Abstract
During oxidative burst, neutrophils selectively generate HOCl to destroy invading microbial pathogens. Excess HOCl reacts with taurine, a semi-essential amino acid, resulting in the formation of the longer-lived biogenerated broad-spectrum antimicrobial agent, N-chlorotaurine (NCT). In the presence of an excess of HOCl or under moderately acidic conditions, NCT can be further chlorinated, or it can disproportionate to produce N,N-dichlorotaurine (NNDCT). In the present study, 2,2-dimethyltaurine was used to prepare a more stable N-chlorotaurine, namely, N,N-dichloro-2,2-dimethyltaurine (NVC-422). In addition, we report on the chemical characterization, in vitro antimicrobial properties, and cytotoxicity of this compound. NVC-422 was shown effectively to kill all 17 microbial strains tested, including antibiotic-resistant Staphylococcus aureus and Enterococcus faecium. The minimum bactericidal concentration of NVC-422 against Gram-negative and Gram-positive bacteria ranged from 0.12 to 4 μg/ml. The minimum fungicidal concentrations against Candida albicans and Candida glabrata were 32 and 16 μg/ml, respectively. NVC-422 has an in vitro cytotoxicity (50% cytotoxicity = 1,440 μg/ml) similar to that of NNDCT. Moreover, our data showed that this agent possesses rapid, pH-dependent antimicrobial activity. At pH 4, NVC-422 completely killed both Escherichia coli and S. aureus within 5 min at a concentration of 32 μg/ml. Finally, the effect of NVC-422 in the treatment of an E. coli-infected granulating wound rat model was evaluated. Treatment of the infected granulating wound with NVC-422 resulted in significant reduction of the bacterial tissue burden and faster wound healing compared to a saline-treated control. These findings suggest that NVC-422 could have potential application as a topical antimicrobial.
INTRODUCTION
As part of the innate immune system, leukocytes are capable of generating and releasing reactive oxygen species. During oxidative burst, neutrophils selectively generate hypochlorous acid to destroy invading microbial pathogens. In a secondary reaction, hypochlorous acid reacts with taurine, a semi-essential amino acid. The resulting N-chlorotaurine (NCT) is a well-known antimicrobial and anti-inflammatory oxidant (12, 15). Furthermore, NCT has shown significant bactericidal and fungicidal properties with relatively low toxicity compared to other oxidants (13, 14). In the presence of an excess of hypochlorous acid or under moderately acidic conditions, NCT can be further chlorinated or, alternatively, it can disproportionate to form N,N-dichlorotaurine (NNDCT), which also has broad-spectrum antimicrobial activity (9). Unfortunately, both NCT and NNDCT have relatively short solution half-lives, decomposing by hydrolytic dehydrochlorination (5, 6, 16). Because of this instability, the potential usefulness of the endogenous N-chlorotaurines as marketable therapeutics is limited. Replacement of the hydrogens at the β-carbon of taurine by methyl groups prevents the dehydrochlorination process, thereby circumventing this instability issue. Using this approach we were able to synthesize a stable analog of N-chlorotaurines, namely, N,N-dichloro-2,2-dimethyltaurine (NVC-422) (19). Importantly, NVC-422 possesses an added feature of long-term stability in solution (19). We therefore characterized this agent's chemical and antimicrobial properties. In addition, the in vivo activity of NVC-422 in the treatment of infected wounds was evaluated using an infected rat granulating wound model. Our data demonstrate that NVC-422 is stable and possesses broad-spectrum antimicrobial activity, and our infected rat wound model data show that NVC-422 is efficacious in vivo, indicating that it has the potential as a topical antimicrobial.
MATERIALS AND METHODS
Reagents.
All chemicals were of reagent grade. Sodium hypochlorite (6%) was purchased from VWR International (West Chester, PA). Hypochlorous acid solution was prepared by acidifying sodium hypochlorite solution to pH ∼6 with hydrochloric acid. Millipore water was used for all solution preparation.
1H-NMR and MS.
