Abstract
The objective of this study was to investigate for the first time tenofovir (TFV) pharmacokinetics in plasma and peripheral blood mononuclear cells (PBMCs) of the neonate. HIV-1-infected pregnant women received two tablets of tenofovir disoproxil fumarate (TDF; 300 mg) and emtricitabine (FTC; 200 mg) at onset of labor and then one tablet daily for 7 days postpartum. A single dose of 13 mg/kg of body weight of TDF was administered to 36 neonates within 12 h of life after the HIV-1-infected mothers had been administered two tablets of TDF-emtricitabine at delivery. A total of 626 samples collected within the 2 days after the drug administration were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and analyzed by a population approach. In the neonate, the median TFV plasma area under the curve and minimal and maximal concentrations, respectively, were 3.73 mg/liter · h and 0.076 and 0.29 mg/liter. In PBMCs, TFV concentrations were detectable in all fetuses, whereas tenofovir diphosphate (TFV-DP) was quantifiable in only two fetuses, suggesting a lag in appearance of TFV-DP. The median TFV-DP neonatal concentration was 146 fmol/106 cells (interquartile range [IQR], 53 to 430 fmol/106 cells); two neonates had very high TFV-DP concentrations (1,530 and 2963 fmol/106 cells). The 13-mg/kg TDF dose given to neonates produced plasma TFV and intracellular active TFV-DP concentrations similar to those in adults. This dose should be given immediately after birth to reduce the delay before the active compound TFV-DP appears in cells.
INTRODUCTION
The combination tenofovir (TFV) disoproxil fumarate (TDF)-emtricitabine (FTC) has been considered during the perinatal period to prevent mother-to-child transmission (PMTCT) and/or to reduce viral resistance to nevirapine (NVP) (3, 8); its administration to pregnant women at the onset of labor followed by a single dose to the neonate was thus proposed. The TEmAA (Tenofovir/Emtricitabine in Africa and Asia) ANRS 12109 Trial study was an open, phase I/II trial built in two steps. In the first step, the TDF-FTC combination was administered to HIV-infected pregnant women, and the pharmacokinetics, safety, and toxicity were evaluated for mothers and their newborns (2). After estimation of the maternal and fetal FTC-tenofovir (TFV) pharmacokinetic profiles, the mother was proposed to renew the dose after 12 h if she had not delivered yet in order to maintain effective TFV fetal concentrations. Then, a 13-mg/kg of body weight TDF dose (at birth) was proposed for neonates to obtain similar exposure to that in adults (16). In the second step of the TEmAA study, the TDF-FTC combination was administered to HIV-infected pregnant women and to their newborns, allowing evaluation of these dose propositions based on the results obtained in the first step of the study. Tolerance and virological results have been reported elsewhere (24).
TFV, like other nucleoside analogues, undergoes intracellular phosphorylation by various cellular kinases to an active diphosphate (TFV-DP), which competitively inhibits reverse transcriptase, by being incorporated into the viral genome, causing termination in DNA chain elongation. Newborns are likely to be overexposed to intracellular phosphorylated nucleoside reverse transcriptase inhibitors (NRTIs), as was shown for lamivudine triphosphate (3TC-TP) and zidovudine triphosphate (AZT-TP) during the first 2 weeks of life (10). Indeed, high levels of AZT/AZT monophosphate (AZ-MP)/AZT-TP, and 3TC-TP were consistent with short-term hematological disturbance observed in clinics (7, 11). If, as suggested by the literature (10), this overexposure was related to a more efficient phosphorylation due to a higher activation in the peripheral blood mononuclear cells (PBMCs) of the neonates than in adults, it could be the same for TFV-DP and could cause toxicity problems. It is important with this background to verify that neonatal intracellular TFV-DP concentrations in PBMCs are not too high following PMTCT exposure.
MATERIALS AND METHODS
Patients.
Eligible for this study were pregnant women (between 28 and 38 weeks of gestation), older than 18 years, infected by HIV-1 and/or HIV-2, and naïve to all antiretroviral treatment, who had an indication for antiretroviral prophylaxis for PMTCT during pregnancy (in line with international or national recommendations, including WHO's clinical stage 1 or stage 2 and a CD4 count of ≥200/mm3 or stage 3 and a CD4 count of ≥350/mm3). Neonates with a gestational age of >37 weeks and a birth weight of >2,000 g were eligible. The study protocol was approved by the national ethics committees of Ivory Coast and Cambodia, by the University of the Witwatersrand Health Research Ethics Committee in South Africa, and by each country's health or medicine regulatory authorities. The mother and, where possible, the father of the child to be born provided signed informed consent.
Treatments.
Mothers were administered zidovudine (ZDV; 300 mg twice a day) from enrollment in the study (between the 28th and 36th weeks of amenorrhea) to delivery, with one tablet of nevirapine (NVP; 200 mg) and two tablets of TDF (300 mg)-FTC (200 mg) given at the onset of labor. Two additional tablets of TDF-FTC were administered to the mothers who did not deliver within the 12 h after the first administration. In postpartum, mothers received one tablet of TDF (300 mg)-FTC (200 mg) per day for 7 days. On the first day of life, neonates received a single dose of NVP syrup (2 mg/kg), within the 12 h after birth a single dose of TDF oral solution (13 mg/kg) and a single dose of FTC oral solution (2 mg/kg), and for 7 days ZDV syrup (4 mg/kg every 12 h). The TDF oral solution was a pediatric formulation developed and ultimately discontinued by Gilead as a powder for reconstitution as a suspension and provided bioequivalent systemic exposures to Viread tablets. Although we recommended administration of the TDF oral dose at birth to the neonate based on our previous findings in the first part of the study (16), for simplification, it was administered at the same time as FTC (i.e., within the first 12 h after birth).
