Abstract
This article analyzes patterns of consumption of fluoroquinolones and documents the in vitro resistances of Streptococcus pneumoniae isolates to fluoroquinolones in the ambulatory care setting in Belgium over time. The volume of fluoroquinolone consumption has fallen consistently since 2003. Fluoroquinolones were used primarily for their registered indications (i.e., urinary tract infections and lower respiratory tract infections). The MIC distributions of moxifloxacin and levofloxacin in S. pneumoniae isolates remained stable during 2004 to 2009, and the level of resistance to moxifloxacin and levofloxacin was low (≤1%).
TEXT
Fluoroquinolones are a class of antibiotics developed in the 1980s. Fluoroquinolones initially demonstrated their effectiveness against Gram-negative bacteria (Haemophilus influenzae and Moraxella catarrhalis), whereas newer fluoroquinolones such as moxifloxacin have increased activity against Gram-positive bacteria, including Streptococcus pneumoniae and Staphylococcus aureus; anaerobes; and atypical microorganisms (Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila). This article aims to analyze patterns of consumption of fluoroquinolones and to document the in vitro resistance of S. pneumoniae to fluoroquinolones in the ambulatory care setting in Belgium over time.
Data on fluoroquinolone consumption in the ambulatory care setting in Belgium were derived from IMS Health during 1993 to 2009. The volume of consumption was expressed in terms of the number of defined daily doses per 1,000 inhabitants per day (DIDs). Defined daily doses of fluoroquinolones originated from the World Health Organization and evolved over the study time period, if applicable. The volume of consumption was valued at public prices pertaining to the month of consumption.
IMS Health consumption data by type of infection originated from a panel of 500 physicians of all specialties offering general medical services, except for hospital inpatients. The composition of the panel of physicians corresponded to the distribution of the Belgian population of physicians by specialty and by region. When extrapolating the number of fluoroquinolone prescriptions by type of infection issued by the panel of 500 physicians to the consumption of fluoroquinolones in the Belgian population, care was taken that the frequency of results adheres to the Gauss curve as closely as possible.
The study analyzed respiratory blood isolates taken from adults to test the in vitro susceptibilities of S. pneumoniae strains to amoxicillin, cefuroxime, levofloxacin, and moxifloxacin. The S. pneumoniae strains were isolated in 15 clinical laboratories throughout Belgium participating in the National Surveillance Programme and were sent to the Belgian Pneumococcal Reference Laboratory. A hundred blood isolates per year were selected at random from the collection that the Belgian Pneumococcal Reference Laboratory received from 2004 to 2009. MICs were determined by the Etest (AB Biodisk). The MIC was the concentration of the antibiotic that inhibited the growth of the pneumococcus completely. Susceptibility and resistance of S. pneumoniae were expressed according to Clinical and Laboratory Standards Institute (CLSI) breakpoints, because these breakpoints represent the “gold standard” and are used internationally in trials (3).
The volume of consumption of fluoroquinolones in the ambulatory care setting in Belgium increased from 1.75 DIDs in 1993 to 3.00 DIDs in 2003 but then fell to 2.66 DIDs in 2009. The annual consumption of fluoroquinolones valued at public prices increased from €24.1 million in 1993 to a maximum of €44.4 million in 2002 and then decreased to €35.0 million in 2009. The decrease in consumption since 2002 and 2003 originated from the falling rates of consumption of norfloxacin and ofloxacin. In 2009, the consumption of fluoroquinolones consisted of 1.07 DIDs of ciprofloxacin (40% of fluoroquinolone consumption), 0.78 DIDs of moxifloxacin (29%), 0.33 DIDs of levofloxacin (13%), 0.27 DIDs of norfloxacin (10%), and 0.21 DIDs of ofloxacin (8%). Fluoroquinolones represented 10% of the volume of antibiotic consumption in 2009. The same trend was demonstrated by European Surveillance of Antimicrobial Consumption (ESAC) data (http://app.esac.ua.ac.be/public/).
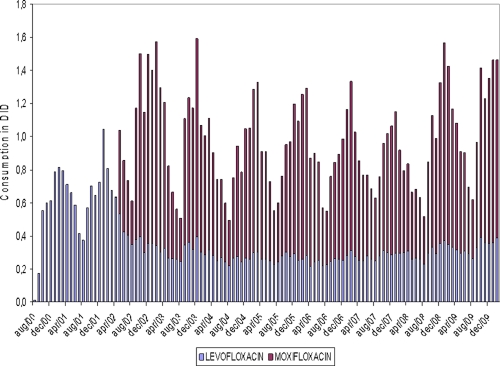
Figure 1 presents monthly data on the volume of consumption of levofloxacin (introduced in July to August 2000) and moxifloxacin (introduced in April 2002). The pattern of seasonal variation observed for levofloxacin prior to the introduction of moxifloxacin almost disappeared after its introduction. On the other hand, the consumption of moxifloxacin exhibited a typical pattern of seasonal variation. This may reflect the fact that levofloxacin was used for treating respiratory tract infections prior to the introduction of moxifloxacin and remained one of the fluoroquinolones used for the treatment of gastrointestinal and urinary tract infections after the introduction of moxifloxacin. Moxifloxacin, a fluoroquinolone typically used for the treatment of respiratory tract infections, followed the pattern of seasonal variation that would be expected.
