Abstract
Influenza virus infections are known to persist longer in patients with underlying diseases, including respiratory tract diseases, and tend to become complicated by secondary influenza-associated infections, such as pneumonia. To assess the efficacy and safety of the novel anti-influenza virus drug peramivir in high-risk patients, we conducted a clinical trial of patients with diabetes or chronic respiratory tract diseases and patients being treated with drugs that suppress immune function. In this multicenter, uncontrolled, randomized, double-blind study, peramivir was intravenously administered at 300 or 600 mg/day for 1 to 5 days, as needed. Efficacy was investigated in 37 patients (300 mg, n = 18 patients; 600 mg, n = 19 patients). The median durations of influenza illness were 68.6 h (90% confidence interval, 41.5 to 113.4 h) overall, 114.4 h (90% confidence interval, 40.2 to 235.3 h) in the 300-mg group, and 42.3 h (90% confidence interval, 30.0 to 82.7 h) in the 600-mg group. The hazard ratio for the 600-mg group compared to the 300-mg group was 0.497 (90% confidence interval, 0.251 to 0.984), and the duration of influenza illness was significantly shorter in the 600-mg group than in the 300-mg group. Among the 42 patients in the safety analysis set, adverse events occurred in 73.8% and adverse drug reactions in 33.3%. No adverse events were particularly problematic clinically, and all patients recovered quickly from all events. The measured blood drug concentrations showed no tendency toward accumulation. Drug accumulation with repeated doses was thus considered to be of little concern. Intravenous peramivir appears to offer a potentially useful treatment for high-risk patients in the future.
INTRODUCTION
Ever since the highly pathogenic avian influenza virus (H5N1) was isolated in Hong Kong in 1997, the number of avian influenza virus-infected patients has continued to rise, albeit sporadically, and measures designed to minimize the damage have been pursued worldwide. In addition, the new swine origin H1N1 strain emerged in Mexico in April 2009 and rapidly spread throughout the world. In June 2009, the World Health Organization (WHO) raised the warning level to phase 6, indicating a global epidemic (pandemic). Subsequently, the H1N1 strain rapidly spread to 214 countries around the globe, and more than 18,000 deaths had been reported by May 2010 (32).
Most people who contract influenza develop a transient fever and respiratory tract symptoms before recovering naturally within 7 to 10 days without developing any complications. However, the elderly, small children, pregnant women, and people with underlying diseases (respiratory tract diseases, heart diseases, diabetes, immunodeficiency, etc.) are known to be at risk of developing influenza-associated complications, such as otitis media, paranasal sinusitis, bronchitis, and pneumonia, and their condition sometimes becomes serious and results in death (28, 33). It is recommended that such high-risk patients be immediately treated with anti-influenza drugs in order to prevent the condition from becoming serious (7, 12). Early treatment with anti-influenza drugs appears essential for both H5N1 influenza (34) and high-risk patients.
To date, four drugs have been used as anti-influenza drugs: oseltamivir phosphate, zanamivir, amantadine, and rimantadine. However, all four are oral or inhaled drugs, and administration to patients with severe symptoms or patients who require respiratory management is often difficult. Concern also remains regarding the degree to which these drugs are absorbed in patients in whom gastrointestinal motility is impacted by influenza symptoms or who cannot inhale properly. One report found that zanamivir had no therapeutic efficacy when used to treat influenza virus infection in bone marrow transplant patients who developed pneumonia as a complication, and pulmonary absorption of zanamivir appeared to be limited in patients with severe immunodeficiency associated with pulmonary infiltrate (19). Another report found that the bioavailability of oseltamivir when administered via a nasogastric tube was unreliable (31), and the development of an anti-influenza drug in an injectable formulation has long been desired for reliable administration to influenza patients in whom oral or inhalation administration is difficult.
Peramivir is an anti-influenza drug that selectively inhibits the neuraminidase (NA) of human type A and type B influenza viruses (2, 3, 5, 6) and exhibits potent NA-inhibitory activity against highly pathogenic influenza virus, such as H5N1 subtypes (8). The drug was developed as an intravenous preparation, and a placebo-controlled double-blind study of seasonal influenza patients without risk factors (referred to here as seasonal influenza patients) found that administration of a single dose of 300 or 600 mg/day significantly shortened the duration of influenza illness in comparison with the placebo. The absence of major safety problems has also been confirmed (13), and use of peramivir was approved in Japan at the beginning of 2010 after a trial in which oseltamivir phosphate was used as the control (14). Clinical use of peramivir has therefore commenced. As an intravenously administered drug, peramivir is expected to be the first-choice pharmacotherapy for high-risk and severely ill patients who require hospital admission. We therefore conducted an uncontrolled, randomized, double-blind study of peramivir at 300 or 600 mg/day for 1 to 5 days in patients with influenza infection who had risk factors in order to assess efficacy and safety when administered intravenously.
MATERIALS AND METHODS
This clinical trial was conducted starting with patient enrollment in January 2009 and finishing with final patient follow-up in May 2009 as a multicenter, uncontrolled, double-blind study at 37 medical institutions in Japan. The study was undertaken in compliance with the Declaration of Helsinki and the Ministerial Ordinances Related to Good Clinical Practice (GCP) of Japan.
Patients.
The subjects in this trial were influenza virus-infected patients ≥20 years old who were influenza rapid antigen test (RAT) positive and had ≥1 risk factor, had experienced onset of influenza symptoms within the previous 48 h, and showed ≥2 of 7 influenza symptoms of moderate or greater severity. Four kinds of RAT kits (Rapid Testa FLU II and FLU stick [Sekisui Medical, Tokyo, Japan], Espline Influenza A&B [Fuji Rebio, Tokyo, Japan], and Capilia FLU A+B [Tauns, Shizuoka, Japan]) were used in the study. Any one of them was used at each hospital. The risk factors were poorly controlled diabetes (hemoglobin A1c [HbA1c] ≥ 7.0%), current pharmacotherapy for chronic respiratory tract disease, and current use of a drug that suppresses immune function (immunosuppressant drug or adrenocortical hormone preparation equivalent to ≥10 mg/day of prednisolone). Influenza symptoms included headache, muscle or joint pain, fever or chills, fatigue, cough, sore throat, and nasal congestion. The onset of influenza symptoms was defined as the time at which body temperature rose ≥1°C over the patient's normal body temperature (<37.0°C) or the time at which the patient experienced the onset of ≥2 of the above-mentioned influenza symptoms.
The following were adopted as exclusion criteria: (i) chronic respiratory failure requiring artificial ventilation; (ii) diabetes with an HbA1c value of ≥10%; (iii) organ transplant or hematopoietic stem cell transplant within the previous 12 months; (iv) requirement for dialysis or presence of nephropathy (estimated creatinine clearance, <50 ml/min); (v) presence of congestive heart failure as a complication; (vi) presence of ischemic heart disease or serious arrhythmia; (vii) QTc (corrected QT) interval of ≥480 ms or bradycardia (heart rate, <40 beats/min); (viii) presence of major circulatory system disease, central nervous system disease, metabolic disease (thyroid or adrenal function), cancer, hepatitis, or liver cirrhosis; (ix) current treatment with a human immunoglobulin preparation or colony-stimulating factor (e.g., granulocyte or macrophage colony-stimulating factor) preparation; and (x) presence of a complicating infection judged to require treatment by systemic administration of an antimicrobial agent.