The 1H-nuclear magnetic resonance (1H-NMR) spectrum of NVC-422 was obtained on a Bruker Avance DRX 400 spectrometer (400 MHz, D2O). NVC-422 powder was analyzed by using electrospray ionization/time-of-flight/mass spectrometry (MS). A full mass spectrum was acquired in negative ionization mode.
Solution preparation and stability studies.
All solutions were prepared in 0.9% physiological saline for biological testing. NVC-422 solutions were prepared by either dissolving the powder (19) in saline or by the reaction of hypochlorous acid with 2,2-dimethyltaurine (19). NNDCT solutions were prepared by the reaction of hypochlorous acid with taurine. The stock NCT and N-chloro-2,2-dimethyltaurine (NVC-612) solutions were prepared by the reaction, under basic conditions (pH ∼9), of hypochlorite with taurine and 2,2-dimethyltaurine (2,2-DMT), respectively. The stock solutions were diluted to the desired concentration, and the pH was adjusted to the target pH with dilute aqueous HCl. The concentrations of NVC-422 (ε304 nm = 297 M−1 cm−1) (19), N-chloro-2,2-dimethyltaurine (ε248 nm = 362 M−1 cm−1) (19), NCT (ε251 nm = 397 M−1 cm−1) (1), NNDCT (ε302 nm = 333 M−1 cm−1) (1), and NaOCl (ε292 nm = 362 M−1 cm−1) (8) were determined spectrophotometrically with an Agilent 8453 UV-visible spectrophotometer (Agilent Technologies Deutschland GmbH, Waldbronn, Germany). The stability of NVC-422 at room temperature over a period of 430 days was monitored spectrophotometrically at its λmax value.
Estimation of pKa of NVC-422.
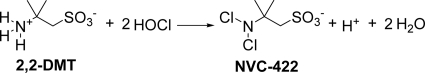
A 25% stoichiometric excess of 2,2-DMT (2.28 mmol or 4.56 meq) was added into 1.0 liter of 3.65 mM (3.65 meq) of HOCl solution at pH 3.36. The excess of 2,2-DMT ensures complete conversion of HOCl, thus allowing only measurement of NVC-422 by UV. The excess of 2,2-DMT does not interfere with the measurement of NVC-422 due to its lack of UV absorbance. The change of pH was used to estimate the pKa based on the stoichiometry of the reaction shown in Fig. 1.
Fig. 1.
Reaction of 2,2-DMT with hypochlorous acid forming NVC-422.
Microbial strains and growth media.
Microbial isolates used in the present study were purchased from the American Type Culture Collection (ATCC; Manassas, VA). The bacterial strains used in the present study (Table 1) were grown on tryptic soy agar (Difco Laboratories, Detroit, MI) at 37°C overnight except that vancomycin-resistant Enterococcus faecium 51559 was grown on brain heart infusion agar (Difco) and Haemophilus influenzae 49144 was cultured overnight on chocolate agar (Difco) at 37°C in a 5% CO2 incubator. Candida albicans 10231 and Candida glabrata 90030 were grown on Sabouraud dextrose agar (Difco) and cultured in Sabouraud dextrose broth (SDB; Difco). Yeasts were grown in SDB overnight at 37°C, centrifuged at 2,300 × g, resuspended in 0.9% NaCl (pH 4), and adjusted to a working stock concentration of 108 yeast/ml by using a hemocytometer.
Table 1.
MBCs of NVC-422 in 0.9% saline solution at pH 4 against a broad spectrum of microorganisms
| Pathogen | ATCC no. | MBC (μg/ml) |
|---|---|---|
| Gram-positive bacteria | ||
| S. aureus | 29213 | 1 |
| S. aureus (MRSA) | 33591 | 4 |
| S. epidermidis | 12228 | 0.25 |
| S. hominis | 27844 | 4 |
| S. sciuri | 49575 | 0.12 |
| E. faecium (VRE) | 51559 | 0.5 |
| Gram-negative bacteria | ||
| E. coli | 25922 | 1 |
| E. aerogenes | 51697 | 0.5 |
| A. baumannii | 19606 | 4 |
| A. calcoaceticus | 51432 | 2 |
| H. influenzae | 49144 | 0.5 |
| K. pneumoniae | 10031 | 0.25 |
| P. mirabilis | 29245 | 1 |
| P. aeruginosa | 27853 | 1 |
| S. marcescens | 13880 | 1 |
| Yeast | ||
| C. albicans | 10231 | 32 |
| C. glabrata | 90030 | 16 |
MBC and MFC.
