Abstract
The aim of the study was to investigate the effect of sesame oil on acute kidney injury induced by the synergistic action of aminoglycoside and iodinated contrast in rats. Acute kidney injury was induced by a 5-day course of daily gentamicin injections (100 mg/kg of body weight, subcutaneously) and then iodinated contrast (4 ml/kg, intravenously) in male specific-pathogen-free Sprague-Dawley rats. Sesame oil (0.5 ml/kg, orally) was given 1 h before iodinated contrast. Renal function and oxidative stress were assessed 6 h after iodinated contrast injection. Renal function was evaluated by measuring serum blood urea nitrogen and creatinine levels. Renal oxidative stress was assessed by determining renal lipid peroxidation, myeloperoxidase, hydroxyl radical, superoxide anion, nitrite/nitrate, and inducible nitric oxide synthase levels. Sesame oil significantly prevented the rise of serum blood urea nitrogen and creatinine levels. Furthermore, there was a parallel inhibition of the rise in levels of expression of renal lipid peroxidation, myeloperoxidase, hydroxyl radicals, superoxide anion, nitrite/nitrate, and inducible nitric oxide synthase in rats with gentamicin-plus-iodinated contrast-induced acute kidney injury. We conclude that sesame oil may attenuate aminoglycoside-plus-iodinated contrast-induced acute kidney injury by inhibiting renal oxidative stress in rats.
INTRODUCTION
The development of radiologic contrast media originated with the use of iodinated contrast medium in radiography in 1918 (18). The use of iodinated contrast medium has been increasing along with the increasing use of diagnostic imaging. It is estimated that more than 80 million patients received iodinated contrast medium in 2003 worldwide (21). Because iodinated contrast is widely used, it is sometimes administered to patients receiving an aminoglycoside. The action of this combination of contrast agents and aminoglycosides against renal hemodynamics and tubular cell function is potentially synergistic (25). In addition, a large cohort study has found that the use of iodinated contrast significantly increased the risk of acute kidney injury (AKI) in patients treated with aminoglycosides (25).
Oxidative stress plays an important role in the pathogenesis of iodinated contrast-associated AKI in renally compromised patients (24). The vasoconstriction and renal ischemia caused by contrast media leads to the generation of reactive oxygen species (ROS) and nitric oxide, both of which are involved in the development of renal oxidative stress and tubular cell damage (1, 2, 20, 24). Therefore, inhibiting contrast-associated oxidative stress may be beneficial in preventing iodinated contrast-plus-aminoglycoside-induced renal injury. Sesame oil is a potent antioxidative agent (9–11, 14). Sesame oil potently reduces renal oxidative stress by inhibiting the generation of ROS and nitric oxide in septic rats (9–11, 14). Further, sesame oil protects against gentamicin-induced renal injury by inhibiting renal oxidative stress in rats (17). However, the effect of sesame oil on renal injury induced by an aminoglycoside combined with iodinated contrast has never been investigated. The aim of the present study was to investigate the effect of sesame oil on AKI induced by the synergistic action of aminoglycoside and iodinated contrast in rats.
MATERIALS AND METHODS
Reagents.
Gentamicin was purchased from China Chemical & Pharmaceutical Co., Ltd. (Taipei, Taiwan). Sesame oil was purchased from Sigma (St. Louis, MO). Contrast medium Conray (iothalamate meglumine injection USP, 60%; each ml contains 600 mg iothalamate meglumine, 0.09 mg edetate calcium disodium as a stabilizer, and 0.125 mg monobasic sodium phosphate as a buffer) was purchased from Mallinckrodt (St. Louis, MO).
Animals.
Thirty male specific-pathogen-free Sprague-Dawley rats weighing 200 to 250 g were obtained from and housed in our institution's Laboratory Animal Center. Rats were housed individually in a room with a 12-h light/12-h dark cycle and with central air conditioning (25°C, 70% humidity). They were allowed free access to tap water and pelleted rodent diet (Richmond Standard; PMI Feeds, St. Louis, MO). The animal care and experimental protocols were in accord with nationally approved guidelines.
Experimental design.