Sampling.
Blood samples were collected for pharmacokinetic analysis: at delivery; 1, 2, 3, 5, 8, 12, and 24 h after the administration of 600 mg TDF at the onset of labor; and 24 h after the 1st, 2nd, or 3rd and 7th administrations of 300 mg TDF. Two cord blood samples were obtained at delivery: the first to measure plasma drug concentrations and the second for the TFV-DP concentration in PBMCs. The neonate had four plasma samplings, in the time windows 0.5 to 1.5 h, 6 to 10 h, and 24 to 36 h after the administration of TDF syrup. The sampling windows were based on the optimal sampling scheme. Three samples, one in each time window, were collected to measure plasma TFV concentrations, and one sample was collected between 24 and 36 h to measure TFV-DP in PBMCs.
Analytical method.
TFV and TFV-DP were obtained from Moravek Biochemicals (Brea, CA). To determine the plasma TFV concentration, a liquid-liquid extraction procedure was performed using 300 μl of methanol-dichloromethane (10/16 [vol/vol]) containing 0.1% hydrochloric acid added to 100 μl of plasma samples. After vortexing and centrifugation at 20,000 × g at +4°C for 20 min, the upper phase was withdrawn and evaporated under nitrogen to dryness. The sample was then reconstituted with 100 μl of the mobile phase, and 40 μl of the extract was injected in the analytical system. TFV concentrations were measured by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method previously described (20). The limit of quantification was 10 ng/ml.
PBMCs were collected according to a previously reported method, including a specific red blood cell lysis step (9). PBMCs were then kept frozen at −80°C and transferred on dry ice to the analytical laboratory. Each PBMC sample was counted by using a validated biochemical test as previously described (5). Then, a previously reported LC-MS/MS assay method (21) was used for the determination of TFV-DP levels with a limit of quantification (LOQ) of 100 fmol/sample (i.e., 10 fmol/106 cells for a sample pellet containing 10 million cells). The limit of quantification of TFV in PBMC samples was 20 pg/sample. For the LC-MS/MS TFV-DP assay, the calibration curve ranged from 100 to 7,000 fmol/sample. The calibration curve was fitted to a 1/x-weighted linear regression model. The mean ionization recovery rates were 62.3% (coefficient of variation [CV], 3.6%) over the calibration range and 67.9% (15.0) for the internal standard (IS) at the working concentration. Mean lysis recoveries were 95.9% (CV, 6.3%) over the calibration range and 86.4% (CV, 11.2%) for IS at the working concentration. Within-assay reproducibility, including the lower limit of quantification (LLOQ) level, ranged from 88.4 to 108% and from 4.6 to 10.3% for accuracy and precision, respectively. Between-assay reproducibility, including the LLOQ level, ranged from 96 to 103% and from 3.4 to 10.4% for accuracy and precision, respectively.
Population pharmacokinetic model.
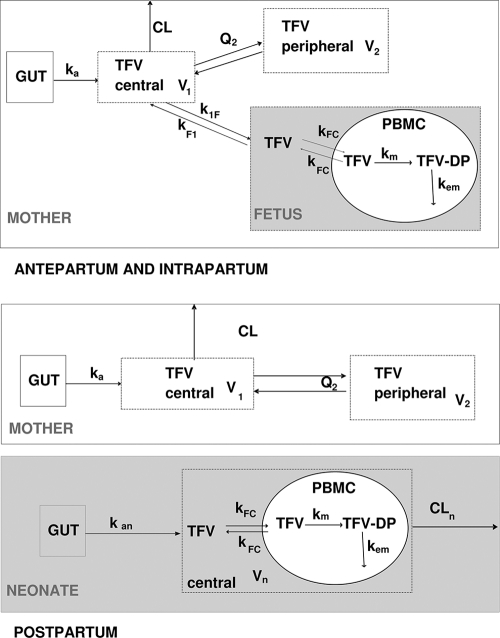
TFV maternal, fetal, and neonatal concentrations in plasma, TFV fetal and neonatal concentrations in PBMCs, and TFV-DP fetal and neonatal concentrations in PBMCs were analyzed simultaneously by a population approach (Fig. 1). To mix these data, all of the doses and concentrations were expressed in homogeneous unities: doses (in mg) and plasma drug concentrations (in mg/liter) were divided by the molar mass (247.3 g/mol) and intracellular drug concentrations (TFV and TFV-DP in pmol/106 cells) were transformed assuming a 0.4-pl cell volume (13). Data were analyzed with the MONOLIX software (version 3.1s; http://wfn.software.monolix.org) (18), and the SAEM algorithm was used. Analytical equations were written in an MLXTRAN script file in MONOLIX to estimate these pharmacokinetic parameters. A two-compartment model with first-order absorption and elimination best described the maternal data. Parameters of this part of the model were the absorption rate constant (ka), maternal apparent elimination clearance from the central compartment (CL/F), apparent volume of the central maternal compartment (V1/F), apparent intercompartmental clearance (Q2/F), and apparent volume of the peripheral maternal compartment (V2/F). An “effect” compartment model linked to the maternal circulation, with different rate constants for maternal-to-fetal (k1F) and fetal-to-maternal (kF1) transfers, best described cord drug concentrations. After delivery, this fetal compartment was disconnected, time was reset to zero, and the neonate had his or her own absorption and elimination. A one-compartment model with first-order absorption and elimination was sufficient to describe neonatal drug concentrations, the parameters used were neonatal absorption (kan), apparent neonatal volume of distribution (Vn/F), and apparent neonatal elimination clearance (CLn/F). An effect compartment linked to the fetal/neonatal circulation by a TFV cell transfer rate constant (kFC) was used to describe TFV in PBMCs. TFV-DP concentrations in