In accordance with GCP, when enrolling subjects in this trial, the principal investigator or subinvestigator provided each patient with an informed consent form that had been examined and approved by the institutional review board at each medical institution. After having been provided with sufficient explanation, each patient gave written consent to participate.
Study procedures.
This trial was designed as an uncontrolled double-blind study in which patients were randomly allocated to receive either 300 or 600 mg of drug/day in a single dose and was conducted as a multicenter collaborative study. Doses of peramivir were administered intravenously over a period of 15 to 60 min for 1 to 5 days in accordance with the repeated-dosing criteria described below. The criteria for repeated dosing by continuing administration from day 2 onward were a body temperature of ≥37.5°C or judgment by the principal investigator or subinvestigator that continued administration was necessary based on clinical manifestations. Combined treatment with other antiviral drugs or antimicrobial drugs was prohibited, although use of acetaminophen to reduce fever was permitted. Dynamic allocation by the minimization method was used to allocate patients to each of the dosage groups. Whether the patient had poorly controlled diabetes, was undergoing pharmacotherapy for a chronic respiratory tract disease, or was using immunosuppressant drugs and patient age (<65 years versus ≥65 years) were used as allocation factors during the allocation process.
Every day from the day of enrollment until day 14, or until discontinuation, the patient recorded body temperature in a patient diary, 4 times/day (morning, noon, evening, and bedtime) until day 5 and twice daily (morning and evening) from day 6 to day 14. The severity of each of the 7 influenza symptoms was evaluated on a 4-grade scale (0, none; 1, mild; 2, moderate; 3, severe) twice daily (morning and evening) from the day of enrollment until day 14. If a patient took acetaminophen to reduce fever, body temperature was measured at least 4 h after the dose of acetaminophen was taken. In addition, a pharyngeal or nasal cavity swab was collected for virus testing on days 2, 3, and 6. Clinical examination (hematology, biochemistry, electrolyte measurements, and urinalysis) and monitoring and assessment of vital signs (blood pressure, heart rate, and respiratory rate) were performed on days 3, 6, and 10 after the start of peramivir administration.
The per-protocol set (PPS) was used as the main subject analysis population for efficacy. Duration of influenza illness was adopted as the primary endpoint for efficacy, representing the time until influenza symptoms in the combined group (with all patients given either 300 or 600 mg/day) resolved (i.e., the point at which all 7 influenza symptoms had become “0, none” or “1, mild” and remained at that level for ≥21.5 h). The following were adopted as secondary endpoints: (i) duration until body temperature normalized (body temperature of <37.0°C for ≥12 h), (ii) influenza virus titer [log10 50% tissue culture infective dose {TCID50}/ml], (iii) proportion of patients who were influenza virus positive (≥0.5 log10 TCID50/ml) after administration (virus titer that did not decrease to below the level of detection after the start of peramivir administration), and (iv) the incidence of influenza-associated complications (pneumonia, bronchitis, otitis media, and paranasal sinusitis). In addition to evaluating endpoints in the entire group (the two groups combined), each endpoint was analyzed according to the dosage group and according to whether a single dose (1 day) or repeated doses (2 to 5 days) had been administered.
The safety analysis set was evaluated with regard to the types, severity, and incidence of adverse events. The Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events (29) was used for rating severity as follows: grade 1, mild; grade 2, moderate; or grade 3, severe.
Viral assessments.
A nasal swab from one naris and a single throat swab were collected at day 1 (baseline) and on days 2, 3, and 6. All samples were taken from the same sites throughout the study. These samples were each transported in 3 ml viral transport medium to a central laboratory and were divided for typing and gene sequence assay using PCR (0.3 ml) and virus titration (0.8 ml). Viral titers were calculated as log10 TCID50/ml of viral transport medium, according to the Spearman-Karber equation. Madin-Darby canine kidney (MDCK) cells were infected in triplicate with 0.1 ml of a 10-fold dilution series of samples (ranging from undiluted to 107) in serum-free medium containing 3 μg of trypsin per ml. Virus was adsorbed for 1 h, and then the cells were washed twice to remove unadsorbed virus and residual peramivir. The MDCK cells were then incubated at 37°C in 5% CO2 for 6 days. Following this incubation period, the appearance of cytopathic effect (CPE) on cell monolayers was scored using light microscopy, and the final titer was expressed as TCID50/ml. When no CPE was observed using undiluted viral solution, it was defined as an undetectable level. In the study, the undetectable level was considered to be 100.5 TCID50/ml. Neuraminidase enzyme-inhibitory assays were performed on the isolated virus using a standard fluorometric assay (30). The 50% inhibitory concentration (IC50) was calculated by plotting the percent inhibition of neuraminidase activity versus the inhibitor concentration. The results are reported as the mean ± standard deviation (SD) of three independent experiments. DNA and amino acid sequence analysis was performed using commercial software at Shionogi and Co., Ltd. The sequences were examined on the amplified NA regions including the 274th and 294th (N2 numbering) amino acids.
NA sequence analysis.
Influenza A (H1N1) viruses isolated from patients before treatment (screening point) were analysis candidates. cDNA was generated using viral RNA as a template and a PrimeScriptII 1st-strand cDNA Synthesis Kit, according to the manufacturer's instructions (TaKaRa Bio Inc.). A DNA fragment of part of the NA region was amplified from the cDNA with the TaKaRa Ex Taq and PCR primers (forward, GAATTGGCTCCAAAGGAGATG; reverse, GGGACGCGGGTTGTCACCGA). The PCR products were purified, sequenced with a BigDye Terminator v3.1 Cycle Sequencing Kit according to the manufacturer's instructions (Applied Biosystems), and then analyzed on a DNA sequencer. The DNA sequences were compared to a reference sequence.
Statistical analysis.
Median values and 90% confidence intervals (CIs) were adopted in this study to assess the primary endpoint of the duration of influenza illness. Since it was deemed to be very difficult to enroll many high-risk patients with influenza virus infections, the 90% CI, not 95%, was utilized. The target number of patients in the 300-mg and 600-mg groups combined, i.e., all patients who were administered either 300 mg or 600 mg, was set at ≥50 patients (upper limit, n = 100), so that the 90% CI for median values of the primary endpoint for efficacy, duration of influenza illness, could be expected to lie within a 72-h interval. The PPS, i.e., patients who had received the trial drug and had been confirmed to have influenza virus infection by the RAT, was adopted as the main subject analysis population for efficacy. The 90% CI for the hazard ratio of the 600-mg-dose group compared to the 300-mg-dose group was calculated, and the significance of the 600-mg-dose group was indicated if the upper confidence limit was less than 1.0. Cases in which influenza symptoms disappeared during the observation period were censored. The time until return to normal body temperature was analyzed in the same way. Patients who were positive for influenza virus were counted, and the positive rate and 90% CI were calculated. The Clopper-Pearson method was used to calculate CIs of rates. Influenza-associated complications were analyzed in the same way.
The safety analysis set comprised patients who had received the trial drug at least once and patients from whom some kind of information related to safety had been obtained. Due to the need to give priority to on-site treatment following the Pandemic A (H1N1) 2009 epidemic, this trial was concluded as of May 2009, even though the target of 50 cases had not been reached at that stage.
RESULTS
Patient characteristics.