Rats were divided into five groups of six. The normal (healthy) (N) group rats were given daily injections of saline (1 ml/kg of body weight/day, subcutaneously [s.c.]) over 5 days, the contrast medium (C) group rats were treated with a single dose of contrast (4 ml/kg) intravenously (i.v.) after 5-day treatments with saline (1 ml/kg/day, s.c.), the gentamicin (G) group rats were treated with gentamicin (100 mg/kg/day, s.c.) daily for 5 days (7), the contrast-combined-with-gentamicin (CG) group rats were treated with contrast after 5-day daily gentamicin injections, and contrast medium-plus-gentamicin- and sesame oil-treated rats (CGS) group rats were given sesame oil (0.5 ml/kg, orally) (17) 1 h before contrast after 5 days of gentamicin treatment. Renal function and lipid peroxidation, myeloperoxidase (MPO), hydroxyl radical, superoxide anion, nitrite/nitrate, and inducible nitric oxide synthase (iNOS) expression were determined 6 h after the injection of contrast.
Blood biochemistry study.
Renal dysfunction was assessed by measuring rises in serum levels of blood urea nitrogen (BUN) and creatinine. Serum samples were spotted onto slides and evaluated for BUN and creatinine using a blood biochemical analyzer (DRI-CHEM 3500s; Fujifilm, Kanagawa, Japan).
Histological evaluation of renal injury.
Renal injury was further assessed by histological examination. Briefly, organ tissues were fixed in 4% formaldehyde buffered with a phosphate solution (0.1 mol/liter, pH 7.4) at room temperature. Organ fragments were washed in phosphate buffer, dehydrated in graded concentrations of ethanol, and then embedded in paraffin. From each tissue, 4-μm thin sections were obtained and stained with hematoxylin and eosin to evaluate renal morphology (26).
The renal histopathological score was evaluated by an experienced pathologist in our institute, who was blinded to the groups when the slides were evaluated, by using the following criteria: 0, no alterations in proximal and distal tubules; 1, slight change in proximal and distal tubules; 2, mild damage to proximal and distal tubules with loss of brush border; 3, moderate proximal and distal tubular damage, loss of brush border with cellular infiltration, and hyaline deposits; 4, severe proximal and distal tubular damage, loss of brush border with cellular infiltration and hyaline deposits; and 5, total loss of proximal and distal tubular structures and hyaline deposition all over the cortex.
Protein assay.
Protein concentration in kidney tissue was determined using a protein assay dye (Bio-Rad Laboratories, Hercules, CA) and bovine serum albumin (BSA) was used as a standard.
Measuring renal lipid peroxidation level.
Kidney tissue was homogenized in Tris-HCl (20 mM, pH 7.4). Blood samples were collected in serum-separation tubes. Tissue homogenate (500 μl) was centrifuged at 2,500 × g for 10 min at 4°C. Supernatant (200 μl) was taken for lipid peroxidation measurement using a kit (Biotech, MDA-586 assay kit; Oxis International Inc., Foster City, CA), and the results were read spectrophotometrically (DU 640B; Beckman, CA) at 586 nm (14).
Measuring renal MPO activity.
Renal tissue was homogenized in 20 mM phosphate buffer (pH 7.4) and then centrifuged (15,400 × g for 10 min at 4°C). The pellet was resuspended in 1 ml of 50 mM phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. The suspension was subjected to four cycles of freezing and thawing and then centrifuged (15,400 × g for 5 min at 4°C). Supernatant (0.5 ml) was mixed with tetramethylbenzidine (0.5 ml), and the mixture was incubated for exactly 1 min. The reaction was stopped by adding 0.5 ml of 2 N H2SO4. The spectrophotometer was then used to measure the absorbance at 405 nm. MPO activity was shown as the absorbance at 405 nm/min/mg protein (23).
Measuring hydroxyl radical and superoxide anion in renal tissue.