Susceptibility testing of various microbial species to the N-chlorotaurine analog was conducted using modified Clinical and Laboratory Standards Institute (CLSI) methods. The CLSI protocol for minimum bactericidal concentration (MBC) (4) testing was modified by substituting 0.9% saline for cation-adjusted Mueller-Hinton broth (CAMHB) to circumvent for the reactivity of chlorine to certain components of CAMHB. The CLSI protocol for broth dilution antifungal susceptibility testing of yeasts (3) was modified by substituting 0.9% saline for RPMI 1640 medium again to circumvent for the reactivity of chlorine to certain components of RPMI 1640. Due to the rapid cidal effects of the chloramines, the MBC and minimum fungicidal concentration (MFC) assays were shortened from 16 to 20 h at 35°C to 60 min at room temperature. The MBC and MFC were defined as the lowest concentrations achieving >99.9% killing, which is equivalent to a 3-log10 CFU reduction of the challenge inoculum. Microorganisms were grown to mid-log phase, centrifuged, and suspended in saline adjusted to the desired pH or phosphate-buffered saline (PBS). Next, the organisms were added to dilutions of test compounds (ranging from 256 to 0.25 μg/ml for NVC-422 and from 1,024 to 1 μg/ml for NCT) in pH-adjusted saline to a final inoculum of 105 to 106 CFU/ml, followed by incubation for 60 min at room temperature. Aliquots of the cell suspension were transferred into 9 volumes of Dey-Engley neutralizing broth (Hardy Diagnostics, Santa Maria, CA), plated on agar with growth medium, and incubated for 24 to 48 h at 35°C (with 5% CO2 for H. influenzae), and the CFU were quantified. The MBC pH dependence was examined in the range of pH 4.0 to pH 7.4.
Time-kill studies.
The kinetics of microbial killing were tested by incubation of the test compound at 0, 5, 15, 30 and 60 min at room temperature using the modified CLSI method (2–4). Modifications included substituting 0.9% saline for CAMHB and shortening the assay from 16 to 20 h at 35°C to 60 min at room temperature as described above. Time-kill kinetics analyses were performed at pH 4.0 and 7.4.
Cytotoxicity assays.
Mammalian cell line L929 (mouse fibroblasts) was purchased from the ATCC. The toxicity of the test compounds was evaluated after a 1-h exposure to various concentrations ranging from 40 to 0.037 mM. Monolayers were grown to ≥80% confluence and treated with 2-fold dilutions of test compounds in 0.9% saline (pH 4) or 4.25 mM PBS (pH 7) for 1 h of incubation at 37°C in a 5% CO2 atmosphere. The test article was then replaced with complete medium, and the plates were incubated overnight at 37°C. Cell viability was measured by using the Dojindo cell proliferation assay (7). The concentration of test compound cytotoxic to 50% of the cells (CT50) was determined by using the GraphPad Prism program.
Chronic granulating wound model.