A total of 42 patients participated in the trial (300-mg group, n = 21; 600-mg group, n = 21). After 1 ineligible case and 4 cases that violated the trial drug administration method were excluded, the PPS consisted of 37 cases (300-mg group, n = 18; 600-mg group, n = 19). The background characteristics of the 37 patients in the PPS and patient information and distribution by hospital are shown in Table 1 and Table 2, respectively. Risk factors comprised poorly controlled diabetes in 4 cases (300-mg group, n = 2; 600-mg group, n = 2), current pharmacotherapy for chronic respiratory tract disease in 29 cases (300-mg group, n = 15; 600-mg group, n = 14), and current use of a drug to suppress immune function in 9 cases (300-mg group, n = 5; 600-mg group, n = 4); all 5 patients with asthma were also taking steroids and thus had more than one risk factor. The influenza virus type as determined by PCR was type A in 30 cases (81.1%) and type B in 3 cases (8.1%), and the subtype of type A virus was A/H1 in 16 cases (43.2%) and A/H3 in 13 cases (35.1%). The IC50 against NA for peramivir was within the range of 0.724 to 25.02 nM. According to age, 28 patients were <65 years old (300-mg group, n = 14; 600-mg group, n = 14), and 9 patients were ≥65 years old (300-mg group, n = 4; 600-mg group, n = 5). The most common duration of administration was 2 days in 23 patients, followed by 1 day in 10 patients, 3 days in 2 patients, and 4 days and 5 days in 1 patient each, as shown in Table 1.
Table 1.
Background characteristics of the patients (PPS population)
| Parameter | Value for group |
||
|---|---|---|---|
| Combined (n = 37) | 300-mg (n = 18) | 600-mg (n = 19) | |
| Gender [no. (%)] | |||
| Male | 15 (40.5) | 7 (38.9) | 8 (42.1) |
| Female | 22 (59.5) | 11 (61.1) | 11 (57.9) |
| Age (yr) | |||
| Mean | 50.9 | 51.5 | 50.4 |
| SD | 16.2 | 16.2 | 16.7 |
| No. (%) ≤65 | 28 (75.7) | 14 (77.8) | 14 (73.7) |
| No. (%) >65 | 9 (24.3) | 4 (22.2) | 5 (26.3) |
| Poorly controlled diabetes [no. (%)] | |||
| Yes | 4 (10.8) | 2 (11.1) | 2 (10.5) |
| No | 33 (89.2) | 16 (88.9) | 17 (89.5) |
| Current drug therapy for a respiratory tract disease [no. (%)] | |||
| Yes | 29 (78.4) | 15 (83.3) | 14 (73.7) |
| No | 8 (21.6) | 3 (16.7) | 5 (26.3) |
| Current use of a drug that suppresses immune function [no. (%)] | |||
| Yes | 9 (24.3) | 5 (27.8) | 4 (21.1) |
| No | 28 (75.7) | 13 (72.2) | 15 (78.9) |
| Time of influenza onset [no. (%)] | |||
| 0–12 h | 2 (5.4) | 1 (5.6) | 1 (5.3) |
| 12–24 h | 15 (40.5) | 8 (44.4) | 7 (36.8) |
| 24–36 h | 10 (27.0) | 5 (27.8) | 5 (26.3) |
| 36–48 h | 10 (27.0) | 4 (22.2) | 6 (31.6) |
| Influenza vaccinationa [no. (%)] | |||
| Yes | 19 (51.4) | 7 (38.9) | 12 (63.2) |
| No | 18 (48.6) | 11 (61.1) | 7 (36.8) |
| Influenza virus subtype [no. (%)] | |||
| A/H1 | 16 (43.2) | 7 (38.9) | 9 (47.4) |
| A/H3 | 13 (35.1) | 4 (22.2) | 9 (47.4) |
| A/H5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| A/- | 1 (2.7) | 1 (5.6) | 0 (0.0) |
| B | 3 (8.1) | 3 (16.7) | 0 (0.0) |
| Unknown | 4 (10.8) | 3 (16.7) | 1 (5.3) |
| IC50 of peramivir | |||
| n | 34 | 15 | 19 |
| Mean | 11.68 | 12.74 | 10.84 |
| SD | 10.48 | 10.52 | 10.66 |
| Minimum | 0.72 | 0.72 | 0.74 |
| Median | 9.97 | 19.20 | 3.75 |
| Maximum | 25.02 | 24.13 | 25.02 |
| Administration [no. (%)] | |||
| 1 day | 10 (27.0) | 7 (38.9) | 3 (15.8) |
| 2 days | 23 (62.2) | 9 (50.0) | 14 (73.7) |
| 3 days | 2 (5.4) | 1 (5.6) | 1 (5.3) |
| 4 days | 1 (2.7) | 0 (0.0) | 1 (5.3) |
| 5 days | 1 (2.7) | 1 (5.6) | 0 (0.0) |
| Mean no. of days of administration (mean dose in mg) | 1.91 (884) | 1.83 (550) | 2.0 (1200) |
Patients had trivalent inactivated influenza vaccination between autumn 2008 and enrollment in the study.
Table 2.
Patient information and distribution by hospital (PPS population)
| Hospital no. | No. of patients | Number with risk factor |
No. of patients treated with acetaminophen | ||
|---|---|---|---|---|---|
| Poorly controlled diabetes | Current drug therapy for respiratory tract disease | Current use of a drug that suppresses immune function | |||
| 1 | 1 | 1 | 1 | ||
| 2 | 1 | 1 | |||
| 3 | 1 | 1 | 1 | ||
| 4 | 1 | 1 | 1 | ||
| 5 | 1 | 1 | |||
| 6 | 2 | 1 | 1 | ||
| 7 | 1 | 1 | 1 | ||
| 8 | 1 | 1 | 1 | ||
| 9 | 2 | 1 | 1 | ||
| 10 | 1 | 1 | |||
| 11 | 1 | 1 | 1 | ||
| 12 | 1 | 1 | 1 | ||
| 13 | 1 | 1 | |||
| 14 | 1 | 1 | 1 | 1 | |
| 15 | 0 | ||||
| 16 | 1 | 1 | |||
| 17 | 1 | 1 | |||
| 18 | 1 | 1 | 1 | ||
| 19 | 2 | 2 | |||
| 20 | 3 | 3 | 2 | 1 | |
| 21 | 1 | 1 | 1 | ||
| 22 | 1 | 1 | 1 | ||
| 23 | 6 | 5 | 1 | 6 | |
| 24 | 2 | 2 | 2 | ||
| 25 | 3 | 3 | 1 | ||
| Total | 37 | 4 | 29 | 9 | 19 |
Efficacy results.
The results of the efficacy evaluation are shown in Table 3. A repeated 600-mg dose of peramivir appears effective in influenza virus-infected patients at high-risk for complications.
Table 3.