Renal hydroxyl radical and superoxide anion were measured using a high-performance chemiluminescence (CL) analyzer (model CLA-2100; Tohoku Electronic Industrial Co. Ltd., Rifu, Japan). Briefly, 400 μl of a tissue homogenate was mixed with 200 μl of phosphate-buffered saline in a stainless dish, and then the background CL count was read for 60 s. One hundred microliters of indoxyl β-d-glucuronide or lucigenin (17 mmol/liter dissolved in phosphate-buffered saline, for determination of hydroxyl radical and superoxide anion, respectively) was injected into the machine, and CL was counted for another 1,200 s at 10-s intervals. The data were analyzed using Chemiluminescence Analyzer data acquisition software (CLA-DAS; Tohoku Electronic Industrial Co.) (13).
Measuring renal nitrite/nitrate level.
Renal levels of nitrite/nitrate, the stable metabolic product of nitric oxide, were measured using a nitrite/nitrate assay kit (Cayman Chemical, Ann Arbor, MI) (5).
Western blotting.
We loaded 50 μg of protein on 8% or 10% sodium dodecyl sulfate-polyacrylamide gels, carried out electrophoresis (SDS-PAGE; we applied 80 mA/gel until the proteins were well into the stacking gel and then 120 mA/gel until the stacking dye reached the bottom of the gel), and then transferred the gel to nitrocellulose sheets (NEN Life Science Products, Inc., Boston, MA) in a transfer apparatus (Bio-Rad) run at 1.2 A for 3 h. After we blocked the blots in 5% nonfat skim milk in Tris-buffered saline–Tween 20, we incubated the blots with primary iNOS polyclonal antibody (dilution, 1:1,000; BD Biosciences, San Diego, CA) against the target protein in 5% nonfat skim milk and then with anti-rabbit IgG conjugated with alkaline phosphatase (dilution, 1:3,000; Jackson ImmunoResearch Laboratories, Inc., Philadelphia, PA). Immunoblots were developed using alkaline phosphatase substrate solution (5-bromo-4-chloro-3-indoyl-phosphate–nitroblue tetrazolium) solution (Kirkegaard and Perry Laboratories, Inc., Baltimore, MD).
Statistical analysis.
Data are expressed as means ± standard deviations (SDs). Comparison between groups was made by using SPSS statistical software. One-way analysis of variance (ANOVA) followed by Tukey honestly significant difference (HSD) post hoc analysis was used to make pairwise comparisons between groups. Statistical significance was set at a P value of <0.05.
RESULTS
Effects of sesame oil on renal injury in contrast medium-plus-gentamicin-treated rats.
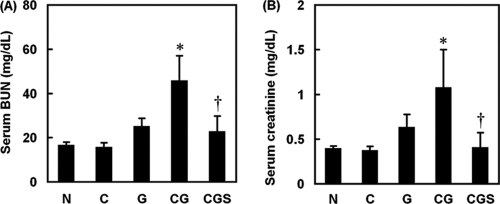
To examine the protective effects of sesame oil on contrast medium-induced renal injury, serum BUN and creatinine levels were measured. The G group, but not the C group, had mildly increased BUN and creatinine levels compared with the N group. The CG group had significantly increased serum BUN and creatinine levels compared with the G group. However, both parameters in the CGS group were significantly lower than the comparable figures in the CG group (Fig. 1A and B).
Fig. 1.
Effects of sesame oil on gentamicin-plus-iodinated contrast-induced acute kidney injury in rats. Rats were divided into five groups, and BUN (A) and creatinine (B) levels were determined 6 h after contrast injection. Data are expressed as means ± SDs (n = 6). Significant differences of measurement traits were analyzed using one-way ANOVA, followed by Tukey HSD post hoc analysis. *, P < 0.05 compared with G group; †, P < 0.05 compared with CG group.
Effects of sesame oil on renal histological changes in contrast-plus-gentamicin-treated rats.
