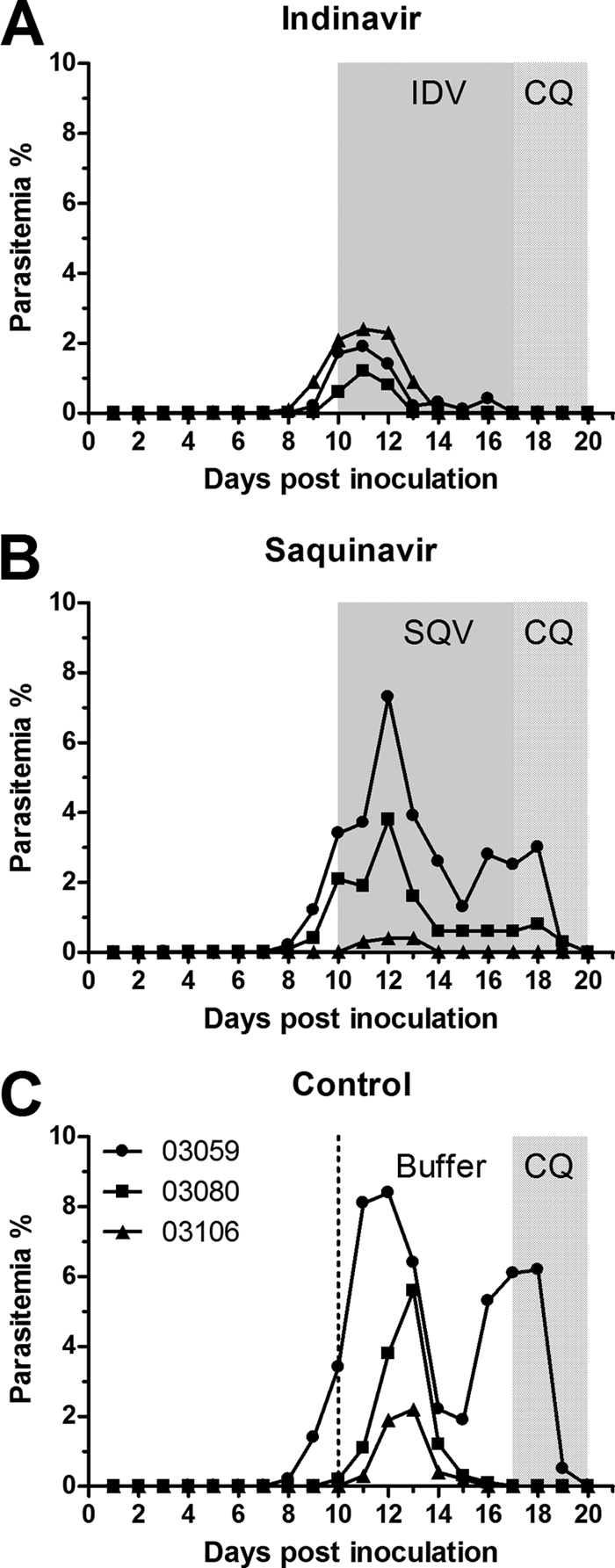
Efficacies of indinavir and saquinavir against P. cynomolgi in rhesus macaques. Monkeys infected with 1 × 107 P. cynomolgi-parasitized RBCs were treated intragastrically, three times daily, for seven consecutive days with either 30 mg/kg indinavir (IDV) (A), 22.5 mg/kg saquinavir (SQV) (B), or control saline buffer (C). After the 7-day treatment, chloroquine phosphate (CQ) (25 mg/kg body weight) was administered once a day for 3 days to eliminate the parasites. The level of parasitemia was recorded daily. The gray areas and the area between the dotted line and the dotted-gray area indicate the duration of indinavir, saquinavir, or buffer treatment, as indicated. The dotted-gray areas indicate the duration of the chloroquine phosphate treatment. The significance of differences in comparison with the control group during treatment (days 11 to 17) was analyzed with the nonparametric Kruskal-Wallis test with Dunn's posttest (IDV, P < 0.01, and SQV, P > 0.05).
We further investigated the effect of indinavir on P. knowlesi, which causes a lethal infection in rhesus macaques. It has been previously reported that inoculations of rhesus monkeys with 104 to 106 P. knowlesi-parasitized RBCs would lead to death in 100% of the animals (22). In this study, one rhesus macaque was inoculated with 1 × 107 P. knowlesi-infected erythrocytes (thawed from liquid nitrogen, so the infectious parasites were probably fewer than 107). Parasitemia was recorded daily and every 3 to 6 h from day 5. On day 5 after inoculation, the parasitemia increased rapidly to 16%, and then the monkey was treated with indinavir at a dose of 40 mg/kg three times daily. After an 18-hour treatment with indinavir, the parasitemia decreased sharply, to 6% (Fig. 3), followed by a slight upswing until 24 h and then a further reduction to 0.3% within 2 days. Parasites were only found in the thick blood smear after the 3-day treatment. Then, chloroquine was used to clear the remaining parasites, as described previously.