The ability of NVC-422 to control tissue bacterial burden and to overcome the inhibition of wound healing caused by infection was evaluated in an established chronic granulating wound model (10, 17). All experiments followed a protocol approved by the Bay Pines VA Healthcare System Animal Care Use Committee and complied with the National Research Council's criteria for humane care of animals. Twenty-four Sprague-Dawley rats weighing 300 to 350 g were acclimatized for a week in the laboratory prior to use. Under intraperitoneal pentobarbital anesthesia (35 mg/kg) using ketamine, xylaine, and acepromazine, each rat dorsum was shaved and depilated. A full-thickness dorsal burn measuring 30 cm2 was created by immersion in boiling water for 15 s. The burned area were then topically inoculated with 5 × 109 CFU of E. coli ATCC 25922 strain after the rats had been allowed to cool for 15 min. Bacteria were obtained from fresh 18-h broth culture, and the inoculum size was confirmed by CFU determination. After 5 days, the eschar was excised from each anesthetized animal, resulting in a chronic granulating wound. Test solutions consisted of sterile normal saline (0.9%) and 4, 12, and 40 mM solutions of NVC-422 in saline. Treatment consisted of a 3-by-3-in. gauze dressing saturated with 20 ml of test solution applied to the wound, covered with one layer of Adaptic and then covered with Coban. All wounds were left dressed between treatments. Five groups (five rats per group) were divided as follows: group I served as the infected control and was treated with 0.9% NaCl dressings changed every 24 h; groups II to V were treated as follows: group II, 4 mM NVC-422 changed every 24 h; group III, 12 mM NVC-422 changed every 24 h; group IV, 12 mM NVC-422 for 0.5 h, followed by gentle, atraumatic wiping every 24 h; and group V, 40 mM NVC-422 every 24 h. Any exudate or scab that formed was atraumatically removed prior to any wound tracings or biopsies. Every 72 h, the outlines of the wounds were traced onto acetate sheets, and area calculations performed using digital planimetry (Sigma Scan; Jandel Scientific, Corte Madera, CA). Care was taken only to record the perimeter of the wound that represented the advancing full-thickness margin rather than the edge of any advancing epithelium (10). This avoided the small component of advancement provided by the smooth, pink, translucent, hairless neoepithelium. Randomized wound biopsy sites were obtained from re-anesthetized rat on days 3, 5, 7, 10, 12, 14, and 17 after escharectomy for quantitative and qualitative bacterial analysis according to the method of Heggers and Robson (11). The wound surface was cleaned with 70% isopropyl alcohol prior to punch biopsy to exclude surface contamination. Biopsies were aseptically weighed, homogenized, serially diluted, and back-plated onto nonselective media. Bacterial counts were completed after 48 h of incubation and are expressed as CFU/g of tissue.
Statistical analysis.
Mean bacterial counts for each group of animals were determined and are expressed as CFU/g of tissue. These values were compared for each experiment using a one-way analysis of variance. Post hoc analyses of differences between groups were carried out using Tukey's test, with P < 0.05 considered significant. Sigma Stat statistical software (Jandel Scientific) was used for data analysis.
RESULTS
NVC-422 structural elucidation.
NVC-422 was characterized by 1H-NMR and MS (described in the supplemental material). The 1H-NMR spectrum displays characteristic signals at δ 1.56 in D2O (6 H, 2 × CH3) and at δ 3.48 ppm (-CH2-SO3Na) consistent with the structure assigned (Fig. 1). The additional peak at ∼4.7 is attributed to the D2O used as the solvent. Negative ion mass spectroscopy shows the molecular ions with m/z values (m-1) of 220, 222, and 224 (ca. 9:6:1), a finding consistent with the presence of two chlorine atoms (35Cl and 37Cl [3:1]) in the anion form of NVC-422.
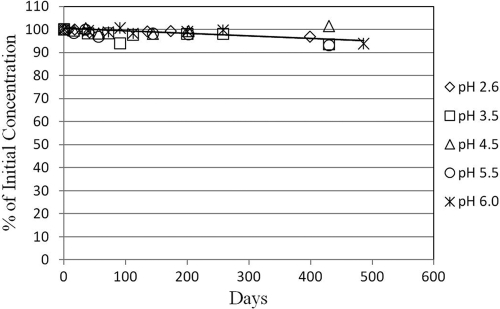
NVC-422 solution stability over a range of pH values.
The stabilities of solutions of NVC-422 at ambient temperatures in the pH range from 2.6 to 6.0 are given in Fig. 2. After storage at ambient room temperature for 430 days, the concentrations of NVC-422 solutions over the pH range tested remained at >93% of the initial concentrations. These data indicate that NVC-422 is stable in solution (>95% of initial concentration) independent of pH for longer than 1 year.
Fig. 2.
Stability of NVC-422 solutions in the pH range from 2.6 to 6.0 stored at ambient temperature. Concentrations: [NVC-422]initial, 2 mM; [NaCl], 150 mM. The container used was a borosilicate glass vial with a fluoropolymer/silicone liner cap.
