Abstract
Voriconazole is an antifungal agent that is currently used as primary therapy for invasive aspergillosis and as a potential treatment for systemic candidiasis. Data on the dosing of voriconazole in obese patients are not available, which is problematic given the increased prevalence of this special population. To address this limitation, we evaluated the steady-state plasma pharmacokinetics of voriconazole through a two-way, crossover design in a cohort of eight healthy volunteers with class II obesity or greater. The median (minimum, maximum) age, weight, and body mass index of obese subjects were 43 (22, 48) years, 133 (105, 155) kg, and 46.2 (38.4, 53.7) kg/m2, respectively. The geometric mean ratios (90% confidence intervals) for the area under the curve from time zero to 12 h (dosing interval; AUC0–τ), maximum concentration (Cmax), minimum concentration at 12 h (Cmin), and time to Cmax (Tmax) of the 300-mg to 200-mg dosing regimens were 2.0 (1.5, 2.7), 1.8 (1.4, 2.2), 2.2 (1.6, 2.9), and 1.6 (1.0, 2.4) in obese subjects, respectively. The AUC0–τ values observed in obese subjects were comparable to those from a historical data set of nonobese subjects. Voriconazole dose-normalized AUC0–τ values had a modestly better correlation with lean body weight (r2 = 0.42) than total body weight (r2 = 0.14). An excellent linear relationship (r2 = 0.96) was identified between Cmin values and AUC0–τ values. Adjustment of voriconazole doses in individuals with class II obesity or greater does not appear to be necessary on the basis of body weight.
INTRODUCTION
Voriconazole is an antifungal agent used for the treatment of fungal infections such as invasive aspergillosis and systemic candidiasis (14). It is available clinically as both intravenous and oral (p.o.) formulations (14). The current recommended oral dosing regimen (DR) for voriconazole includes use of 200 mg every 12 h for patients who are over 40 kg in body weight (14). The dosage can be increased to 300 mg by mouth every 12 h in situations where a sufficient clinical response is not observed. No specific recommendation currently exists to modify the oral dose of voriconazole in patients to accommodate extremes in body weight. For patients on an intravenous regimen, however, a weight-based (actual or total body weight [TBW]) voriconazole dosing strategy is recommended (3 to 6 mg/kg intravenously every 12 h) to treat serious infections such as invasive aspergillosis.
Given that voriconazole is administered either as a fixed dose (by mouth) or as a weight-based dose (intravenously), a clinical conundrum exists when obese patients are dosed. Based on the product label, a 100-kg patient may be treated with 600 mg every 12 h if the patient is dosed intravenously (6 mg/kg of body weight) or, alternatively, may be treated with 2 mg/kg every 12 h if the patient is dosed by mouth (200 mg every 12 h). This discordance between intravenous and oral dosing may prompt clinicians to adopt a weight-based dosing approach for obese patients when using oral doses of voriconazole. However, use of a weight-based dosing approach for obese subjects is concerning given its nonlinear pharmacokinetics: small changes in the dosage can result in a disproportional increase in drug exposure (16). For example, a 1.5-fold dose increment in voriconazole from 200 mg to 300 mg every 12 h results in an approximately 2.5-fold increase in exposure in normal-weight subjects (14). This dosing discrepancy could lead to toxicity due to high plasma exposures (intravenous dosing) or failure due to low plasma exposures (oral fixed dosing).
Unfortunately, the oral dosing of voriconazole has not been systematically evaluated in obese patients (12). The current pilot study was conducted to characterize the pharmacokinetic (PK) profile of voriconazole in obese subjects using two fixed-dose regimens. The specific objectives of this study were to (i) compare the steady-state pharmacokinetics of voriconazole administered by mouth as a loading dose (400 mg for 2 doses on day −1) and as two fixed maintenance doses (200 mg or 300 mg every 12 h for 7 doses) in obese subjects and (ii) compare the pharmacokinetics of voriconazole among our cohort of obese subjects to an extant data set of healthy normal-weight subjects who have received similar oral voriconazole regimens.