PBMCs were modeled by using an additional compartment with first-order reactions for the TFV-to-TFV-DP metabolism constant rate (km) and TFV-DP elimination constant rate (kem). A median intracellular half-life of TFV-DP of approximately 150 h has already been reported in adults (15, 20). As no neonatal PBMC samples were collected after 50 h, we could not evaluate this half-life in neonates, so it was fixed to the same adult value (kem = 0.005 h−1). Residual variabilities were best described by an additive error model for maternal plasma and for fetal plasma, intracellular TFV and TFV-DP concentrations, and by a proportional error model for neonatal plasma, intracellular TFV, and TFV-DP concentrations. An exponential model was used for intersubject variability (ISV). The available data were not sufficient to estimate intersubject variability for ka, k1F, kF1, kFC, kem, and km, and fixing the variance of these random effects to zero had no influence on the goodness-of-fit criteria. Maternal body weight and the type of delivery (vaginal delivery versus caesarian section) were tested on maternal pharmacokinetic parameter with significant intersubject variability (CL/F, V1/F, V2/F, and Q/F). Neonatal body weight, postnatal age of administration, gestational age, sex, and the type of alimentation (breast-feeding versus formula feeding) were tested on neonatal pharmacokinetic parameter with random effects (kan, CLn/F, and Vn/F).These effects were systematically tested via generalized additive modeling on the basic model as previously described (16).
Fig. 1.
Population pharmacokinetic model for the simultaneous prediction of plasma tenofovir (TFV) concentrations in the mother, the cord, and the neonate and intracellular TFV and TFV-DP in the fetus and the neonate during intrapartum and postpartum. A two-compartment model with first-order absorption and elimination best described the maternal data. An “effect” compartment model linked to the maternal circulation best described cord concentrations. A one-compartment model with first-order absorption and elimination was sufficient to describe neonatal concentrations. An effect compartment linked to the fetal/neonatal circulation was used to describe TFV in PBMCs. An additional compartment was used for TFV-DP in PBMCs with first-order reactions for TFV to TFV-DP metabolism and TFV-DP elimination. F denotes the bioavailability, ka the maternal absorption rate constant, CL the maternal elimination clearance from the central compartment, V1 the volume of the central maternal compartment, Q2 the maternal intercompartmental clearance, V2 the volume of the peripheral maternal compartment, k1F the maternal-to-fetal rate constant, kF1 the fetal-to-maternal rate constant, kan the neonatal absorption rate constant, Vn/F the apparent neonatal volume of distribution, CLn/F the apparent neonatal elimination clearance; kFC the TFV cell transfer rate constant, km the TFV to TFV-DP metabolism constant rate, and kem the TFV-DP elimination constant rate.
Evaluation and validation.
For evaluation of the goodness of fit, the following graphs were performed: observed and predicted concentrations versus time, observed concentrations versus population predictions, weighted residuals versus time, and weighted residuals versus predictions. Similar graphs using individual predictive post hoc estimation were displayed. The diagnostic graphs were performed with RfN (S. Urien, RFN-831-20070911; https://sourceforge.net/project/showfiles.php?group_id=29501&package_id=140129&release_id=538680) with the R program (23).
TFV concentration profiles were simulated and compared with the observed data thanks to a visual predictive check in order to validate the model. More precisely, the vector of pharmacokinetic parameters from 1,000 patients was simulated using the final model. Each vector parameter was drawn in a log-normal distribution with a variance corresponding to the ISV previously estimated. A simulated residual error was added to each simulated concentration. The 5th, 50th, and 95th percentiles of the simulated concentrations at each time were then overlaid on the observed concentration data by using the R program, and a visual inspection was performed.
Proposed dose evaluation.
Maternal and fetal plasma TFV concentrations in mother who were readministered two tablets of TDF-FTC 12 h after the first intake were compared with those of women who had a single administration at the onset of labor. After the administration of TDF syrup to each neonate, the area under the concentration curve (AUC) and minimal (Cmin) and maximal (Cmax) plasma TFV concentrations were derived from the estimated individual pharmacokinetic parameters.
Profiles of TFV in plasma and TFV/TFV-DP in PBMCs.
Observed and predicted TFV concentrations in plasma and TFV and TFV-DP concentrations in PBMCs were drawn as a function of time in the fetus/neonate. Then, TFV cell transfer and TFV phosphorylation to TFV-DP could be evaluated.
RESULTS
Demographic data.