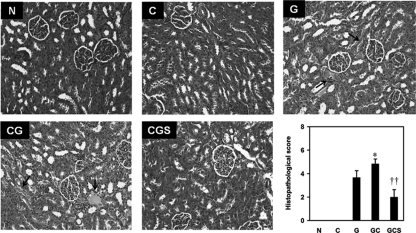
To confirm further the protective effect of sesame oil on contrast-augmented renal injury, we conducted histological examination. Histological sections from the N group showed normal histological structures of the proximal and distal tubules. Renal sections from the C group showed no significant changes in histological features. Renal sections from the G group showed hyaline deposits and loss of the brush border in tubules with mild cellular infiltration in the cortex. In the CG group, renal sections showed severe brush border loss, cellular infiltration, cellular swelling, and sloughs in the cortex. Further, renal sections from the CGS group showed less inflammatory cell infiltration, dilation, and congestion in the tubules (Fig. 2).
Fig. 2.
Effect of sesame oil on histological changes in gentamicin-plus-iodinated contrast-induced acute kidney injury. Rats were divided into five groups, and histological changes were examined 6 h after contrast injection. The arrows indicate hyaline deposits with renal tubular cell necrosis. Hematoxylin and eosin stain. Magnification = ×100. For histopathological score, data are expressed as means ± SDs (n = 6). Significant differences of measurement traits were analyzed using one-way ANOVA, followed by Tukey HSD post hoc analysis. *, P < 0.05 compared with G group; ††, P < 0.01 compared with CG group.
Effects of sesame oil on oxidative stress in contrast-plus-gentamicin-treated rats.
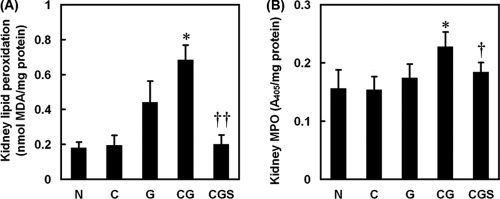
To examine the involvement of oxidative stress, we assessed the oxidative stress markers lipid peroxidation and MPO in the kidney. The G group, but not the C group, showed increased renal lipid peroxidation compared with the rats in the N group. Contrast increased the renal lipid peroxidation induced by gentamicin (CG group). Further, sesame oil significantly inhibited renal lipid peroxidation induced by the contrast and gentamicin combination in the CGS group compared with that in the CG group (Fig. 3A). On the other hand, the MPO level in the CG group was significantly higher than the levels in the other groups (Fig. 3B).
Fig. 3.
Effect of sesame oil on oxidative stress in gentamicin-plus-iodinated contrast-induced acute kidney injury. Rats were divided into five groups, and lipid peroxidation (A) and myeloperoxidase (MPO) (B) levels were determined 6 h after contrast injection. Data are expressed as means ± SDs (n = 6). Significant differences of measurement traits were analyzed using one-way ANOVA, followed by Tukey HSD post hoc analysis. MDA, malondialdehyde; *, P < 0.05 compared with G group; †, P < 0.05 compared with CG group; ††, P < 0.01 compared with CG group.
Effects of sesame oil on generation of renal reactive oxygen species in contrast-plus-gentamicin-treated rats.
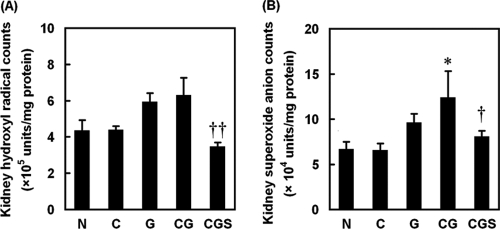
To investigate further the effect of sesame oil on contrast-augmented renal oxidative stress, we assessed renal hydroxyl radicals and superoxide anion. Renal samples from the G or CG group showed an increase in renal hydroxyl radicals, whereas the sesame oil treatment (CGS group) significantly inhibited this increase compared with that in the G or CG group (Fig. 4A). Further, sesame oil (CGS group) also inhibited renal superoxide anion generation compared with that in the CG group (Fig. 4B).
Fig. 4.
Effect of sesame oil on oxygen free radical generation in gentamicin-plus-iodinated contrast-induced acute kidney injury. Rats were divided into five groups, and renal hydroxyl radical (A) and superoxide (B) levels were determined 6 h after contrast injection. Data are expressed as means ± SDs (n = 6). Significant differences of measurement traits were analyzed using one-way ANOVA, followed by Tukey HSD post hoc analysis. *, P < 0.05 compared with G group; †, P < 0.05 compared with CG group; ††, P < 0.01 compared with CG group.