(This work was presented in part at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 12 to 15 September 2010.)
MATERIALS AND METHODS
Regulatory compliance.
The current study was approved by the Institutional Review Board of the Albany College of Pharmacy and Health Sciences prior to the enrollment of any subjects. An Investigational New Drug (IND) application exemption was obtained on 26 May 2009 (IND 105,565). The study protocol was registered through www.clinicaltrials.gov (NCT01030653).
Study subjects.
Ten obese subjects were recruited with the assumption that up to two subjects may not complete both pharmacokinetic study phases. The inclusion criteria included the following: (i) males and females 18 to 50 years of age, (ii) nonsmoking or light smoking (≤5 cigarettes per day), and (iii) body mass index (BMI) of ≥35 kg/m2. To be considered for inclusion, female subjects of childbearing potential had to be either surgically sterilized or using an effective method of contraception. The exclusion criteria included (i) history of significant hypersensitivity reaction or intolerance to any triazole, (ii) history of significant clinical illness requiring pharmacological management, (iii) abnormal serum electrolyte or complete blood count requiring further clinical workup, (iv) transaminase levels >2.5 times the upper limit of normal, (v) estimated creatinine clearance of <50 ml/min (Cockcroft-Gault equation [3]), (vi) positive urine pregnancy test (if female), (vii) abnormal electrocardiogram (ECG), (viii) inability to tolerate venipuncture and multiple blood draws, and (ix) clinically significant abnormal physical examination. The use of any concurrent medications or herbal supplements was not permitted during the study.
Experimental design.
This was a phase 1, open-label, dose-sequence-randomized, multiple-dose, crossover, pharmacokinetic study in subjects with class II obesity or greater (BMI ≥ 35 kg/m2). Qualifying subjects who completed an informed consent were randomized (permuted block) to initiate one of two voriconazole DRs with a 7-day washout period between phases: (i) DR-1 was 400 mg p.o. every 12 h for 2 doses (day 1), followed by 200 mg p.o. every 12 h for 7 doses, and (ii) DR-2 was 400 mg p.o. every 12 h for 2 doses (day 1), followed by 300 mg p.o. every 12 h for 7 doses. The first and last doses administered within each DR were directly observed by the study personnel. Subjects self-administered all subsequent voriconazole doses by mouth with about 8 fluid ounces of water in the fasted state (no food 1 h prior to and after dosing).
Pharmacokinetic sampling and analysis.
A blood sample was collected within 15 min prior to the scheduled 7th maintenance dose, and the time was recorded as time 0 h. Blood samples were then collected at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, and 12 h in sodium heparin-containing tubes after the last dose (7th maintenance dose). Plasma was harvested and stored at −70°C within 60 min of blood collection until analysis. A minimum 7-day washout period (no voriconazole dosing) followed the initial sampling phase. Plasma was analyzed for voriconazole by PPD (Middleton, WI), according to PPD method P968.000, “Determination of voriconazole and voriconazole n-oxide in human plasma by LC/MS/MS [liquid chromatography-tandem mass spectrometry].” All samples were stored at −20°C or colder prior to assay and analyzed as a single batch. Details of this assay methodology have been described previously (4). This validated assay included lower and upper limits of quantitation (10 ng/ml and 5,000 ng/ml, respectively). Samples with concentrations above the upper limits of quantitation were diluted and reassayed. For analytical runs which contained diluted subject samples, the appropriate-level quality control pool was also diluted and analyzed in a similar manner to validate the dilution of study samples. Finally, incurred sample reproducibility was performed on 10% of the study samples. The coefficient of variation for the interassay precision and accuracy was less than 5%. Incurred sample reproducibility was also performed on 10% of the samples.