Data from the 36 enrolled women and their neonates were available for TFV pharmacokinetic evaluation. Table 1 summarizes the patients' characteristics. Two mothers did not deliver within the 12 h after the first administration and thus received another two-tablet dose of TDF-FTC.
Table 1.
Characteristics of the 36 HIV-infected pregnant women enrolled in the TFV pharmacokinetic study of the TEmAA ANRS 12109 Trial, Step 2
| Covariate | Median value (range) |
|---|---|
| Maternal body wt at delivery (kg) | 67.5 (48–95.9) |
| Gestational age (wk) | 38 (37–44) |
| Delivery: vaginal, caesarian section (n) | 16, 20 |
| Neonatal body wt at birth (kg) | 3 (2.4–3.7) |
| Delay (h) between: | |
| Last maternal TFV administration and delivery | 6 (0.4–12) |
| Birth and neonatal TDF administration | 9.2 (1.5–48.6) |
| Neonatal TDF administration and sampling | 25.5 (10–45.5) |
Population pharmacokinetics.
A total of 626 concentrations (398 TFV concentrations from maternal plasma, 36 TFV concentrations from cord plasma, 108 TFV concentrations from neonatal plasma, 20 TFV concentrations from cord PBMCs, 22 TFV concentrations from neonatal PBMCs, 20 TFV-DP concentrations from cord PBMCs, and 22 TFV-DP concentrations from neonatal PBMCs) were available for pharmacokinetic analysis. The numbers of concentrations below the limit of quantification (LOQ) and below the limit of detection (LOD) are reported in Table 2. Table 3 summarizes the final population pharmacokinetic estimates. In the postpartum period, only residual concentrations were collected; thus, the estimation of the absorption corresponds only to the 600 mg during labor, not to the 400 mg postpartum. A covariance between neonatal apparent clearance and volume of distribution was significant (leading to a 14.3-U decrease in objective function value [OFV]); the correlation between these parameters was equal to 1. The effect of gestational age (from 37 to 44 weeks) on neonatal volume of distribution was significant, leading to a 10.6-U decrease in OFV and a decrease in the intersubject variability for the volume of distribution from 50 to 46%. None of the other covariates tested decreased the OFV by more than 6.63 U (khi2, 1 degree of freedom, 1%). The postnatal age of TDF administration was less than 15 h for all children (except one, 48.6 h); thus, we could not find evidence of the effect of the huge changes in the gut and liver over the first few days of life on neonatal pharmacokinetic parameters. Five infants out of 36 were breast-fed; this covariate had no significant effect on the neonatal absorption constant rate or on bioavailability (CLn/F and Vn/F at the same time). The median TFV breast milk dose received by the neonate was very low (0.03% of the proposed oral infant dose) (4); thus, it was not taken into account in our model.
Table 2.
Number of TFV and TFV-DP concentrations in plasma and PBMCs below the LOQ and below the LOD for the mother, the cord, and the neonate
| Concn type | Limit used | No. of concns below limit/total in: |
||
|---|---|---|---|---|
| Mother | Fetus | Neonate | ||
| TFV in plasma | LOQ | 13/398 | 5/36 | 1/108 |
| TFV in PBMCs | LOQ | 13/20 | 5/22 | |
| TFV-DP in PBMCs | LOQ | 18/20 | 6/22 | |
| LOD | 6/20 | 3/22 | ||
Table 3.
Population pharmacokinetic parameters of tenofovir from the final model for 36 HIV-infected pregnant women after receiving 600 mg of TDF at the onset of labor and for their 36 neonates enrolled in the TEmAA ANRS 12109 Trial, Step 2a
| Structural model parameter | Estimate by structural model (% RSE) | Statistical model |
|
|---|---|---|---|
| Parameter | Estimate (% RSE) | ||
| ka | 0.651 h−1 (13) | ωCL/F | 39% (22) |
| CL/F | 189 liters/h (11) | ωV1/F | 165% (13) |
| V1/F | 482 liters (34) | ωQ/F | 63% (19) |
| Q/F | 230 liters/h (16) | ||
| V2/F | 3,500 liters (23) | ωkan | 177% (15) |
| k1F | 0.259 h−1 (38) | ωCLn/F | 68% (9) |
| kF1 | 0.691 h−1 (31) | ωVn/F | 46% (18) |
| kFC | 0.157 h−1 (49) | σmother plasma | 0.03 mg · liter−1 (4) |
| km | 0.0495 h−1 (29) | σcord plasma | 0.014 mg · liter−1 (12) |
| kem | 0.005 h−1 (fixed) | σneonate plasma | 47% (12) |
| kan | 0.518 h−1 (38) | σcord TFV cells | 63.2 fmol/106 cells (16) |
| CLn/F | 3.01 liters/h (13) | σneonate TFV cells | 105% (16) |
| Vn/F | 37.7 liters (17) | σcord TFV-DP cells | 33.4 fmol/106 cells (16) |
| Vn/F, θGA | 8.5 (32) | σneonate TFV-DP cells | 114% (16) |
RSE, relative standard error (standard error of estimate/estimate · 100); ka, absorption rate constant; CL/F, maternal apparent elimination clearance from the central compartment; V1/F, apparent volume of distribution of the central maternal compartment; Q/F, apparent maternal intercompartmental clearance; V2/F, apparent volume of distribution of the peripheral maternal compartment; k1F, maternal-to-fetal rate constant; kF1, fetal-to-maternal rate constant; kFC, fetal-to-cord rate constant; kan, neonatal absorption; CLn/F, neonatal apparent elimination clearance; Vn/F, apparent neonatal volume of distribution; Vn/F, θGA, influential factor for gestational age on neonatal apparent volume of distribution; km, TFV-to-intracellular TFV-DP metabolism constant rate; kem, TFV-DP elimination constant rate; σ, residual variability estimates (CV of residual variability [%]); and ω, interindividual variability estimates (CV of intersubject variability [%]).