Fig. 1.
Consumption of levofloxacin-moxifloxacin in ambulatory care settings in Belgium. DID, defined daily dose per 1,000 inhabitants per day.
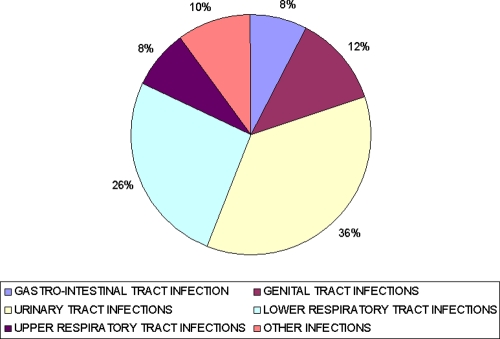
A breakdown of the volume of consumption of fluoroquinolones by type of infection in Belgium in 2009 is portrayed in Fig. 2. This shows that the principal use of fluoroquinolones was for the treatment of urinary tract infections (36% of consumption; volume of 0.95 DIDs) and lower respiratory tract infections (26% of consumption; volume of 0.70 DIDs). This suggests that fluoroquinolones were used primarily for those indications where they have been shown to yield clinical benefit (1, 2).
Fig. 2.
Consumption of fluoroquinolones by type of infection in Belgium in 2009.
Fluoroquinolones were used marginally for the management of upper respiratory tract infections, with a share of consumption of 2% (volume of 0.21 DIDs). Upper respiratory tract infections were managed mainly by broad-spectrum penicillins (72% of consumption in 2009; volume of 8.36 DIDs), macrolides (12% of consumption; volume of 1.33 DIDs), and oral cephalosporins (8% of consumption; volume of 0.92 DIDs). Therefore, this study recommends the identification of those indications, such as upper respiratory tract infections, where the use of antibiotics is not recommended and introduce policy measures such as clinical guidelines, peer review with feedback, educational campaigns, or financial incentives to discourage the use of antibiotics for the treatment of those infections.
The MIC distributions (MIC10 to MIC100) of S. pneumoniae strains and susceptibility rates during 2004 to 2009 are presented in Tables 1 and 2 for moxifloxacin, levofloxacin, amoxicillin, and cefuroxime. The levels of resistance to moxifloxacin and levofloxacin were low (≤1%). The MIC distributions of moxifloxacin as well as levofloxacin remained stable during 2004 to 2009. The percentage of S. pneumoniae strains that were not susceptible to amoxicillin was equal to or less than 1% for the six different years. The rate of resistance of S. pneumoniae to cefuroxime was 9% to 11% in 2004 to 2005 but decreased to 4% in 2006 to 2009.
Table 1.
MIC distributions for S. pneumoniae
| Drug and yr | MIC distribution (mg/liter) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC10 | MIC20 | MIC30 | MIC40 | MIC50 | MIC60 | MIC70 | MIC80 | MIC90 | MIC100 | |
| Moxifloxacin | ||||||||||
| 2004 | 0.094 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.19 | 0.19 | 3 |
| 2005 | 0.094 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.19 | 4 |
| 2006 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.19 | 0.19 | 0.19 | 0.25 | 0.25 |
| 2007 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.19 | 0.19 | 0.25 | 0.25 |
| 2008 | 0.094 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.19 | 0.19 | 4 |
| 2009 | 0.094 | 0.094 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.19 | 0.38 |
| Levofloxacin | ||||||||||
| 2004 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1 | 1.5 | >32 |
| 2005 | 0.5 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1 | >32 |
| 2006 | 0.75 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1 | 1.5 |
| 2007 | 0.5 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1.5 | 1.5 |
| 2008 | 0.5 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1 | 1.5 | >32 |
| 2009 | 0.5 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1 | 1 | 2 |
| Amoxicillin | ||||||||||
| 2004 | <0.016 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.094 | 2 |
| 2005 | <0.016 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.75 | 1.5 |
| 2006 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.032 | 1.5 |
| 2007 | <0.016 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 4 |
| 2008 | <0.016 | <0.016 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.032 | 1.5 |
| 2009 | <0.016 | <0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.032 | 1 |
| Cefuroxime | ||||||||||
| 2004 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.023 | 0.032 | 0.12 | 12 |
| 2005 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.023 | 0.047 | 1.5 | 3 |
| 2006 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.023 | 0.064 | 4 |
| 2007 | 0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.023 | 0.064 | 8 |
| 2008 | <0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.023 | 0.023 | 0.032 | 4 |
| 2009 | <0.016 | 0.016 | 0.016 | 0.016 | 0.023 | 0.023 | 0.023 | 0.032 | 0.094 | 3 |
Table 2.