NVC-422 pKa.
The pKa of NNDCT has been estimated to be ca. −10 (1). Although the pKa of NVC-422 was not directly determined, the acidity of protonated NVC-422 was estimated by a measurement of the released amount of H+ from the reaction between 2,2-DMT and HOCl (Fig. 1). In the test, a stoichiometric excess of 2,2-DMT was added to HOCl solution at pH 3.36. Upon completion of the reaction, the pH dropped to 2.65, suggesting that the formation of NVC-422 was accompanied by the release of H+. From the initial and final pH values, the [H+] generated from the reaction was found to correspond to the concentration of NVC-422 formed. It appears that the dichlorinated amine functionality was completely nonbasic under these conditions. No proton attaches to the -NCl2 or -SO3− groups in NVC-422. Therefore, the pKa of NVC-422 is ca. −10, which is similar to the reported pKa of NNDCT.
NVC-422 has broad-spectrum antimicrobial activity.
The microbicidal activity of NVC-422 in saline solution was investigated against a panel of pathogens, including bacteria and yeast. NVC-422 was shown to be highly effective in killing all microbial strains tested, including antibiotic-resistant S. aureus and E. faecium (Table 1). The MBC of NVC-422 against Gram-negative and Gram-positive bacteria ranged from 0.12 to 4 μg/ml. MFC values against C. albicans and C. glabrata were higher—32 and 16 μg/ml, respectively.
NVC-422 antimicrobial activity is pH dependent.
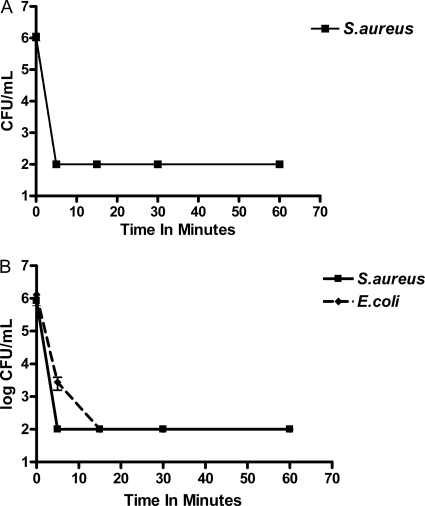
The effect of pH on the antimicrobial activities of NVC-422 was tested by determining its MBCs against representative Gram-positive (S. aureus ATCC 29213) and Gram-negative (E. coli ATCC 25922) strains over a pH range of 4.0 to 7.4. A profound pH dependence of antimicrobial activities for NVC-422 was observed (Table 2). The MBC of NVC-422 at pH 7.4 was 256- to 512-fold higher than at pH 4.0. The antimicrobial activity of 0.9% saline alone in the pH range from 4.0 to 7.4 was also tested as a control, and no killing was observed. The killing rate of NVC-422 was determined against two test organisms, E. coli ATCC 25922 and S. aureus ATCC 29213. Figure 3A and B shows an average results for at least two independent experiments. Since we observed almost identical results with both E. coli and S. aureus at pH 4, Figure 3A shows the time-kill curve of S. aureus only. Our data showed a significant pH effect on the ability of NVC-422 to kill microorganisms. At pH 4, NVC-422 demonstrates rapid bactericidal activity (4-log10 CFU reduction for both organisms within 5 min at a concentration of 32 μg/ml). At pH 7, the concentration of NVC-422 has to be increased to 512 μg/ml to completely kill both organisms within 5 to 15 min.
Table 2.
pH dependence of NVC-422 activity
| pH | NVC-422 MBC (μg/ml) |
|
|---|---|---|
| S. aureus ATCC 29213 | E. coli ATCC 25922 | |
| 4.0 | 1 | 1 |
| 4.7 | 2 | 2 |
| 5.4 | 8 | 4 |
| 5.9 | 16 | 8 |
| 7.4 | 256 | 512 |
Fig. 3.