Validation.
A visual predictive check of the final population pharmacokinetic model allowed the comparison of the 5th, 50th, and 95th predicted percentiles from the 1,000 simulations and the observed concentrations (Fig. 2). The percentage of observed points out of the 90% prediction interval of the simulations was not significantly different from the theoretical value of 10%, and with repartition, the observed points in the 0 to 5th, 5th to 50th, 50th to 95th, and 95th to 100th percentiles of the simulations were not significantly different from the theoretical 5, 45%, 45, and 5% for all types of concentrations, except for the fetal TFV-DP. The validation problem for fetal TFV-DP came from the high number of unquantifiable concentrations (18/20) and their treatment. Indeed the limit of quantification was 100 fmol per sample; thus, a patient with few cells in the sample would have very high concentrations.
Fig. 2.
Evaluation of the final model: comparison between the 5th (dashed line), 50th (full line), and 95th (dashed line) percentiles obtained from 1,000 simulations and the observed data (points) for tenofovir plasma concentrations (top four panels) in the mother during all treatments (i.e., samples at delivery; 1, 2, 3, 5, 8, 12, and 24 h after the administration of 600 mg TDF; and 24 h after the 1st, 2nd or 3rd, and 7th administrations of 300 mg TDF [top left]) and at delivery only (top right), in the cord (bottom left) and neonate (bottom right), and for intracellular concentrations in PBMCs (bottom four panels) with TFV in the fetus (top left), TFV in the neonate (top right), TFV-DP in the fetus (bottom left), and TFV-DP in the neonate (bottom right). Solid symbols represent plasma TFV concentrations in the two mothers (at delivery) and fetuses for which the mother did not deliver within the 12 h after drug intake and to whom was readministered two tablets of TDF-FTC. Crosses with the data points represent the concentrations under the limit of quantification.
Proposed dose evaluation.
As shown in Fig. 2, the two mothers and fetuses for whom TDF was readministered due to prolonged labor had comparable concentrations compared to those mothers who received a single administration at the onset of labor.
The neonate was administered a 13-mg/kg TDF dose. The resulting median plasma tenofovir AUC, Cmin, and Cmax, respectively, were 3.73 mg/liter · h (interquartile range [IQR], 2.51 to 4.93 mg/liter · h), 0.076 mg/liter (IQR, 0.056 to 0.131 mg/liter), and 0.29 mg/liter (IQR, 0.21 to 0.42 mg/liter).
Profiles of TFV in plasma and TFV/TFV-DP in PBMCs.
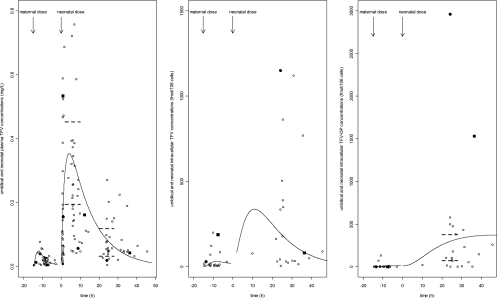
Figure 3 displays for fetuses and neonates the observed and predicted TFV concentrations in plasma, TFV concentrations in PBMCs, and TFV-DP concentrations in PBMCs as a function of time in our study and the plasma TFV and TFV-DP concentrations reported in previous studies of adults (15, 17, 19, 20). Fetal and neonatal plasma TFV and IC TFV-DP concentrations were compared to adult values measured in the same laboratory with the same method of dosage (20, 21).
Fig. 3.
Fetus/neonate observed (data points) and predicted (lines) TFV concentrations in plasma (left), TFV concentrations in PBMCs (middle), and TFV-DP concentrations in PBMCs (right), as a function of time. A median delay of 6 h between maternal administration and delivery and a median delay of 9 h between birth and neonatal administration was used to represent fetal/neonatal concentrations. In the left panel, the dashed lines correspond to the IQR for the plasma TFV peak concentrations (0.195 to 0.453 mg/liter) (17, 20) and the IQR for the plasma TFV trough concentrations (0.033 to 0.119 mg/liter (17, 20) in adults. In the right panel, the dashed lines correspond to the IQR for IC TFV-DP concentrations in adults (70 to 376.5 fmol/106 cells) (17, 20). Black points and black squares correspond to concentrations of TFV in plasma, TFV in PBMCs, and TFV-DP in PBMCs of the two neonates who had very high TFV-DP concentrations.
The variability of TFV concentrations in PBMCs was important (from 5 to 1,151 fmol/106 cells). TFV concentrations in PBMCs were significantly correlated to TFV concentrations in plasma (r = 0.53, P = 7.10−4).