Fig. 3.
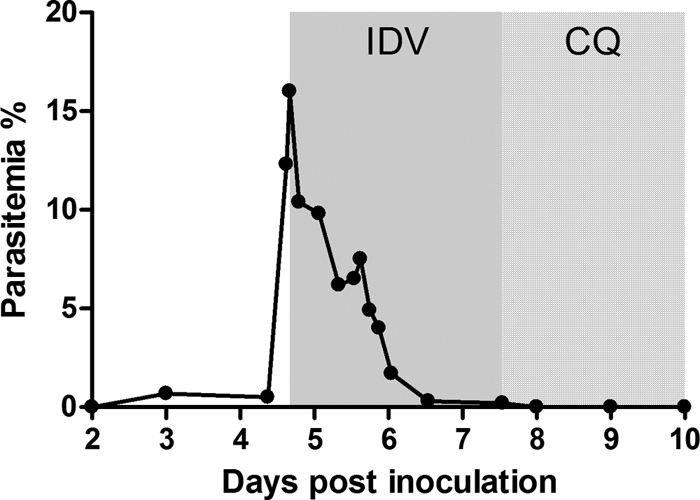
Efficacy of indinavir against P. knowlesi in rhesus macaques. One macaque inoculated with 1 × 107 P. knowlesi-parasitized RBCs was treated intragastrically with 40 mg/kg indinavir (IDV) three times daily for three consecutive days starting on day 5 after the inoculation. To eliminate the parasites, chloroquine phosphate (CQ) was administered. The gray area indicates the duration of the indinavir treatment. The dotted area indicates the duration of the chloroquine phosphate treatment.
Finally, we investigated the preventative effects of indinavir for P. cynomolgi infection in monkeys. One rhesus macaque was administered indinavir at a dose of 60 mg/kg twice a day for 17 days and was inoculated intravenously with 7 × 107 P. cynomolgi-parasitized erythrocytes on the fourth day of indinavir treatment. The parasites were not detected in thick smears until 3 days after the treatment terminated (data not shown).
Our result that indinavir can suppress P. cynomolgi growth is consistent with the previous findings on P. falciparum or P. vivax in vitro (1, 16, 19, 21). Although saquinavir can inhibit human malaria parasites in vitro (1, 15, 16, 19, 21), it displayed only weak activity against malaria in our monkey model. Saquinavir exhibited in vivo antimalarial activity only when combined with ritonavir (1). Thus, its antimalarial efficiency of combination administration needs to be investigated further in primates. More importantly, indinavir has potent activity against P. knowlesi in monkeys; P. knowlesi causes severe quotidian malaria in Southeast Asia (3, 9, 14). This is the first case of indinavir suppressing a human malaria parasite in an in vivo primate model, although the effect needs to be confirmed in more monkeys.
Some epidemiological studies have shown that concomitant HIV infection increased the incidence of malaria (8) and the severity of malaria in adults with low CD4+ T lymphocyte counts (10). The treatment with indinavir in the present study was able to suppress P. cynomolgi to an undetectable level or prevent P. cynomolgi infection in monkeys. These results suggest that an indinavir-containing or an appropriate HIV PI-containing HAART regimen may protect HIV-positive individuals from severe malaria once the malaria infection is established or may prevent clinical malaria. The interactions between HIV PIs and some antimalarial drugs have been investigated. Combinations of indinavir (15a), ritonavir, or saquinavir and chloroquine or mefloquine displayed strong synergistic antimalarial effects, especially on drug-resistant parasites (10–12, 20). Ritonavir has also shown a potent inhibitive effect on cytochrome P450 (6) that catabolizes most HIV PIs (2); therefore, an appropriate ritonavir-boosted HIV PI regimen in combination with current antimalarial drugs should be taken into consideration in future studies. In addition, the appearance of HIV PI-resistant plasmodia is a concern given the recrudescence seen in the current study and that these drugs are usually administered to HIV-infected individuals life-long. Overall, an appropriate HIV PI-containing HAART regimen may kill two birds with one stone: a treatment for HIV infection may reduce the malaria morbidity and mortality in regions where malaria and HIV are coendemic.
Acknowledgments
We thank the staff at Non-human Primate Center of Guangzhou Institutes of Biomedicine and Health for excellent care of the animals.
This work was supported by the National Natural Science Foundation of China (grants 30800986 and 30870132) and Natural Science Foundation of Guangdong Province, China (grants 07118293, 9151051501000073, and 9251064001000002).