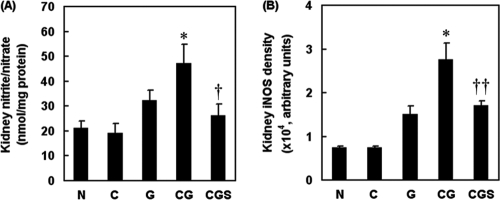
Effects of sesame oil on renal nitric oxide production in contrast-plus-gentamicin-treated rats.
To examine the involvement of nitric oxide in the protection against contrast-augmented renal injury exerted by sesame oil, we determined renal nitrite/nitrate levels and iNOS expression. The C group did not have altered renal nitrite/nitrate levels compared with those in the N group. The G group and CG group had increased renal nitrite/nitrate levels compared with the level in the N or C group, whereas sesame oil (in the CGS group) significantly inhibited the contrast-enhanced renal nitrite/nitrate level compared with that in the CG group (Fig. 5A). Further, gentamicin (G group) but not contrast medium (C group) increased renal iNOS expression compared with that in the N group. Contrast significantly increased the renal iNOS expression induced by gentamicin (CG group). Further, sesame oil (CGS group) significantly inhibited contrast-induced iNOS expression compared with that in the CG group (Fig. 5B).
Fig. 5.
Effect of sesame oil on nitric oxide and iNOS expression in gentamicin-plus-iodinated contrast-induced acute kidney injury. Rats were divided into five groups, and the renal nitrite/nitrate level (A) and iNOS expression (B) were determined 6 h after contrast injection. Data are expressed as means ± SDs (n = 6). Significant differences of measurement traits were analyzed using one-way ANOVA, followed by Tukey HSD post hoc analysis. *, P < 0.05 compared with G group; †, P < 0.05 compared with CG group; ††, P < 0.01 compared with CG group.
DISCUSSION
We demonstrated that sesame oil protected against aminoglycoside-plus-iodinated contrast-induced AKI in rats. Sesame oil reduced renal damage, oxidative stress, ROS generation, nitric oxide levels, and iNOS expression levels in rats with AKI. We suggest that sesame oil may prevent aminoglycoside-plus-iodinated contrast-induced AKI by inhibiting renal oxidative stress in rats.
Inhibiting oxidative stress may be involved in the protection against AKI exerted by sesame oil. Oxidative stress plays an important role in the pathogenesis of contrast-associated renal injury (26). Antioxidants such as ascorbic acid (32), atorvastatin (33), probucol (22), and N-acetylcysteine (NAC) (29) have shown beneficial effects against contrast medium-induced AKI. Sesame oil is a potent antioxidant in various animal models of disease, including endotoxemia (10, 11, 15), lead intoxication (12), and gentamicin-induced renal injury (17). In the present study, sesame oil potently decreased renal injury and oxidative stress. It is likely that sesame oil exerts the renal protection through the inhibition of renal oxidative stress in our AKI animal model. Further, sesame oil may decrease the oxidative stress by inhibiting the generation of hydroxyl radicals, one of the toxic free radicals. Hydroxyl radical is involved in the development of oxidative stress and in the pathogenesis of contrast-associated renal injury (6, 34). Previous studies indicated that sesame oil is a potent scavenger of hydroxyl radicals (9–11, 16). Sesame oil significantly decreased renal oxidative stress and hydroxyl radical generation in contrast-plus-gentamicin-treated rats. We suggest that the inhibition of hydroxyl radical-associated oxidative stress is at least partially involved in the sesame oil-exerted protection against AKI induced by the combination of aminoglycoside and iodinated contrast.