Bactericidal activity of NVC-422. (A) Bacterial activity of 32 μg of NVC-422/ml against S. aureus ATCC 29213 at pH 4. The kinetics of killing was tested by incubation of the test compound for 0, 5, 15, 30, and 60 min in unbuffered saline (pH 4) at room temperature. The results are means ± the standard deviations of three separate experiments. (B) Bactericidal activity of 512 μg of NVC-422/ml against S. aureus ATCC 29213 and E. coli ATCC 25922 at pH 7. The kinetics of killing were tested by incubation of the test compound for 0, 5, 15, 30, and 60 min in PBS (pH 7) at room temperature. The results are the means ± the standard deviations of three separate experiments.
Comparative antimicrobial activities of NVC-422, NNDCT, NVC-612, and NCT.
The MBCs of NVC-422 and NNDCT in 0.9% saline solution at pH 4 against E. coli and NVC-612 and NCT in PBS against E. coli are shown in Table 3. The MBCs of the precursor compounds 2,2-DMT and taurine, which have negligible antimicrobial activity, were also tested. The MBCs of NVC-422 and NNDCT against E. coli were found to be similar. Moreover, NVC-612 and NCT also displayed essentially identical antimicrobial activities against E. coli.
Table 3.
Antimicrobial activities and relative CT50s of various compoundsa
| Compound | Concn (μg/ml) |
|
|---|---|---|
| MBC (E. coli ATCC 25922) | CT50 (L929 cells) | |
| 2,2-DMT | >1,024 | >6,128 |
| Taurine | >1,024 | >5,004 |
| NNDCT | 1,024 | 901 |
| NVC-422 | 512 | 1,440 |
| NCT | 3,300 | 270 |
| NVC-612 | 3,300 | 311 |
NVC-422, NNDCT, NCT, and NVC-612 were tested in PBS (pH 7.4) at 25°C (n = 2).
Comparative cytotoxicities of NVC-422, NVC-612, NNDCT, and NCT.
The relative mammalian cell toxicities of NVC-422, NVC-612, NNDCT, and NCT were assessed according to a standard method (7). Table 3 summarizes the CT50 values obtained. The higher CT50 values for NVC-422 and NNDCT (1,440 and 901 μg/ml) relative to NVC-612 and NCT (311 and 270 μg/ml) indicate a lower cytotoxicity to L929 cells for the first two compounds.
Evaluation of the antimicrobial properties of NVC-422 in vivo using an E. coli-infected rat animal model.
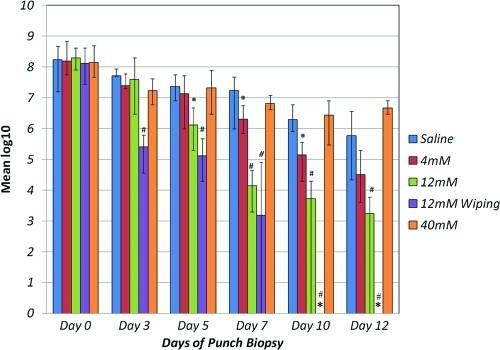
The results of quantitative bacteriology performed on biopsy specimens before and during treatment are shown in Fig. 4. For the day 0 pretreatment day and for each biopsy day the mean CFU/g for each group was calculated and transformed to log10 for ease of presentation. Figure 4 charts the mean biopsy values (up to day 12), including the range (high/low) of values for each treatment group. These data suggest that a 12 mM NVC-422 concentration (2,900 μg/ml) was better at lowering the bacterial burden than the other two concentrations tested (4 and 40 mM). Notably, when 12 mM NVC-422 was applied, followed by gentle wiping (group IV), there was a reduction in the bacterial count to <106 CFU/g by day 3, and no bacteria were recovered by days 10 to 17.
Fig. 4.
Mean bacterial counts for each study group. The bars represent the mean log10 CFU/g for each group; the lines at the tops of the bars represent the ranges (high/low) of the values. *, No bacterial growth was observed. Significant differences from group I, the saline-treated infected control, are indicated by the symbols “#” (P < 0.001) or “ ” (P < 0.05).
” (P < 0.05).
The improvement due to NVC-422 (12 mM, 2,900 μg/ml) applied for 24 h was statistically significant, with a P value of <0.05 beginning at day 5 and a P value of <0.001 level beginning day 7. However, the data for 12 mM NVC-422 applied for 30 min with wiping was statistically significant, with a P value of <0.001 beginning on day 3.