In the fetus, TFV phosphorylation was very low. No TFV-DP was found in the PBMCs of six children; the concentration was under the LOQ in 12 children and quantifiable only in 2 children, whereas TFV was detected in all of the fetal PBMCs. In the neonate, TFV-DP was measured in PBMCs 22 to 38 h after the neonatal drug intake for 20 children and 10 h and 45 h after neonatal drug intake for 2 other children; the median TFV-DP concentration was 146 fmol/106 cells (IQR, 53 to 430 fmol/106 cells). TFV-DP concentrations in the neonates were in the same order of magnitude as adult TFV-DP concentrations, except for two children who had very high concentrations (1,530 and 2,963 fmol/106 cells). As no TFV-DP samples were collected after 50 h, no point allowed us to estimate TFV-DP half-life; however, whatever the value was for kem, all other parameters remained stable.
Two neonates had very high TFV-DP concentrations 24 to 36 h after their drug intake. As shown in Fig. 3, the first neonate had a 6-times-higher concentration of TFV-DP in plasma and a cell concentration of TFV comparable to the mean plasma TFV concentrations of the other neonates. The second neonate had a 12-times-higher TFV-DP concentration than the mean and a 4-times-higher intracellular TFV concentration than the mean, but its plasma TFV concentration was lower than the mean. No adverse event was reported in these two neonates.
DISCUSSION
This paper provides data on TFV pharmacokinetics in plasma and PBMCs of the neonate. As no pharmacokinetic data were available for studying TFV in neonates, the TEmAA (Tenofovir/Emtricitabine in Africa and Asia) ANRS 12109 Trial study was built in two steps. In the first one, TDF was administered only to the mother at the onset of labor (16). A cord sample allowed the estimation of the dose that reached to the fetus and a few neonatal samplings allowed estimation of the TFV neonatal half-life. The first-order kinetic was used to describe maternal/fetal transfer; however, at higher concentrations of the drug, this assumption of linearity may not be checked. Indeed, tenofovir is a substrate of influx transporters human organic anion transporter type 1 (OAT1) and OAT3 and of efflux transporter MRP4 in the kidney. OAT1 and OAT3 are also expressed in the placenta and could transport tenofovir according to a saturable process. Furthermore, biological processes affecting placental blood flow and placental transfer are also not captured in the first-order kinetics presented. Using the data from step 1 and making a hypothesis about neonatal absorption and distribution, we evaluated the neonatal dose of TDF that would produce the same plasma drug exposure and minimal concentrations in neonates as those observed in adults with the recommended dose (6, 22). To apply this strategy, a population approach was developed to determine a neonatal dose in the first part of the study, which allowed us to progress to the second step. This approach was validated by the results obtained in the second part reported here.
Based on the results obtained in the first step, a readministration of two tablets of TDF-FTC to the mother was proposed after 12 h if the mother had not delivered yet, and a first dose of 13 mg/kg at birth was proposed for the neonate. These propositions could be evaluated here. First, the two mothers and fetuses, for which the mother was readministered TDF 12 h after the first intake, had comparable concentrations compared to the mothers and fetuses for which the mother had a single administration at the onset of labor. Second, the dose of 13 mg/kg administered to the neonate within 12 h after birth produced plasma TFV concentrations close to the adult values: 3.73 mg/liter · h versus 2.88 and 2.65 mg/liter · h for AUC, 0.076 mg/liter versus 0.060 and 0.053 mg/liter for Cmin, and 0.29 mg/liter versus 0.31 and 0.29 mg/liter for Cmax (6, 22). In conclusion, as expected by our simulations based on the first step of the study, with the neonatal TDF dose of 13 mg/kg, plasma drug concentrations were comparable between neonates and adults.
The method used to obtain similar adult plasma drug exposure is currently used to determine the dosage in children. However, concentrations of the drug at the site of action could be different in children and adults for the same plasma concentrations (14). Indeed, as HIV acts inside the lymphocyte and as TFV is not active by itself but undergoes intracellular phosphorylation by various cellular kinases to give the active diphosphate TFV-DP, we aimed to compare not only plasma drug concentrations but also intracellular drug concentrations between adults and neonates in the second step. A previous study showed that newborns are likely to be overexposed to AZT/AZT-MP/AZT-TP and 3TC-TP during the first 2 weeks of life (10). These observations are consistent with short-term hematological disturbances seen in clinics (7, 11). However, no correlation was found between intracellular nucleoside reverse transcriptase inhibitor monophosphate (NRTI-MP)/TP concentrations and short-term toxicity data at birth and at 1, 3, and 6 months of age. The authors suggested that this overexposure could be related to differences in the PBMC activation state between adults and neonates, some NRTIs being more efficiently phosphorylated in activated cells (12). Thus, by collecting and dosing TFV and TFV-DP in PBMCs extracted from one fetal and one neonatal sample and by using a population approach, TFV cell transfer and TFV phosphorylation were described.