In addition, inhibiting superoxide anion and nitric oxide generation may be associated with sesame oil's protection against AKI. During oxidative stress, most of the hydroxyl radical is generated via the reaction of superoxide anion and nitric oxide (3, 8), whereas nitric oxide is mostly generated by iNOS (35). Sesame oil reduced renal superoxide anion generation and iNOS expression. This observation is in accord with the findings of previous studies that show that sesame oil acts as an inhibitor of iNOS expression in various oxidative stress models (12, 15, 17). Therefore, we suggest that inhibition of iNOS expression plays a crucial role in sesame oil-associated renal protection against aminoglycoside-plus-iodinated contrast-induced AKI.
Sesame oil might have the potential to prevent AKI clinically in the future. To evaluate the renoprotective effects of various compounds, the experimental AKI rat model is useful and the results can be extrapolated to clinical AKI (4, 31, 36). Few compounds have been used in clinical trials and found to have therapeutic value. For example, NAC and ascorbic acid, both of which are potent antioxidants, have been successfully used in preventing contrast-induced AKI in clinical trials (29, 30, 32). Sesame oil has been used orally in clinical trials for treating cough in 2- to 12-year-old children (5 ml/individual) (28) and small bowel obstruction (150 ml/adult individual) (19). In the present study, the effective dose of sesame oil for preventing contrast-induced AKI is about 0.12 ml/kg in human (according to body surface area calculation [27]), which is far less than that which has been used in clinical trials. In addition, sesame oil is a natural product with no reported toxicity, and it can easily be given before a scheduled imaging study involving i.v. contrast. On this basis, we suggest that the results of this study can be extrapolated to human clinical trials for preventing contrast-induced AKI. We concluded that sesame oil may prevent AKI induced by exposure to aminoglycosides and iodinated contrast by inhibiting oxidative stress in rats.
ACKNOWLEDGMENTS
This study was supported by the National Science Council Taiwan (grant 98-2312-B-006-002-MY3 to D.-Z. Hsu and grant 96-2628-B-006-038-MY3 to M.-Y. Liu).
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Bakris G., Lass N., Gaber A., Jones J., Burnett J. 1990. Radiocontrast medium-induced declines in renal function: a role for oxygen-free radicals. Am. J. Physiol. 258:F115–F120 [DOI] [PubMed] [Google Scholar]
- 2. Bakris G., Lass N., Glock D. 1999. Renal hemodynamics in radiocontrast medium-induced renal dysfunction: a role for dopamine-1 receptors. Kidney Int. 56:206–210 [DOI] [PubMed] [Google Scholar]
- 3. Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman A. 1990. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 87:1620–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cetin M., et al. 2008. Ionic high-osmolar contrast medium causes oxidant stress in kidney tissue: partial protective role of ascorbic acid. Ren. Fail. 30:567–572 [DOI] [PubMed] [Google Scholar]
- 5. Ding J., Song D., Ye X., Liu S. F. 2009. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J. Immunol. 183:4031–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ebina Y., et al. 1986. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J. Natl. Cancer Inst. 76:107–113 [PubMed] [Google Scholar]
- 7. Gossrau R., Gunther T., Graf R. 1989. Enhancement of gentamicin-induced nephrotoxicity by Mg deficiency in non-pregnant rats. Histochemistry 90:489–496 [DOI] [PubMed] [Google Scholar]
- 8. Gross S. S., Wolin M. S. 1995. Nitric oxide: pathophysiological mechanisms. Annu. Rev. Physiol. 57:739–769 [DOI] [PubMed] [Google Scholar]