NVC-422 at the two lower concentrations (4 and 12 mM) allowed the wound to heal at a faster rate than did the saline control. However, the 40 mM concentration inhibited healing due to its damaging effect on the wound and its failure to control the bacterial bioburden because of the excessive necrotic tissue in the wounds.
DISCUSSION
We show here that the new N,N-dichloro-2,2-dimethyltaurine, NVC-422, has greatly enhanced stability compared to NNDCT and NCT. The similarity of NVC-422 and NNDCT, NVC-612, and NCT in their MBCs against E. coli and CT50 values suggests that substituting methyl groups for the two hydrogens on the β-carbon of taurine does not significantly change the antimicrobial and cytotoxic properties of the corresponding endogenous N-chlorotaurines. The lower MBCs of NVC-422 and NNDCT than NVC-612 and NCT (Table 3) against E. coli suggest that dichloroamines have more potent antimicrobial activity than the corresponding monochloroamines. At pH 7, NVC-422 achieved complete killing of E. coli at 512 μg/ml, whereas 3,300 μg of NVC-612/ml was required to kill the organism. This difference in potency of dichloroamine and monochloroamine might be explained by the difference in their oxidation potentials, assuming that their action is due to their electrochemical nature rather than diffusion. The antimicrobial activity of NVC-422 exhibits a potent pH dependence. A similar pH influence was also observed previously on the antimicrobial activities of hypochlorite (18) and NNDCT and NCT (9). Rudolph and Levine (18) found a decrease in pH substantially increased the biocidal activity of hypochlorite. These authors attributed the higher activity at low pH to the undissociated hypochlorous acid, which is a neutral molecule and able to penetrate into the bacterial cell. However, the pKa of NVC-422 found in the present study indicates that at pH 4, neither the chloramine function group nor the sulfonate group of NVC-422 is protonated; therefore, the electric charge of the molecule (−1) remains the same in the tested pH range (pH 7.4 to 4). The explanation for the pH effect on the antimicrobial activity of hypochlorite does not apply to the pH effect on NVC-422 activity. Gottardi et al. (9) proposed an explanation for the enhancement of antimicrobial activity of NCT and NNDCT at low pH. Since bacterial membranes bear negative charges, the approach of the anions NCT and NDCT is hampered in a neutral environment. At low pH, the negative charges on the bacterial surface are partially neutralized by the protons, resulting in better access for the chlorination agents. This hypothesis still needs to be confirmed.
The MBC and MFC values reported here for NVC-422 are not comparable to values obtained with conventional testing because of changes to the medium used, a higher inoculum challenge, and the duration of the testing. The in vitro antibacterial properties and tissue safety profile of NVC-422 suggests its potential as a wound care agent. From a review of the quantitative bacteriology data from the experiment using the chronic granulating wound model, 12 mM NVC-422 applied for 30 min and then removed from the wound (group IV) appeared to be the most effective regimen for decreasing the bacterial load. The role of the atraumatic wiping between applications is not very clear. If NVC-422 kills bacteria rapidly after initial contact, the gentle wiping may remove the devitalized bacterial and any possible debris, allowing the second application of NVC-422 to be in immediate contact with any remaining viable bacteria.
Conclusions.
We have shown that the strategic design of NVC-422 avoids the hydrolytic dehydrochlorination exhibited by N-chlorotaurines while retaining the broad-spectrum antimicrobial activity of N-chlorotaurines. Furthermore, this agent shows rapid antimicrobial activity that is also characteristic of this class of compounds. Substituting methyl groups for the two hydrogens on the β-carbon of taurine does not significantly change either the antimicrobial or the cytotoxic properties of the corresponding endogenous N-chlorotaurines. The in vivo study results are very encouraging and suggest that NVC-422 could have potential application as a topical antimicrobial.
Supplementary Material
ACKNOWLEDGMENTS
We thank John A. Soderquist, University of Puerto Rico, and Mahmoud Ghannoum, Case Western Reserve University, for their valuable comments and critical review of the manuscript. We also thank Mark B. Anderson, Tim Shiau, and Jungjoo Yoon of NovaBay for their assistance in the preparation of the manuscript.