In our study, TFV-DP in PBMCs was measured 22 to 38 h after the neonatal drug intake for 20 children and 10 h and 45 h after neonatal drug intake for two other children, and neonates were not likely to be overexposed to the intracellular phosphorylated TFV-DP. Indeed, median TFV-DP concentrations were 146 fmol/106 cells (IQR, 53 to 430 fmol/106 cells), these concentrations were comparable to adult therapeutic values obtained at steady state and were lower than the values obtained after administration of a single TDF dose to an adult (15, 17, 19, 20). As TFV-DP elimination could not be estimated in the neonates due to the lack of points after 50 h, the exposition between neonates and adults could not be compared. Although it was not evidenced by the model, we think that the difference between NRTI phosphorylation (TFV-DP versus AZT-TP or 3TC-TP) could be due to the enzymes implicated. The last phosphorylation is performed by the same enzyme (5′ diphosphatase kinase), but the enzymes implicated differ for the first or second phosphorylation of the compound: adenylate kinase for TFV, thymidine and thymidylate kinase for ZDV, and deoxycytidine kinase and monophosphate kinase for 3TC (1). Furthermore, it seems that PBMC activation in neonates is more effective by some enzymes than by others (12).
Two children had very high TFV-DP concentrations (1,530 and 2,963 fmol/106 cells—i.e., 6 to 12 times the median TFV-DP concentration) but normal plasma drug concentrations, so these children could not be identified by a simple plasma sample. None of them had serious adverse events, while four serious adverse events were reported in this study (24).
Both fetal and neonatal intracellular drug concentrations were measured and allowed estimation of TFV-DP generation. In the fetus, samples were taken from 0.4 to 12 h after maternal administration, and in all the neonates except one, samples were taken from 22 to 45.5 h after neonatal administration. The lack of information on the neonate before 24 h has been compensated for by information on the fetus for which concentrations were collected between 0.4 and 12 h. We have a hole between 12 and 22 h, but we could estimate TFV-DP generation. With the exception of two samples, no TFV-DP was quantifiable in fetal PBMCs. This could be due to too-low fetal plasma drug concentrations, a low rate of cell transfer, or a delay before the phosphorylation. Although drug concentrations were quite low in the plasma of the fetus, TFV was present and quantifiable in fetal PBMCs, and thus the low TFV-DP concentrations at birth seem to be attributable to a delay before phosphorylation. This delay could not be estimated precisely due to the lack of TFV-DP concentrations between 12 and 22 h. Although in the first step of the TEmAA Study, we recommended administration of TDF to the neonate at birth, for practical reasons, it was given at the same time as emtricitabine (at a median delay of 9.2 h after birth). Taking into account the relatively low plasma TFV concentrations in the fetus and the delay before the drug starts to be active, we again recommend giving the 13-mg/kg dose of TDF to the neonate at birth.
In conclusion, the 13-mg/kg TDF dose given to the neonate produced similar plasma TFV and intracellular active TFV-DP concentrations in neonates compared to those in adults. This dose should be given immediately after birth to reduce the delay before the active compound TFV-DP appears.
ACKNOWLEDGMENTS
We acknowledge the French Agence Nationale de Recherches sur le VIH/SIDA et les Hépatites Virales (ANRS) for sponsoring the trial, as well as the European and Developing Country Clinical Trials Partnership (EDCTP) and the French charity Sidaction for additional financial support.
We greatly thank the local investigators and their staff in the Formations Sanitaires Urbaines de Youpougon-Attié and Abobo-Avocatier, the Centre Hospitalier Universitaire de Yopougon and the Centre de Diagnostic et de Recherches sur le SIDA in Abidjan, in the Calmette Hospital, and the Pasteur Institute in Phnom Penh, and in the Perinatal HIV Research Unit, Lesedi Clinic in Soweto, and BARC SA Clinical Trials in Johannesburg. We also thank the women who agreed to participate in the trial and their infants. We acknowledge Gilead Sciences for providing the study drugs, with special thanks to Jim Rooney and Camille Aubron-Olivier.
The TEmAA Study Group is constituted as follows. The primary investigators are François Dabis (INSERM U897, ISPED, Bordeaux, France) and Didier Koumavi Ekouévi (PACCI, Abidjan, Ivory Coast). The coinvestigators are Christine Rouzioux, Stéphane Blanche, Jean-Marc Treluyer, Marie-Laure Chaix, and Elisabeth Rey (Paris, France); N′Dri-Yoman (Abidjan, Ivory Coast); Leang Sim Kruy and Eric Nerrienet (Phnom Penh, Cambodia); and Glenda Gray and James McIntyre (Soweto, South Africa). The trial coordinator is Elise Arrivé (Bordeaux, France).
The other members of the TEmAA ANRS 12109 Study Group are as follows: from Paris, Déborah Hirt, Saik Urien, and Alain Pruvost; from Abidjan, Gérard Allou, Divine Avit, Clarisse Amani-Bosse, Kouakou Brou, Patrick Coffie, Patrice Fian, Eulalie Kanga, Broulaye Kone, Suzanne Kouadio, Jeanne Eliam Kouakou, Sidonie Ngatchou, Touré Pety, Zenica Seoue, and Mamourou Kone; from Phnom Penh, Laurence Borand, Pinn Chou, Kearena Chhim, Meng Ly Ek, Leakhena Say, Seng Hout, Sethikar Im, Saroeum Keo, Vannith Lim, Sopheak Ngin, Vara Ouk, Vibol Ung, and the Magna and Maryknoll Associations; and from Soweto, Promise Duma, Portia Duma, Sarita Lalsab, Shini Legote, Joseph Makhura, Modise Maphutha, Selvan Naidoo, Mandisa Nyati, and Ravindre Panchia.