Footnotes
Published ahead of print on 12 April 2011.
REFERENCES
- 1. Andrews K. T., et al. 2006. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 50:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barry M., Mulcahy F., Merry C., Gibbons S., Back D. 1999. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin. Pharmacokinet. 36:289–304 [DOI] [PubMed] [Google Scholar]
- 3. Cox-Singh J., et al. 2008. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 46:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorsey B. D., et al. 1994. L-735,524: the design of a potent and orally bioavailable HIV protease inhibitor. J. Med. Chem. 37:3443–3451 [DOI] [PubMed] [Google Scholar]
- 5. Duval L., et al. 2009. Chimpanzee malaria parasites related to Plasmodium ovale in Africa. PLoS One 4:e5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eagling V. A., Back D. J., Barry M. G. 1997. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br. J. Clin. Pharmacol. 44:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. FDA 2005. Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers, p. 7 U.S. Food and Drug Administration, Washington, DC: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf [Google Scholar]
- 8. Gallagher M., et al. 2005. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS 19:1849–1855 [DOI] [PubMed] [Google Scholar]
- 9. Garcia L. S. 2010. Malaria update for the clinical microbiology laboratory: a new species, Plasmodium knowlesi, and new diagnostic tests. Clin. Microbiol. Newsl. 32:127–133 [Google Scholar]
- 10. Grimwade K., et al. 2004. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 18:547–554 [DOI] [PubMed] [Google Scholar]
- 11. He Z., Chen L., You J., Qin L., Chen X. 2009. Antiretroviral protease inhibitors potentiate chloroquine antimalarial activity in malaria parasites by regulating intracellular glutathione metabolism. Exp. Parasitol. 123:122–127 [DOI] [PubMed] [Google Scholar]
- 12. He Z., et al. 2008. Synergy of human immunodeficiency virus protease inhibitors with chloroquine against Plasmodium falciparum in vitro and Plasmodium chabaudi in vivo. Antimicrob. Agents Chemother. 52:2653–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hobbs C. V., et al. 2009. HIV protease inhibitors inhibit the development of preerythrocytic-stage Plasmodium parasites. J. Infect. Dis. 199:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jongwutiwes S., Putaporntip C., Iwasaki T., Sata T., Kanbara H. 2004. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg. Infect. Dis. 10:2211–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lek-Uthai U., et al. 2008. Stronger activity of human immunodeficiency virus type 1 protease inhibitors against clinical isolates of Plasmodium vivax than against those of P. falciparum. Antimicrob. Agents Chemother. 52:2435–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a. Li X., et al. 2011. Synergy of the antiretroviral protease inhibitor indinavir and chloroquine against malaria parasites in vitro and in vivo. Parasitol. Res. [Epub ahead of print.] doi:10.1007/s00436-011-2427-Z [DOI] [PubMed] [Google Scholar]
- 16. Parikh S., et al. 2005. Antimalarial activity of human immunodeficiency virus type 1 protease inhibitors. Antimicrob. Agents Chemother. 49:2983–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peatey C. L., et al. 2010. Antimalarial asexual stage-specific and gametocytocidal activities of HIV protease inhibitors. Antimicrob. Agents Chemother. 54:1334–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts N. A., et al. 1990. Rational design of peptide-based HIV proteinase-inhibitors. Science 248:358–361 [DOI] [PubMed] [Google Scholar]
- 19. Savarino A., Cauda R., Cassone A. 2005. Aspartic proteases of Plasmodium falciparum as the target of HIV-1 protease inhibitors. J. Infect. Dis. 191:1381–1382 [DOI] [PubMed] [Google Scholar]
- 20. Skinner-Adams T. S., Andrews K. T., Melville L., McCarthy J., Gardiner D. L. 2007. Synergistic interactions of the antiretroviral protease inhibitors saquinavir and ritonavir with chloroquine and mefloquine against Plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 51:759–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skinner-Adams T. S., McCarthy J. S., Gardiner D. L., Hilton P. M., Andrews K. T. 2004. Antiretrovirals as antimalarial agents. J. Infect. Dis. 190:1998–2000 [DOI] [PubMed] [Google Scholar]
- 22. Tripathi R., Mishra D., Rizvi A., Singh C. 2007. Evaluation of some adamantane-based synthetic trioxanes against Plasmodium knowlesi in rhesus monkeys. Life Sci. 81:1544–1548 [DOI] [PubMed] [Google Scholar]
- 23. Waters A. P., Higgins D. G., McCutchan T. F. 1993. Evolutionary relatedness of some primate models of Plasmodium. Mol. Biol. Evol. 10:914–923 [DOI] [PubMed] [Google Scholar]
- 24. WHO 2010. World malaria report 2010, p. xii World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241564106_eng.pdf [Google Scholar]