Efficacy parameters of peramivir (PPS population)
| Parameter | Value for group |
||
|---|---|---|---|
| Combined (n = 37) | 300 mg (n = 18) | 600 mg (n = 19) | |
| Duration of influenza illness | |||
| n | 37 | 18 | 19 |
| Median (h) (90% CI) | 68.6 (41.5, 113.4) | 114.4 (40.2, 235.3) | 42.3 (30.0, 82.7) |
| Hazard ratio of 600-mg group to 300-mg group (90% CI) | 0.497 (0.251, 0.984) | ||
| Time to return to a normal body temp | |||
| n | 36 | 17 | 19 |
| Median (h) (90% CI) | 40.2 (34.2, 53.8) | 57.1 (34.2, 75.1) | 37.6 (22.3, 46.8) |
| Hazard ratio of 600-mg group to 300-mg group (90% CI) | 0.375a (0.182, 0.770)a | ||
| Time until capable of returning to everyday life | |||
| n | 37 | 18 | 19 |
| Median (h) (90% CI) | 266.5 (169.0, 308.8) | 233.0 (169.0, +∞) | 266.5 (147.6, 308.8) |
| Hazard ratio of 600-mg group to 300-mg group (90% CI) | 0.698a (0.357,1.364)a | ||
| Incidence rate of influenza-associated complications [% (no. of new cases/no. of cases)] | |||
| Total (90% CI) | 10.8 (4/37) (3.8, 23.1) | 11.1 (2/18) (2.0, 31.0) | 10.5 (2/19) (1.9, 29.6) |
| Pneumonia (90% CI) | 8.1 (3/37) (2.2, 19.6) | 11.1 (2/18) (2.0, 31.0) | 5.3 (1/19) (0.3, 22.6) |
| Bronchitis (90% CI) | 2.7 (1/37) (0.1, 12.2) | 0.0 (0/18) (0.0, 15.3) | 5.3 (1/19) (0.3, 22.6) |
| Otitis media (90% CI) | 0.0 (0/37) (0.0, 7.8) | 0.0 (0/18) (0.0, 15.3) | 0.0 (0/19) (0.0, 14.6) |
| Paranasal sinusitis (90% CI) | 2.7 (1/37) (0.1, 12.2) | 5.6 (1/18) (0.3, 23.8) | 0.0 (0/19) (0.0, 14.6) |
| Virus titer [log10 (TCID50/ml)]b | |||
| Baseline | |||
| n | 37 | 18 | 19 |
| Mean | 3.98 | 3.66 | 4.29 |
| SD | 2.28 | 2.45 | 2.12 |
| Min | 0.50 | 0.50 | 0.50 |
| Median | 3.80 | 3.50 | 4.50 |
| Max | 8.50 | 8.50 | 8.50 |
| Day 2 | |||
| n | 31 | 14 | 17 |
| Mean | 1.90 | 2.29 | 1.59 |
| SD | 1.88 | 2.52 | 1.12 |
| Min | 0.50 | 0.50 | 0.50 |
| Median | 0.80 | 1.00 | 0.80 |
| Max | 8.20 | 8.20 | 3.50 |
| Day 3 | |||
| n | 32 | 15 | 17 |
| Mean | 1.35 | 2.09 | 0.69 |
| SD | 1.83 | 2.42 | 0.65 |
| Min | 0.50 | 0.50 | 0.50 |
| Median | 0.50 | 0.50 | 0.50 |
| Max | 7.20 | 7.20 | 3.20 |
| Day 6 | |||
| n | 32 | 15 | 17 |
| Mean | 0.50 | 0.50 | 0.50 |
| SD | 0.00 | 0.00 | 0.00 |
| Min | 0.50 | 0.50 | 0.50 |
| Median | 0.50 | 0.50 | 0.50 |
| Max | 0.50 | 0.50 | 0.50 |
| % Influenza virus-positive patients [% (no. of virus-positive patients/no. of patients)] | |||
| Day 2 (90% CI) | 71.0 (22/31) (54.8, 83.9) | 71.4 (10/14) (46.0, 89.6) | 70.6 (12/17) (47.8, 87.6) |
| Day 3 (90% CI) | 31.3 (10/32) (18.0, 47.2) | 46.7 (7/15) (24.4, 70.0) | 17.6 (3/17) (5.0, 39.6) |
| Day 6 (90% CI) | 0.0 (0/32) (0.0, 8.9) | 0.0 (0/15) (0.0, 18.1) | 0.0 (0/17) (0.0, 16.2) |
Calculated by ad hoc analysis after completion of our clinical study report.
Min, minimum; Max, maximum.
Clinical efficacy.
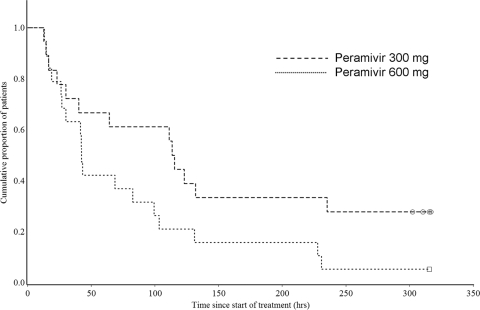
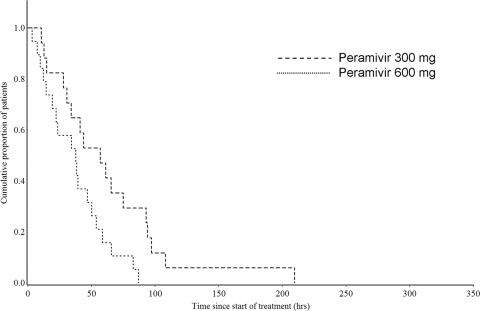
The median duration of influenza illness was 68.6 h (90% CI, 41.5 to 113.4 h) in the PPS, 114.4 h (90% CI, 40.2 to 235.3 h) in the 300-mg group, and 42.3 h (90% CI, 30.0 to 82.7 h) in the 600-mg group (Fig. 1). The hazard ratio for the 600-mg group compared to the 300-mg group was 0.497 (90% CI, 0.251 to 0.984), and the duration of influenza illness was thus significantly shorter in the 600-mg group because the upper confidence limit was less than 1.0 (Table 3). The median duration of influenza illness was 92.0 h (90% CI, 14.6 to 235.3 h; n = 10) in single-dose patients (either the 300-mg or the 600-mg group) and 64.1 h (90% CI, 41.5 to 111.2 h; n = 27) in multiple-dose patients (either the 300-mg or the 600-mg group). The median time until body temperature normalized (<37.0°C) in the PPS was 40.2 h (90% CI, 34.2 to 53.8 h) for the total group, 57.1 h (90% CI, 34.2 to 75.1 h) for the 300-mg group, and 37.6 h (90% CI, 22.3 to 46.8 h) for the 600-mg group (Fig. 2). The time for the 600-mg group was also significantly shorter than that for the 300-mg group (hazard ratio of the 600-mg group compared to the 300-mg group, 0.375 [90% CI, 0.182 to 0.770]).
Fig. 1.
Kaplan-Meier curve of duration of influenza illness in the influenza virus-infected high-risk population.
Fig. 2.
Kaplan-Meier curve of time to return to normal body temperature in the influenza virus-infected high-risk population.
The incidence of influenza-associated complications in the PPS was 10.8% (4/37 cases). The most common influenza-associated complication was pneumonia, in 8.1% of the patients.
Virological results.
The mean influenza virus titers [log10 (TCID50/ml)] on day 2 in patients with positive virus titers at the time of enrollment were 1.90 ± 1.88 overall, 2.29 ± 2.52 in the 300-mg group, and 1.59 ± 1.12 in the 600-mg group. The titers on day 3 were 1.90 ± 1.88 overall, 2.09 ± 2.42 in the 300-mg group, and 0.69 ± 0.65 in the 600-mg group. The titers on day 6 were below the limit of detection (0.50) in both groups. The proportion of influenza virus-positive patients in the PPS was 71.0% on day 2 after the start of administration, 31.3% on day 3, and 0% on day 6. According to dosage group, the proportions were 71.4% in the 300-mg group and 70.6% in the 600-mg group on day 2 and 46.7% in the 300-mg group and 17.6% in the 600-mg group on day 3. The distributions of the IC50s of peramivir, oseltamivir, and zanamivir by virus subtype at baseline are shown in Table 4. The A/H1N1 type was identified in 16 patients (PPS). Except for 4 failures in identification due to small sample volume, the H274Y mutation (N2 numbering; the same as the H275Y mutation by N1 numbering) was identified in 12 patients (300-mg group, 5 patients; 600-mg group, 7 patients). There was no DNA mutation resulting in the N294S substitution. There were no patients who showed an increase in the IC50 (≥3-fold) between baseline and postdose.