Pharmacokinetic analyses were performed using the WinNonLin program (Pharsight Corp., Mountain View, CA). The following pharmacokinetic parameters were estimated: maximum concentration (Cmax), time to Cmax (Tmax), area under the curve from time zero to 12 h (dosing interval; AUC0–τ), apparent clearance (CL/F), apparent volume of distribution (V/F), and minimum concentration at 12 h (Cmin).
Nonobese control data.
Subject-level, steady-state pharmacokinetic parameter data for voriconazole that were obtained from a nonobese healthy volunteer population were provided by Pfizer, Inc. (15). The nonobese population included subjects treated with 200 mg by mouth every 12 h (n = 14) and 300 mg by mouth every 12 h (n = 7).
Evaluation of alternative body size descriptors (ABSDs) as predictors of exposure.
The predictive value of voriconazole doses indexed to TBW, ideal body weight (IBW), adjusted body weight (ABW), lean body weight (LBW), and body surface area (BSA) on AUC0–τ was determined by linear regression for the combined obese and nonobese pharmacokinetic data set. Ideal body weight was estimated using the simple height-based rule (13). Adjusted body weight was set as TBW when the ratio of TBW/IBW was <1.3 and as 0.4 · (TBW − IBW) + IBW when TBW/IBW was ≥1.3 (11). Lean body weight was estimated using the LBW2005 equations by Janmahasatian and colleagues (7). The LBW2005 equations by sex are as follows: LBW2005 for males = (9,270 × TBW)/(6,680 + 216 × BMI) and LBW2005 for females = (9,270 × TBW)/(8,780 + 244 × BMI).
Finally, BSA was estimated using Mosteller's adaptation (10). All statistical analyses were completed using Stata SE (version 11) software (StataCorp LLC, College Station, TX).
RESULTS
Study subjects.
Ten subjects were enrolled into the study prior to the initial data analysis, with data from two phases available for eight subjects. Two subjects withdrew from the study for reasons unrelated to the study medication or procedures. The eight subjects (2 males, 6 females) included in this analysis had a mean ± standard deviation (SD) age, TBW, height, and BMI of 41.6 ± 8.3 years, 133.4 ± 16.8 kg, 1.70 ± 0.09 m, and 46.3 ± 5.8 kg/m2, respectively. Participant race was equally distributed as 50% black and 50% white.
Voriconazole concentration-time profiles.
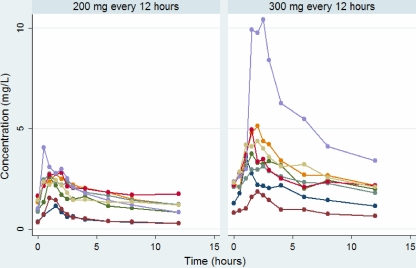
The individual plasma concentration-time profiles for each subject are illustrated in Fig. 1 by dosing regimen. As expected, a nonlinear increase in exposure was visible with the 1.5-fold increase in daily dose. The geometric mean ratios (90% confidence intervals [CIs]) of the 300-mg to 200-mg dosing regimens in obese subjects were 2.0 (1.5, 2.7), 1.8 (1.4, 2.2), 2.2 (1.6, 2.9), 1.6 (1.0, 2.4) for the AUC0–τ, Cmax, Cmin, and Tmax values, respectively. One subject in particular demonstrated a marked increase in exposure with the higher dosage of voriconazole (Fig. 1).
Fig. 1.
Individual voriconazole steady-state concentration-time profiles for obese subjects over the dosing interval by the oral dose regimen.
Comparison of voriconazole pharmacokinetics between obese subjects and nonobese subjects (historical reference group).
The geometric mean (95% CI) plasma pharmacokinetic parameters are included in Table 1 by dosing regimen and group (obese, nonobese). The mean ± SD age, TBW, and BMI of the nonobese referent group were 25.9 ± 5.7 years, 76.9 ± 7.1 kg, and 23.7 ± 1.9 kg/m2, respectively. Despite a 72% difference in the mean TBW, the absolute V/F and Cmax were very similar (±25%) between the two groups when they were compared by dosing regimen. The geometric mean AUC0–τ values were similar between the two groups when the results for the 300-mg dose of voriconazole were compared. In contrast, the geometric mean absolute CL/F was approximately 50% lower, and as a consequence, the AUC0–τ was approximately 50% higher with the 200-mg dose of voriconazole in the obese compared to the nonobese group.