This material is the result, in part, of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System.
The contents of this publication do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 21 March 2011.
REFERENCES
- 1. Antelo J. M., Arce F., Calvo P., Crugeiras J., Rios A. 2000. General acid-base catalysis in the reversible disproportionation reaction of N-chlorotaurine. J. Chem. Soc. Perkin Trans. 2:2109–2114 [Google Scholar]
- 2. Clinical Laboratory Standards Institute 1999. Methods for determining bactericidal activity of antimicrobial agents; approved guideline M26-A. Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Clinical Laboratory Standards Institute 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. M27–A2 Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Clinical Laboratory Standards Institute 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7–A6. Clinical Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Coker M. S., Hu W., Senthilmohan S. T., Kettle A. 2008. Pathways for the decay of organic dichloramines and liberation of antimicrobial chloramine gases. Chem. Res. Toxicol. 21:2334–2343 [DOI] [PubMed] [Google Scholar]
- 6. Cunningham C., Tipton K. F., Dixon H. F. 1998. Conversion of taurine into N-chlorotaurine (taurine chloramine) and sulphoacetaldehyde in response to oxidative stress. Biochem. J. 330:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dojindo Laboratories 2002. Cell proliferation assay and cytotoxicity assay: cell count kit 8. Dojindo Laboratories, Kumamoto, Japan [Google Scholar]
- 8. Furman C. S., Margerum D. W. 1998. Mechanisms of chlorine dioxide and chlorate ion formation from the reaction of hypobromous acid and chlorite ion. Inorg. Chem. 37:4321–4327 [DOI] [PubMed] [Google Scholar]
- 9. Gottardi W., Hagleitner M., Nagl M. 2005. N,N-Dichlorotaurine: chemical and bactericidal properties. Arch. Pharm. Chem. Life Sci. 338:1–11 [DOI] [PubMed] [Google Scholar]
- 10. Hayward P. G., Robson M. C. 1991. Animal models of wound healing contraction, p. 305–312 In Barbul A., et al. (ed.), Clinical and experimental approaches to dermal and epidermal repair: normal and chronic wounds. Wiley-Liss, New York, NY [Google Scholar]
- 11. Heggers J. P., Robson M. C. 1991. Quantitative bacteriology: its role in the armamentarium of the surgeon. CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 12. Marcinkiewicz J. 1997. Neutrophil chloramines: missing links between immate and acquired immunity. Immun. Today 18:577–580 [DOI] [PubMed] [Google Scholar]
- 13. Marcinkiewicz J., et al. 2000. Antimicrobial and cytotoxic activity of hypochlorous acid: interactions with taurine and nitrite. Inflamm. Res. 49:280–289 [DOI] [PubMed] [Google Scholar]
- 14. Nagl M., et al. 2002. Impact of N-chlorotaurine on viability and production of secreted aspartyl proteinases of Candida. J. Antimicrob. Chemother. 46:1996–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagl M., Hess M. W., Pfaller K., Hengster P., Gottardi W. 2000. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. J. Antimicrob. Chemother. 44:2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Olszowski S., Olszowska E., Kusior O., Szneler E. 2002. Sulphoacetaldehyde as a product of taurine chloramine peroxidation at site of inflammation. Amino Acids 22:145–153 [DOI] [PubMed] [Google Scholar]
- 17. Robson M. C., Edstrom L. E., Krizek T. J., Groskin M. G. 1974. The efficacy of systematic antibiotics in the treatment of granulating wounds. J. Surg. Res. 16:299–306 [DOI] [PubMed] [Google Scholar]
- 18. Rudolph A. S., Levine M. 1941. Factors affecting the germicidal efficiency of hypochlorite solutions, bulletin 150, p. 1– 48 Iowa Engineering Experiment Station, Iowa State College, Ames, IA [Google Scholar]
- 19. Wang L., Khosrovi B., Najafi R. 2008. N-Chloro-2,2-dimethyltaurines: stable homologues of N-chlorotaurines. Tetrahedron Lett. 49:2193–2195 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.