Table 4.
Distribution by influenza virus subtype and IC50 of neuraminidase inhibitors at baseline
| Virus subtype and parametera | Value for group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall |
300 mg |
600 mg |
||||||||||
| n | Peramivir | Oseltamivir | Zanamivir | n | Peramivir | Oseltamivir | Zanamivir | n | Peramivir | Oseltamivir | Zanamivir | |
| A/H1 | 16b | 7b | 9b | |||||||||
| Mean | 21.79 | 87.11 | 1.42 | 22.02 | 83.52 | 1.44 | 21.62 | 89.91 | 1.41 | |||
| SD | 2.12 | 16.07 | 0.10 | 1.53 | 16.73 | 0.11 | 2.57 | 15.94 | 0.09 | |||
| Min | 16.19 | 57.42 | 1.26 | 19.20 | 57.42 | 1.26 | 16.19 | 58.28 | 1.27 | |||
| Median | 21.97 | 100.0 | 1.42 | 21.94 | 76.39 | 1.42 | 21.99 | 100.0 | 1.41 | |||
| Max | 25.02 | 100.0 | 1.62 | 24.13 | 100.0 | 1.62 | 25.02 | 100.0 | 1.56 | |||
| A/H3 | 13 | 4 | 9 | |||||||||
| Mean | 0.85 | 0.71 | 2.11 | 0.83 | 0.66 | 2.12 | 0.86 | 0.73 | 2.11 | |||
| SD | 0.10 | 0.11 | 0.16 | 0.10 | 0.11 | 0.08 | 0.10 | 0.11 | 0.19 | |||
| Min | 0.72 | 0.54 | 1.90 | 0.72 | 0.54 | 2.03 | 0.74 | 0.54 | 1.90 | |||
| Median | 0.83 | 0.70 | 2.07 | 0.82 | 0.64 | 2.13 | 0.83 | 0.73 | 2.07 | |||
| Max | 1.02 | 0.88 | 2.43 | 0.95 | 0.81 | 2.19 | 1.02 | 0.88 | 2.43 | |||
| A/- | 1 | 1 | 0 | |||||||||
| Mean | 23.21 | 68.41 | 1.46 | 23.21 | 68.41 | 1.46 | ||||||
| SD | ||||||||||||
| Min | 23.21 | 68.41 | 1.46 | 23.21 | 68.41 | 1.46 | ||||||
| Median | 23.21 | 68.41 | 1.46 | 23.21 | 68.41 | 1.46 | ||||||
| Max | 23.21 | 68.41 | 1.46 | 23.21 | 68.41 | 1.46 | ||||||
| B | 3 | 3 | 0 | |||||||||
| Mean | 3.48 | 18.06 | 9.43 | 3.48 | 18.06 | 9.43 | ||||||
| SD | 0.06 | 2.53 | 0.30 | 0.06 | 2.53 | 0.30 | ||||||
| Min | 3.41 | 15.14 | 9.12 | 3.41 | 15.14 | 9.12 | ||||||
| Median | 3.49 | 19.33 | 9.46 | 3.49 | 19.33 | 9.46 | ||||||
| Max | 3.53 | 19.70 | 9.72 | 3.53 | 19.70 | 9.72 | ||||||
| Unknown | 1 | 0 | 1 | |||||||||
| Mean | 3.75 | 14.76 | 9.48 | 3.75 | 14.76 | 9.48 | ||||||
| SD | ||||||||||||
| Min | 3.75 | 14.76 | 9.48 | 3.75 | 14.76 | 9.48 | ||||||
| Median | 3.75 | 14.76 | 9.48 | 3.75 | 14.76 | 9.48 | ||||||
| Max | 3.75 | 14.76 | 9.48 | 3.75 | 14.76 | 9.48 | ||||||
Min, minimum; Max, maximum.
Except for 4 failures in identification due to small sample volume, the H274Y mutation was identified in 12 patients (300-mg group, 5 patients; 600-mg group, 7 patients).
Safety results.
The incidences of adverse events and adverse drug reactions and types of events in the 42 patients included in the safety analysis set in this trial are shown in Table 5. The incidence of adverse events was 73.8% (31/42 cases), and the incidence of adverse drug reactions was 33.3% (14/42 cases). By dosage group, 44 adverse events were encountered in 15 of the 21 patients (71.4%) in the 300-mg group, and 38 adverse events were encountered in 16 of the 21 patients (76.2%) in the 600-mg group. Eleven adverse drug reactions occurred in 6 patients (28.6%) from the 300-mg group, and 10 adverse drug reactions occurred in 8 patients (38.1%) from the 600-mg group. The major events (≥3 instances of either an adverse event or an adverse drug reaction) were abnormal changes in clinical test values, including increased blood glucose levels and elevated neutrophil counts, in addition to diarrhea, pneumonia, and oral herpes infection. Of the adverse events that developed, 95.1% (78/82) were mild or moderate in severity, and those evaluated as severe consisted of 2 events each of increased blood glucose levels and positive urine glucose levels. Patients rapidly recovered from all events without treatment. Serious adverse events comprised development of bacterial pneumonia and pneumonia in 1 subject each, but causal relationships to peramivir were ruled out because of influenza-related secondary infection. In addition, adverse events occurred in 7 of the 11 patients (63.6%) in the single-dose group and 24 of the 31 subjects (77.4%) in the repeated-dose group (2 to 5 days). No clear increases in the incidence of any adverse event were identified as a result of repeated dosing.
Table 5.
Adverse events and adverse drug reactions in patients with risk factors (safety population)
| Parameter | Adverse events for group |
Adverse drug reactions for group |
||||
|---|---|---|---|---|---|---|
| Total (n = 42) | 300 mg (n = 21) | 600 mg (n = 21) | Total (n = 42) | 300 mg (n = 21) | 600 mg (n = 21) | |
| No. of events | 82 | 44 | 38 | 21 | 11 | 10 |
| No. of patients (%) | 31 (73.8) | 15 (71.4) | 16 (76.2) | 14 (33.3) | 6 (28.6) | 8 (38.1) |
| 95% CI (%) | 58.0, 86.1 | 47.8, 88.7 | 52.8, 91.8 | 19.6, 49.5 | 11.3, 52.2 | 18.1, 61.6 |
| P value (for Peramivir 300 mg) | 1.0000 | 0.7442 | ||||
| Adverse event clinical symptom [no. (%)] | ||||||
| Diarrhea | 2 (4.8) | 1 (4.8) | 1 (4.8) | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Pneumonia | 3 (7.1) | 1 (4.8) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Oral herpes infection | 3 (7.1) | 1 (4.8) | 2 (9.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abnormal laboratory value change [no. (%)] | ||||||
| Blood glucose increased | 10 (23.8) | 6 (28.6) | 4 (19.0) | 3 (7.1) | 2 (9.5) | 1 (4.8) |
| Eosinophil count increased | 4 (9.5) | 3 (14.3) | 1 (4.8) | 1 (2.4) | 1 (4.8) | 0 (0.0) |
| Glucose urine present | 4 (9.5) | 1 (4.8) | 3 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutrophil count decreased | 5 (11.9) | 4 (19.0) | 1 (4.8) | 3 (7.1) | 3 (14.3) | 0 (0.0) |
| Protein total decreased | 4 (9.5) | 3 (14.3) | 1 (4.8) | 1 (2.4) | 1 (4.8) | 0 (0.0) |
| White blood cells urine positive | 3 (7.1) | 2 (9.5) | 1 (4.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Blood phosphorus decreased | 4 (9.5) | 2 (9.5) | 2 (9.5) | 1 (2.4) | 1 (4.8) | 0 (0.0) |
Note: Only events that occurred more than 3.times are included in the list.