Table 1.
Comparison of voriconazole plasma pharmacokinetic parameters by dosage regimen between two groups of healthy volunteersa
| Plasma PK parameter | Geometric mean (95% CI) |
|||
|---|---|---|---|---|
| Obese group |
Nonobese (reference) group |
|||
| 200 mg q12 (n = 8) | 300 mg q12 (n = 8) | 200 mg q12 (n = 14) | 300 mg q12 (n = 7) | |
| AUC0–τ (mg · h/liter) | 14.6 (9.21, 23.1) | 29.2 (19.4, 43.8) | 9.76 (6.99, 13.7) | 30.9 (17.8, 53.8) |
| CL/F (liters/h) | 13.4 (8.45, 21.3) | 10.1 (6.75, 15.2) | 20.0 (14.1, 25.8) | 8.36 (3.88, 12.8) |
| Cmax (mg/liter) | 2.36 (1.70, 3.28) | 4.16 (2.76, 6.27) | 1.89 (1.52, 2.35) | 4.83 (3.50, 6.70) |
| Cmin (mg/liter) | 0.81 (0.46, 1.44) | 1.76 (1.17, 2.66) | 0.35 (0.22, 0.54) | 1.43 (0.60, 3.41) |
| Tmax (h) | 1.16 (0.80, 1.67) | 1.79 (1.37, 2.32) | 1.50 (1.25, 1.75) | 1.43 (1.25, 1.61) |
| V/F (liters) | 163 (88.6, 237) | 118 (76.3, 160) | 160 (124, 196) | 107 (86.1, 128) |
The two groups of healthy volunteers were classified as having class II obesity or greater (obese group) and a body mass index of ≤30 kg/m2 (nonobese group). q12h, every 12 h.
ABSDs and Cmin as predictors of exposure.
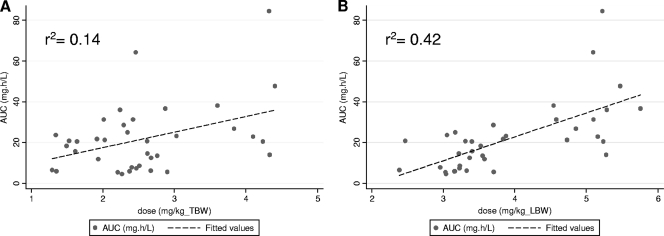
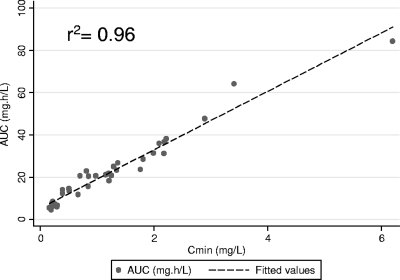
The adjusted r2 values were 0.14, 0.31, 0.38, and 0.42 for the linear regression of AUC0–τ to dose indexed to weight (mg/kg) by TBW, IBW, ABW, or LBW. Figure 2 illustrates the relationship of AUC0–τ to dose (mg/kg) when the current standard of TBW relative to LBW estimated using the LBW2005 equation is considered. As illustrated, dose indexed to LBW had a modestly better correlation to AUC0–τ than a similar indexation with TBW (range, 66 to 155 kg). The voriconazole dose indexed to BSA (mg/m2) was associated with an adjusted r2 value of 0.30 when it was regressed to AUC0–τ. Although LBW was a better predictor of voriconazole exposure across a TBW range of 66 to 155 kg, all assessed ABSDs demonstrated a poor overall correlation (r2 < 0.5). Figure 3 illustrates the relationship of AUC0–τ to Cmin. As demonstrated, a very strong linear relationship exists between voriconazole Cmin and AUC0–τ values. The mean (95% CI) for the coefficient of Cmin was 16.09 (15.06, 17.11) by ordinary least-squares regression (no constant) with an r2 value of 0.96.