S. pneumoniae susceptibility rates
| Drug and yr | % of isolates with susceptibility profile at breakpointa |
||
|---|---|---|---|
| S | I | R | |
| Moxifloxacin | ≤1 mg/liter | 2 mg/liter | ≥4 mg/liter |
| 2004 | 99 | 1 | 0 |
| 2005 | 99 | 0 | 1 |
| 2006 | 100 | 0 | 0 |
| 2007 | 100 | 0 | 0 |
| 2008 | 99 | 0 | 1 |
| 2009 | 100 | 0 | 0 |
| Levofloxacin | ≤1 mg/liter | 2 mg/liter | ≥4 mg/liter |
| 2004 | 98 | 1 | 1 |
| 2005 | 98 | 1 | 1 |
| 2006 | 100 | 0 | 0 |
| 2007 | 100 | 0 | 0 |
| 2008 | 99 | 0 | 1 |
| 2009 | 100 | 0 | 0 |
| Amoxicillin | ≤2 mg/liter | 4 mg/liter | ≥8 mg/liter |
| 2004 | 100 | 0 | 0 |
| 2005 | 100 | 0 | 0 |
| 2006 | 100 | 0 | 0 |
| 2007 | 99 | 1 | 0 |
| 2008 | 100 | 0 | 0 |
| 2009 | 100 | 0 | 0 |
| Cefuroxime | ≤0.5 mg/liter | 1 mg/liter | ≥2 mg/liter |
| 2004 | 91 | 0 | 9 |
| 2005 | 87 | 3 | 10 |
| 2006 | 95 | 1 | 4 |
| 2007 | 96 | 0 | 4 |
| 2008 | 95 | 1 | 4 |
| 2009 | 96 | 0 | 4 |
S, susceptible; I, intermediate resistant; R, resistant.
The in vitro results of this study indicate that moxifloxacin is the most potent fluoroquinolone available for the treatment of S. pneumoniae infections in Belgium, with an MIC90 of 0.19 mg/liter. The use of new fluoroquinolones (levofloxacin and moxifloxacin) and the ongoing use of the older fluoroquinolones (mainly for urinary tract infections) has not led, to date, to an increase in the rate of pneumococcal resistance to fluoroquinolones. It remains at or below 1% for levofloxacin and moxifloxacin. In this multiyear surveillance study, a rightward shift in the distributions of MICs of the fluoroquinolones was not observed. This finding also suggests that first-step mutants have not yet become more prevalent in Belgian strains.
Our results mirror the findings of a recent survey of antibiotic resistance in S. pneumoniae using 3,262 noninvasive clinical isolates in Belgium from 1995 to 2008 (5). The rate of resistance of S. pneumoniae to levofloxacin and to moxifloxacin was less than 1%, and no rightward shift in the MIC distributions of the fluoroquinolones was noted. Even though worldwide resistance to moxifloxacin is low in general, resistance does appear in some regions with a high prevalence of multidrug-resistant S. pneumoniae. In Belgium, however, multidrug resistance remains relatively low, with 2.6% resistance to more than five drug classes and 7% of isolates being resistant to any four drug classes (4).
Acknowledgments
Financial support for this research was received from Bayer Schering Pharma. We have no conflicts of interest that are directly relevant to the content of the manuscript.
We express our gratitude to Eric Mostrey (Market Research, Bayer Schering Pharma) for his assistance.
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Bailey R. R. 1992. Quinolones in the treatment of uncomplicated urinary tract infections. Int. J. Antimicrob. Agents 2:19–28 [DOI] [PubMed] [Google Scholar]
- 2. Ball P. 2003. Efficacy and safety of levofloxacin in the context of other contemporary fluoroquinolones: a review. Diagn. Microbiol. Infect. Dis. 64:646–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Jacobs M. R., Felmingham D., Appelbaum P. C., Gruneberg R. N. 2003. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J. Antimicrob. Chemother. 52:229–246 [DOI] [PubMed] [Google Scholar]
- 5. Vanhoof R., et al. 2009. Survey of antibiotic resistance in Streptococcus pneumoniae collected in Belgium during winter 2008. Follow-up of resistance since 1995 and possible effects of antibiotic campaigns. Abstr. 19th Eur. Soc. Clin. Microbiol. Infect. Dis. Conf., Helsinki, Finland, 16 May 2009 [Google Scholar]