Pharmacokinetics.
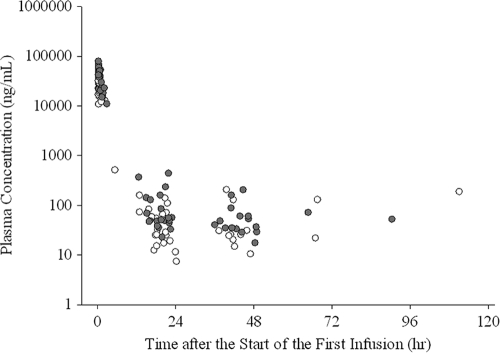
Blood sample collection was required at 3 points: immediately before the end of the first infusion, immediately before the start of the second infusion, and the day after the final infusion. Whenever possible, a blood sample was also collected any time between 30 min and 12 h after completion of the initial infusion. Changes in plasma concentrations of peramivir after the start of the initial infusion are shown in Fig. 3. The median plasma concentration immediately before the end of infusion was 25,500 ng/ml (range, 15,600 to 26,200 ng/ml) in the 300-mg group and 51,500 ng/ml (range, 37,300 to 78,900 ng/ml) in the 600-mg group. Levels immediately before the start of the second infusion (at 18 to 24 h after the end of the first infusion) were 45.7 ng/ml (range, 7.37 to 139 ng/ml) in the 300-mg group and 86.7 ng/ml (range, 22.7 to 435 ng/ml) in the 600-mg group. Plasma concentrations on the day after the final infusion were 10.4 to 129 ng/ml in the 300-mg group and 17.3 to 435 ng/ml in the 600-mg group. All analyses were performed using SAS version 8.2 (or higher) software (SAS Institute, Cary, NC).
Fig. 3.
Plasma concentration of peramivir (open circles, 300-mg dose; solid circles, 600-mg dose). The conversion factor between the plasma concentration (ng/ml) and the IC50 (nM) was as follows: 1 ng/ml = 2.84 nM.
DISCUSSION
Few studies in the past have shown the usefulness of NA inhibitors using the duration of influenza illness as the primary endpoint and only high-risk patients as subjects. An open study of Chinese subjects found a significant difference in the median duration of influenza illness between oseltamivir (110 h) and placebo (174 h; P = 0.0479) (16), while a study of febrile patients found that the median duration of illness on the basis of principal symptoms (e.g., fever) was shorter with oseltamivir (40.8 h) than with placebo (57.9 h; P = 0.0005) (18). Another report found a significantly reduced duration of influenza illness, from 7.0 days with placebo to 5.5 days (P = 0.009) with oseltamivir, among influenza-positive patients ≥12 years old who had asthma or chronic obstructive pulmonary disease as a complication (26). In addition, although not all subjects were high-risk patients, subgroup analysis of high-risk patients who had been included with ordinary seasonal influenza infection patients showed a median duration of influenza illness of 5.5 days with zanamivir compared to 8.0 days with placebo (P = 0.048) (22). Nevertheless, no other studies were successful in demonstrating such superiority (11, 17, 23). Moreover, although zanamivir has been shown to shorten the duration of influenza illness among high-risk patients in pooled analyses combining results from several trials (9, 15, 24, 25), a meta-analysis of 6 trials (4) did not yield clear results with regard to oseltamivir (4). Complicating respiratory tract diseases or influenza-associated complications are possible factors underlying the difficulty in showing the efficacy of drugs in shortening the duration of influenza illness among high-risk patients.
As the duration of influenza illness was longer in high-risk patients than in ordinary patients in the trials described above and deterioration of conditions with the development of influenza-associated complications was feared, the present study administered repeated doses of peramivir to high-risk patients instead of the single dose used in ordinary seasonal influenza patients (non-high-risk patients).
Assessment of the efficacy of peramivir administered at 300 or 600 mg/day for 1 to 5 days to high-risk patients showed that the median duration of influenza illness in all patients was 68.6 h (Table 2).
Since the duration of illness in the present study was significantly shorter (hazard ratio, 0.497; 90% CI 0.25 to 0.984) in the peramivir 600-mg group (mean, 2 doses/patient = 1,200 mg/patient) than in the 300-mg group (mean, 1.83 doses/patient = 550 mg/patient), the 600-mg repeated dose of peramivir can be concluded to represent an appropriate therapy for high-risk patients. Moreover, the present study was conducted during an epidemic season for oseltamivir-resistant strains, and given longer duration of illness with similar drugs in the past for high-risk patients before the prevalence of oseltamivir-resistant strains (4, 9, 15–18, 22–25), a 300-mg repeated dose seems unlikely to have had no efficacy in high-risk patients. However, as the present study was not placebo controlled, evaluation of this possibility was not possible.
On the other hand, a comparison between single-dose and repeated-dose groups showed median durations of influenza illness of 92.0 h and 64.1 h, respectively, so the duration of influenza illness tended to be shorter in the repeated-dose group than in the single-dose group. Repeated dosing can thus be expected to enhance the therapeutic efficacy of peramivir.
In addition, the observed virus titer [log10 (TCID50/ml)] was lower in the 600-mg group than in the 300-mg group on both days 2 and 3, and this finding was consistent with the finding that the duration of influenza illness was significantly shorter in the 600-mg group than in the 300-mg group. Moreover, as shown in Table 2, the virus-positive rate on day 6 was 0% in both groups, indicating complete elimination of the virus. These results suggest that a repeated 600-mg dose is most appropriate for high-risk patients. As shown in the recent clinical report (27), the repeated 600-mg dose of peramivir allowed the patient with severe influenza infection with diabetic nephropathy to show a decline of fever and rapid improvement in respiratory failure, followed by improvement of chest X-ray with pleural effusion and pulmonary consolidation (27).
An approximately 13% incidence of influenza-associated complications has been reported among patients treated with zanamivir (18). As the present trial had no control group, accurate evaluation of the magnitude of the incidence was difficult, but rates of influenza-associated complications as a whole and of pneumonia were 10.8% and 8.1%, respectively. Since these rates were comparable to the results obtained with several drugs in the past, influenza-associated complications may be preventable using repeated doses of peramivir.
Following the phase 2 (Ph2) clinical trial (13), a phase 3 (Ph3) clinical trial in which oseltamivir phosphate was used for comparison (14) again showed that when 300 mg and 600 mg of peramivir were administered to non-high-risk patients in a single dose, the durations of influenza illness were almost the same. Two verification trials in non-high-risk patients thus yielded similar results with 300 mg and 600 mg, and a saturation phenomenon was observed in the results of both studies, whereas high-risk patients showed virus-suppressing effects and influenza symptom-ameliorating effects more clearly with 600 mg than with 300 mg. These effects were even more marked with repeated doses.