Fig. 2.
Scatter and linear fit plots of AUC0–τ (mg · h/liter) compared to the dose (200 mg or 300 mg) indexed to the individual subject's total body weight (A) or lean body weight (B).
Fig. 3.
Scatter and linear fit plots of AUC0–τ (mg · h/liter) compared to the Cmin (mg/liter) of voriconazole.
DISCUSSION
One-third of the U.S. population is now classified as obese (body mass index ≥ 30 kg/m2) (5). Although the prevalence of obesity has increased over the past 2 decades, studies that evaluate the disposition of antimicrobials in this population are scant (6, 12). The U.S. Food and Drug Administration (FDA) does not presently recognize obese patients as a special population, and thus, no specific guidance to evaluate drug disposition in this population exists (6). As a result, clinical trials in the early phases of drug development often exclude obese subjects. Despite this exclusion, drugs are ultimately used in a broader population than that studied in controlled trials. Anecdotal evidence suggests that clinicians may arbitrarily adjust doses on the basis of TBW. This approach may be reasonable for drugs with linear pharmacokinetics and PK parameters that are weight dependent. However, this approach may be unreasonable for drugs like voriconazole with nonlinear pharmacokinetics: for this drug, doubling the dose leads to greater than twice the exposure. Hence, the possibility of under- or overdosing voriconazole in obese subjects is an unfortunate reality given the paucity of clinical pharmacokinetic data in this population.
The current study was designed to provide clinical pharmacokinetic data in a small cohort of obese subjects. We demonstrated that the volume of distribution and clearance of voriconazole between obese and nonobese subjects were indistinguishable, in spite of marked differences in TBW. The similar pharmacokinetic estimates were reflected in highly comparable estimates of AUC0–τ, Cmax, and Cmin between weight groups, especially with the 300-mg twice-daily maintenance dosing schedule. For the 200-mg twice-daily regimen, the mean plasma exposures were numerically higher in the obese group relative to the nonobese group, contrary to what you would expect if weight influenced the pharmacokinetics of an agent. Although a higher mean exposure profile was noted in the obese group, the difference was not statistically significant and perhaps not clinically relevant, as the AUC0–τ distributions were largely overlapping.
Several important inferences can be drawn from the similar pharmacokinetic profiles observed between weight groups. Although voriconazole is a relatively lipophilic compound, the comparable volumes of distribution suggest that it does not necessarily distribute to adipose tissue differently in obese versus nonobese individuals. Further exploration of this theory is warranted through the conduct of noninvasive (19F magnetic resonance spectroscopy) or semi-invasive (microdialysis) studies in obese subjects treated with voriconazole (2). The similar clearances indicate that the clearance capacity of voriconazole does not directly scale to TBW. Exploration of ABSDs demonstrated this to be the case, and LBW was found to be a better scalar than TBW. However, neither weight parameter truly predicted (r2 < 0.5) the variability in voriconazole exposure. The lack of a clinically meaningful relationship in the ABSD analysis indicates that there are more important factors than weight in accounting for both inter- and intrapatient variability in exposure profiles.