The Scientific Board comprises Bernard Koffi Ngoran (Abidjan, Ivory Coast), Koum Kanal (Phnom Penh, Cambodia), Lynn Morris (Johannesburg, South Africa), Séverine Blesson (ANRS, Paris, France), Camille Aubron-Olivier (Gilead Sciences, Paris, France), Gilles Peytavin (Paris, France), Koen Van Rompay (Davis, CA), and Valériane Leroy (Bordeaux, France).
The Independent Committee comprises John Sullivan (Worcester, MA), Philippe Lepage (Brussels, Belgium), Laurent Mandelbrot (Paris, France), Marie-Louise Newell (London, United Kingdom), and Anne-Marie Taburet (Paris, France).
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Anderson P. L., Kakuda T. N., Lichtenstein K. A. 2004. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin. Infect. Dis. 38:743–753 [DOI] [PubMed] [Google Scholar]
- 2. Arrive E., et al. 2009. Tolerance and viral resistance after single-dose nevirapine with tenofovir and emtricitabine to prevent vertical transmission of HIV-1. AIDS 23:825–833 [DOI] [PubMed] [Google Scholar]
- 3. Arrive E., et al. 2007. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int. J. Epidemiol. 36:1009–1021 [DOI] [PubMed] [Google Scholar]
- 4. Benaboud S., et al. 2011. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1 infected women in Abidjan, Côte d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob. Agents Chemother. 55:1315–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benech H., et al. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172–174 [DOI] [PubMed] [Google Scholar]
- 6. Blum M. R., Chittick G. E., Begley J. A., Zong J. 2007. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J. Clin. Pharmacol. 47:751–759 [DOI] [PubMed] [Google Scholar]
- 7. Capparelli E. V., et al. 2003. Population pharmacokinetics and pharmacodynamics of zidovudine in HIV-infected infants and children. J. Clin. Pharmacol. 43:133–140 [DOI] [PubMed] [Google Scholar]
- 8. Chi B. H., et al. 2007. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: an open-label randomised trial. Lancet 370:1698–1705 [DOI] [PubMed] [Google Scholar]
- 9. Durand-Gasselin L., Da Silva D., Benech H., Pruvost A., Grassi J. 2007. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 51:2105–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durand-Gasselin L., et al. 2008. High levels of zidovudine (AZT) and its intracellular phosphate metabolites in AZT- and AZT-lamivudine-treated newborns of human immunodeficiency virus-infected mothers. Antimicrob. Agents Chemother. 52:2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feiterna-Sperling C., et al. 2007. Hematologic effects of maternal antiretroviral therapy and transmission prophylaxis in HIV-1-exposed uninfected newborn infants. J. Acquir. Immune Defic. Syndr. 45:43–51 [DOI] [PubMed] [Google Scholar]
- 12. Gao W. Y., Agbaria R., Driscoll J. S., Mitsuya H. 1994. Divergent anti-human immunodeficiency virus activity and anabolic phosphorylation of 2′,3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633–12638 [PubMed] [Google Scholar]
- 13. Gao W. Y., Cara A., Gallo R. C., Lori F. 1993. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc. Natl. Acad. Sci. U. S. A. 90:8925–8928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giraud C., et al. 2009. High levels of P-glycoprotein activity in human lymphocytes in the first 6 months of life. Clin. Pharmacol. Ther. 85:289–295 [DOI] [PubMed] [Google Scholar]
- 15. Hawkins T., et al. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406–411 [DOI] [PubMed] [Google Scholar]
- 16. Hirt D., et al. 2009. Population pharmacokinetics of tenofovir in HIV-1-infected pregnant women and their neonates (ANRS 12109). Clin. Pharmacol. Ther. 85:182–189 [DOI] [PubMed] [Google Scholar]
- 17. Kiser J. J., et al. 2008. Pharmacokinetics of antiretroviral regimens containing tenofovir disoproxil fumarate and atazanavir-ritonavir in adolescents and young adults with human immunodeficiency virus infection. Antimicrob. Agents Chemother. 52:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn E., Lavielle M. 2005. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 49:1020–1038 [Google Scholar]
- 19. Pruvost A., et al. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pruvost A., et al. 2009. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob. Agents Chemother. 53:1937–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pruvost A., Theodoro F., Agrofoglio L., Negredo E., Benech H. 2008. Specificity enhancement with LC-positive ESI-MS/MS for the measurement of nucleotides: application to the quantitative determination of carbovir triphosphate, lamivudine triphosphate and tenofovir diphosphate in human peripheral blood mononuclear cells. J. Mass Spectrom. 43:224–233 [DOI] [PubMed] [Google Scholar]
- 22. Ramanathan S., Shen G., Cheng A., Kearney B. P. 2007. Pharmacokinetics of emtricitabine, tenofovir, and GS-9137 following coadministration of emtricitabine/tenofovir disoproxil fumarate and ritonavir-boosted GS-9137. J. Acquir. Immune Defic. Syndr. 45:274–279 [DOI] [PubMed] [Google Scholar]
- 23. R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 24. TEmAA ANRS 12109 Study Group 2010. Maternal and nenonatal nevirapine, tenofovir and emtricitabine to prevent vertical transmission of HIV-1: tolerance and viral resistance. AIDS 24:2481–2488 [DOI] [PubMed] [Google Scholar]