In the phase 2 and 3 studies, a correlation with the dose was observed among high-risk patients, even though no difference was observed in the degree of improvement of influenza duration between 300- and 600-mg doses among non-high-risk patients. The reason for this finding may lie in differences between the immunological activities operating in the patients. In other words, immunity, in addition to antiviral activity, seems to make a major contribution to the cure in ordinary seasonal influenza patients, whereas repeated administration of a larger dose with superior antiviral effects was inferred to have accelerated the improvement in influenza symptoms in high-risk patients with weakened immune systems.
On the other hand, since 2008, the H274Y mutant A/H1N1 virus, which is less sensitive to oseltamivir, has become prevalent. This mutant strain reportedly exhibits cross-resistance to peramivir (21). The H274Y mutant A/H1N1 virus prevailed worldwide in the 2008-2009 season, when the trial was conducted. The IC50 of peramivir against the A/H1N1 viruses isolated in the present study is 16.19 to 25.02 nM. Considering that the IC50s were higher than that of wild-type virus, all 16 patients with A/H1N1 virus in the present study were assumed to have been infected with the resistant virus. The H274Y mutation was identified in A/H1N1 viruses from 12 patients before treatment with peramivir (4 patients failed identification due to the small sample volume). As previously reported (1), two interacting groups that bind to active sites of NA have been found in the peramivir molecule, in addition to the carboxyl group possessed by all NA inhibitors. One is a hydrophobic group that is the same as that in oseltamivir, while the other is a guanidino group that is the same as that in zanamivir. Even though the interaction becomes weaker between the hydrophobic group and active site of the NA with oseltamivir-resistant viruses, drug binding to NA is retained by the guanidino and carboxyl groups. Oseltamivir-resistant viruses are thought to lack complete resistance to peramivir, resulting in a limited increase in the IC50 to approximately several tens of nanomoles (21). Not only in the present study, but also in our Ph3 single-dose study conducted in the same influenza season, 428 A/H1N1 viruses, including 427 of the viruses with a H274Y mutation, showed the median (minimum-maximum) IC50s (limits of quantification, 100 nM) of peramivir and oseltamivir were 21.59 (0.41 to 100.0) and 100.0 (0.41 to 100.0) nM, respectively. It was also reported by Hurt et al. that viruses with the H274Y mutation showing higher IC50s are spreading in Oceania, Southeast Asia, and South Africa (10). These values are much lower than the result of the present study, where median plasma concentrations of peramivir immediately after intravenous drip infusion of 300 mg and 600 mg were 72,400 nM (25,500 ng/ml) and 146,300 nM (51,500 ng/ml), respectively. Moreover, 3 to 9% of the drug distributed into the nasal cavity and pharyngeal mucus of healthy subjects, and corresponding local concentrations that sufficiently exceeded the IC50 of the oseltamivir-resistant strain, was maintained as late as 12 h after administration in our Ph1 study (the calculated drug concentrations in pharyngeal mucus after administration of a 600-mg dose of peramivir were 5,280 nM at 2 h and 220 nM at 12 h). The data for the Ph3 single-dose study and the Ph1 study for confirmation of the penetration into the upper respiratory tract will be available in future articles.
It was reported by Memoli et al. that an immunocompromised patient infected by pandemic 2009 A/H1N1 with a H274Y mutation showed clinically significant resistance to peramivir (20). Administration of peramivir started when the virus had increased, despite 24 days of treatment with oseltamivir. Considering that the action mechanism of neuraminidase inhibitors shows virustatic, not virucidal, effects, it is unlikely that peramivir has no effect in immunocompromised patients infected by H274Y resistant virus in the early stages of influenza infection. However, it is reasonable to say that a neuraminidase inhibitor should be administered before critical worsening, especially in immunodeficient patients, whenever possible.
Given the present results, a repeated 600-mg dose of peramivir appears to be effective in patients infected with influenza virus, including oseltamivir-resistant virus, at high risk for complications, and no major safety issues were identified. Reliable treatment of a broader group of patients may be possible using a 600-mg repeated dose, in addition to using a 300-mg single dose for seasonal influenza patients.
ACKNOWLEDGMENTS
This trial was conducted with the financial support of Shionogi & Co. Ltd (Osaka, Japan).
We thank Tohru Ohe, Okayama University, Okayama, Japan, for his help with the evaluation of electrocardiograms obtained in the study, the subjects for their participation, and all those who acted as coworkers and monitors in the participating centers for their dedicated work.
The members of the S-021812 Clinical Study Group were as follows. Writing committee: S. Kohno, H. Kida, M. Mizuguchi, J. Shimada, and Masafumi Seki (Nagasaki University, Nagasaki, Japan), who take responsibility for the content and accuracy of the paper. Investigator group: Japan, Yoshitaka Sugawara, Obihiro Respiratory and Medical Hospital, Obihiro; Noriharu Shijubo, JR Sapporo Hospital, Sapporo; Hideya Iijima, Sendai Open Hospital, Sendai; Takao Ishizuka, Tomioka General Hospital, Tomioka; Michihiko Masuda, Tokyo Women's Medical University Yachiyo Medical Center, Yachiyo; Masaru Oritsu, Japanese Red Cross Medical Center, Tokyo; Kazuma Kishi, Toranomon Hospital, Tokyo; Eri Hagiwara, Kanagawa Cardiovascular and Respiratory Center, Yokohama; Yoshihisa Watanabe, Kawasaki Rinko General Hospital, Kawasaki; Ryoji Nakamura, Takatsu Central Hospital, Kawasaki; Nobuki Aoki, Shinrakuen Hospital, Niigata; Yoshinari Tanabe, Niigata University Medical and Dental Hospital, Niigata; Tetsuo Ozawa, National Hospital Organization Niigata Hospital, Kashiwazaki; Mafumi Owa, Japanese Red Cross Society Suwa Hospital, Suwa; Sekiya Koyama, Matsumoto Medical Center, Matsumoto; Hisao Muto, Kanazawa Social Insurance Hospital, Kanazawa; Takuya Watanabe, Seirei Hamamatsu General Hospital, Hamamatsu; Masashi Yamamoto, Nagoya Ekisaikai Hospital, Nagoya; Motonari Fukui, Kitano Hospital, Osaka; Hiroshi Kubota, Osaka Gyoumeikan Hospital, Osaka; Motokazu Kato, Kishiwada City Hospital, Kishiwada; Tadashi Ishida, Kurashiki Central Hospital, Kurashiki; Hideto Yamakido, Hiroshima City Funairi Hospital, Hiroshima; Toru Rikimaru, Saiseikai Futsukaichi Hospital, Chikushino; Yasuhiro Sako, Saiseikai Fukuoka General Hospital, Fukuoka; Tetsuo Hisadome, Shin Komonji Hospital, Kitakyushu; Kyouta Higashi, Fukuoka Wajiro Hospital, Fukuoka; Masayoshi Abe, Kishida Clinic, Fukuoka; Kenichi Yamamoto, Saga Memorial Hospital, Saga; Yoshihiro Yamamoto, Nagasaki University Hospital, Nagasaki; Kiyoyasu Fukushima, Japanese Red Cross Nagasaki Genbaku Isahaya Hospital, Isahaya; Moritaka Suga, Saiseikai Kumamoto Hospital, Kumamoto; Issei Tokimatsu, Oita University Hospital, Yufu; Kaoru Okada, Oita Red Cross Hospital, Oita; Masaharu Kawabata, Minami Kyushu National Hospital, Aira; Masanobu Ishigaki, Urasoe General Hospital, Urasoe; and Hiroshi Sakugawa, Heart Life Hospital, Nakagami, Japan.