Although weight was not found to be an important pharmacokinetic covariate for voriconazole, a strong linear relationship between voriconazole Cmin values and AUC0–τ values was noted. This has very practical implications for clinicians. In a neutropenic murine model of disseminated candidiasis, a free 24-hour AUC/MIC ratio of 20 to 25 has been demonstrated to be predictive of treatment success (1). Similarly, Mavridou and colleagues have demonstrated the effectiveness of voriconazole to be closely related to free 24-hour AUC/MIC values in a neutropenic murine model of disseminated aspergillosis (9). Consequently, in patients a simple function such as 16.09 · Cmin = AUC0–τ could be used to derive a practical clinical estimate of AUC0–τ. This translation may aid future research groups to identify a clinical AUC/MIC target range that can be used to validate the current preclinical AUC/MIC targets outlined above. Again, adoption of such an approach requires refinement of the estimate of this coefficient (16.09) through analyses of larger data sets. At a minimum, our study at least reveals that the clinical measurement of Cmin values may be easily transformed to the more robust PK parameter of AUC in order to better predict clinical effect. This is especially important because a specific therapeutic range for voriconazole Cmin values (for any drug, for that matter) is likely to remain elusive. Lewis has eloquently framed the rationale of this premise by guiding clinicians to dissociate the concept of a therapeutic range as “an absolute entity; rather, as a concept of probability” (8).
In conclusion, this pilot study provides, for the first time, a glimpse into the disposition of voriconazole in an emerging special population of obese individuals. The knowledge gained from the current study should be utilized to improve population pharmacokinetic models of voriconazole. Oral dosing of voriconazole should not be based on TBW, especially in obese patients, given the risk of disproportional exposure and toxicity. Although our study did not evaluate intravenous dosing of voriconazole, dosing this antifungal solely on the basis of TBW will also lead to disproportional exposure and toxicity in obese patients. A reappraisal of weight-based voriconazole dosing practices in adults is needed. Additional prospective trials in obese patients with invasive fungal infections who are treated with voriconazole are necessary to validate the conclusions of our study.
ACKNOWLEDGMENTS
This study was funded in part through an investigator-initiated grant (WS435452) from Pfizer, Inc.
We acknowledge the support of Danielle Pensa for her excellent coordination of the current study.
Footnotes
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Andes D., Marchillo K., Stamstad T., Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunner M., Langer O. 2006. Microdialysis versus other techniques for the clinical assessment of in vivo tissue drug distribution. AAPS J. 8:E263–E271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cockcroft D. W., Gault M. H. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 4. Damle B., LaBadie R., Crownover P., Glue P. 2008. Pharmacokinetic interactions of efavirenz and voriconazole in healthy volunteers. Br. J. Clin. Pharmacol. 65:523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 6. Hanley M. J., Abernethy D. R., Greenblatt D. J. Effect of obesity on the pharmacokinetics of drugs in humans. Clin. Pharmacokinet 49:71–87 [DOI] [PubMed] [Google Scholar]
- 7. Janmahasatian S., et al. 2005. Quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051–1065 [DOI] [PubMed] [Google Scholar]
- 8. Lewis R. E. 2008. What is the “therapeutic range” for voriconazole? Clin. Infect. Dis. 46:212–214 [DOI] [PubMed] [Google Scholar]
- 9. Mavridou E., Bruggemann R. J., Melchers W. J., Verweij P. E., Mouton J. W. 2010. Impact of cyp51a mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mosteller R. D. 1987. Simplified calculation of body-surface area. N. Engl. J. Med. 317:1098. [DOI] [PubMed] [Google Scholar]
- 11. Pai M. P. 2010. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv. Chronic Kidney Dis. 17:e53–362 [DOI] [PubMed] [Google Scholar]
- 12. Pai M. P., Bearden D. T. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091 [DOI] [PubMed] [Google Scholar]
- 13. Pai M. P., Paloucek F. P. 2000. The origin of the “ideal” body weight equations. Ann. Pharmacother. 34:1066–1069 [DOI] [PubMed] [Google Scholar]
- 14. Pfizer, Inc. 2010. Vfend® (voriconazole for injection, tablets and for oral suspension) product label. Pfizer, Inc., New York, NY [Google Scholar]
- 15. Purkins L., et al. 2002. Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob. Agents Chemother. 46:2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Theuretzbacher U., Ihle F., Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649–663 [DOI] [PubMed] [Google Scholar]