Footnotes
Published ahead of print on 4 April 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Babu Y. S., et al. 2000. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J. Med. Chem. 43:3482–3486 [DOI] [PubMed] [Google Scholar]
- 2. Bantia S., Arnold C. S., Parker C. D., Upshaw R., Chand P. 2006. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antiviral Res. 69:39–45 [DOI] [PubMed] [Google Scholar]
- 3. Bantia S., et al. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burch J., et al. 2009. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect. Dis. 9:537–545 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2009. Drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:433–435 http://www.cdc.gov/mmwr/PDF/wk/mm5816.pdf [PubMed] [Google Scholar]
- 6. Gubareva L. V., Webster R. G., Hayden F. G. 2001. Comparison of the activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob. Agents Chemother. 45:3403–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harper S. A., et al. 2009. Seasonal influenza in adults and children—diagnosis, treatment, chemoprophylaxis, and institutional outbreak management: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1003–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayden F. 2009. Developing new antiviral agents for influenza treatment: what does the future hold? Clin. Infect. Dis. 48:S3–S13 [DOI] [PubMed] [Google Scholar]
- 9. Höffken G., Gillissen A. 2002. Efficacy and safety of zanamivir in patients with influenza—impact of age, severity of infections and specific risk factors. Med. Microbiol. Immunol. 191:169–173 [DOI] [PubMed] [Google Scholar]
- 10. Hurt A. C., et al. 2009. Emergence and Spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 83:90–93 [DOI] [PubMed] [Google Scholar]
- 11. Johnston S. L., Ferrero F., Garcia M. L., Dutkowski R. 2005. Oral oseltamivir improves pulmonary function and reduces exacerbation frequency for influenza-infected children with asthma. Pediatr. Infect. Dis. J. 24:225–232 [DOI] [PubMed] [Google Scholar]
- 12. Kaiser L., Keene O. N., Hammond J. M. J., Elliot M., Hayden F. G. 2000. Impact of zanamivir on antibiotic use for respiratory events following acute influenza in adolescents and adults. Arch. Intern. Med. 160:3234–3240 [DOI] [PubMed] [Google Scholar]
- 13. Kohno S., Kida H., Mizuguchi M., Himada S J. 2010. Efficacy and safety of intravenous peramivir for the treatment of seasonal influenza. Antimicrob. Agents Chemother. 54:4568–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohno S., et al. 2009. Single-intravenous peramivir vs. oral oseltamivir to treat acute, uncomplicated influenza in the outpatient setting: a phase III, randomized, double-blind trial, abstr. V-537a. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother [Google Scholar]
- 15. Lalezari J., Campion K., Keene O., Silagy C. 2001. Zanamivir for the treatment of influenza A and B infection in high-risk patients: a pooled analysis of randomized controlled trials. Arch. Intern. Med. 161:212–217 [DOI] [PubMed] [Google Scholar]
- 16. Lin J. T., et al. 2006. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr. Med. Res. Opin. 22:75–82 [DOI] [PubMed] [Google Scholar]
- 17. Mäkelä M. J., et al. 2000. Clinical efficacy and safety of the orally inhaled neuraminidase inhibitor zanamivir in the treatment of influenza: a randomized, double-blind, placebo-controlled European study. J. Infect. 40:42–48 [DOI] [PubMed] [Google Scholar]
- 18. Martin C., Mahoney P., Ward P. 2001. Oral oseltamivir reduces febrile illness in patients considered at high risk of influenza complications. Int. Congr. Ser. 1219:807–811 [Google Scholar]
- 19. Medeiros R., et al. 2007. Failure of zanamivir therapy for pneumonia in a bone-marrow transplant recipient infected by a zanamivir-sensitive influenza A (H1N1) virus. Antivir. Ther. 12:571–576 [PubMed] [Google Scholar]
- 20. Memoli M. J., Hrabal R. J., Hassontoufighi A., Eichelberger M. C., Taubenberger J. K. 2010. Rapid selection of oseltamivir- and peramivir-resistant pandemic H1N1 virus during therapy in 2 immunocompromised hosts. Clin. Infect. Dis. 50:1252–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mishin V. P., Hyden F. G., Gubareva L. V. 2005. Susceptibilities of antiviral-resistant influenza viruses to novel veuraminidase inhibitors. Antimicrob. Agents Chemother. 49:4515–4520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. M.I.S.T. Study Group 1998. Randomised trial of efficacy and safety of inhaled zanamivir in treatment of influenza A and B virus infections. Lancet 352:1877–1881 [PubMed] [Google Scholar]
- 23. Monto A. S., et al. 1999. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza A and B virus infections. J. Infect. Dis. 180:254–261 [DOI] [PubMed] [Google Scholar]
- 24. Monto A. S., Moult A. B., Sharp S. J. 2000. Effect of zanamivir on duration and resolution of influenza symptoms. Clin. Ther. 22:1294–1305 [DOI] [PubMed] [Google Scholar]
- 25. Monto A. S., Webster A., Keene O. 1999. Randomised, placebo-controlled studies of inhaled zanamivir in the treatment of influenza A and B: pooled efficacy analysis. J. Antimicrob. Chemother. 44(Suppl. B):23–29 [DOI] [PubMed] [Google Scholar]
- 26. Murphy K. R., et al. 2000. Efficacy and safety of inhaled zanamivir for the treatment of influenza in patients with asthma or chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled, multicentre study. Clin. Drug Invest. 20:337–349 [Google Scholar]
- 27. Nasu T., Ogawa D., Wada J., Makino H. 2010. Peramivir for severe influenza infection in a patient with diabetic nephropathy. Am. J. Respir. Crit. Care Med. 182:1209–1210 [DOI] [PubMed] [Google Scholar]
- 28. National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention 2009. Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recommend Rep. 58:1–8 [PubMed] [Google Scholar]
- 29. National Institute of Allergy and Infectious Diseases December 2004. Division of Aids table for grading the severity of adult and pediatric adverse events 1:1–20. http://www.hptn.org/web%20documents/HPTN046/SSP/Appendices/AppendixE-ToxicityTables_DAIDS_AE_GradingTable_FinalDec2004.pdf
- 30. Potier M., Mameli L., Belislem M., Dallaire L., Melanxon S. B. 1979. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyla-D-N-acetylneuraminate) substrate. Anal. Biochem. 94:287–296 [DOI] [PubMed] [Google Scholar]
- 31. Taylor W. R. J., et al. 2008. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS. One 3:e3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization May 2010, posting date Pandemic (H1N1) 2009, update 102. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/don/2010_05_28/en/index.html [Google Scholar]
- 33. World Health Organization February 2010. WHO guidelines for pharmacological management of pandemic (H1N1) 2009 influenza and other influenza viruses. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf [PubMed] [Google Scholar]
- 34. World Health Organization April 2011, posting date Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_04_26/en/index.html [Google Scholar]