- 9. Hsu D. Z., Liu M. Y. 2002. Sesame oil attenuates multiple organ failure and increase survival rate during endotoxemia in rats. Crit. Care Med. 30:1859–1862 [DOI] [PubMed] [Google Scholar]
- 10. Hsu D. Z., Liu M. Y. 2004. Effects of sesame oil on oxidative stress after the onset of sepsis in rats. Shock 22:582–585 [DOI] [PubMed] [Google Scholar]
- 11. Hsu D. Z., Liu M. Y. 2004. Sesame oil protects against lipopolysaccharide-stimulated oxidative stress in rats. Crit. Care Med. 32:227–231 [DOI] [PubMed] [Google Scholar]
- 12. Hsu D. Z., Chen K. T., Chu P. Y., Li Y. H., Liu M. Y. 2007. Sesame oil protects against lead-plus-lipopolysaccharide-induced acute hepatic injury. Shock 27:334–337 [DOI] [PubMed] [Google Scholar]
- 13. Hsu D. Z., Chen K. T., Li Y. H., Chuang Y. C., Liu M. Y. 2006. Sesamol delays mortality and attenuates hepatic injury after cecal ligation and puncture in rats: role of oxidative stress. Shock 25:528–532 [DOI] [PubMed] [Google Scholar]
- 14. Hsu D. Z., et al. 2005. Effect of sesame oil on oxidative-stress-associated renal injury in endotoxemic rats: involvement of nitric oxide and proinflammatory cytokines. Shock 24:276–280 [DOI] [PubMed] [Google Scholar]
- 15. Hsu D. Z., et al. 2008. Sesame oil attenuates hepatic lipid peroxidation by inhibiting nitric oxide and superoxide anion generation in septic rats. JPEN J. Parenter. Enteral Nutr. 32:154–159 [DOI] [PubMed] [Google Scholar]
- 16. Hsu D. Z., Li Y. H., Chien S. P., Liu M. Y. 2004. Effects of sesame oil on oxidative stress and hepatic injury after cecal ligation and puncture in rats. Shock 21:466–469 [DOI] [PubMed] [Google Scholar]
- 17. Hsu D. Z., Liu C. T., Li Y. H., Chu P. Y., Liu M. Y. 2010. Protective effect of daily sesame oil supplement on gentamicin-induced renal injury in rats. Shock 33:88–92 [DOI] [PubMed] [Google Scholar]
- 18. Itoh Y., Yano T., Sendo T., Oishi R. 2005. Clinical and experimental evidence for prevention of acute renal failure induced by radiographic contrast media. J. Pharmacol. Sci. 97:473–488 [DOI] [PubMed] [Google Scholar]
- 19. Ji Z. L., et al. 2010. Therapeutic value of sesame oil in the treatment of adhesive small bowel obstruction. Am. J. Surg. 19:160–165 [DOI] [PubMed] [Google Scholar]
- 20. Katholi R. E., et al. 1998. Oxygen free radicals and contrast nephropathy. Am. J. Kidney Dis. 32:64–71 [DOI] [PubMed] [Google Scholar]
- 21. Katzberg R. W., Haller C. 2006. Contrast-induced nephrotoxicity: clinical landscape. Kidney Int. Suppl. 100:S3–S7 [DOI] [PubMed] [Google Scholar]
- 22. Li G., et al. 2008. Role of probucol in preventing contrast-induced acute kidney injury after coronary interventional procedure. Am. J. Cardiol. 103:512–514 [DOI] [PubMed] [Google Scholar]
- 23. Liu F. C., Day Y. J., Liou J. T., Lau Y. T., Yu H. P. 2008. Sirtinol attenuates hepatic injury and pro-inflammatory cytokine production following trauma-hemorrhage in male Sprague-Dawley rats. Acta Anaesthesiol. Scand. 52:635–640 [DOI] [PubMed] [Google Scholar]
- 24. McCullough P. A. 2008. Multimodality prevention of contrast-induced acute kidney injury. Am. J. Kidney Dis. 51:169–172 [DOI] [PubMed] [Google Scholar]
- 25. Oliveira J. F. P., et al. 2009. Prevalence and risk factors for aminoglycoside nephrotoxicity in intensive care units. Antimicrob. Agents Chemother. 53:2887–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parlakpinar H., et al. 2005. Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology 207:169–177 [DOI] [PubMed] [Google Scholar]
- 27. Reagan-Shaw S., Nihal M., Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J. 22:659–661 [DOI] [PubMed] [Google Scholar]
- 28. Saab B. R., Pashayan N., El-Chemaly S., Sabra R. 2006. Sesame oil use in ameliorating cough in children: a randomised controlled trial. Complement Ther. Med. 14:92–99 [DOI] [PubMed] [Google Scholar]
- 29. Sar F., et al. 2010. The efficacy of N-acetylcysteine in preventing contrast-induced nephropathy in type 2 diabetic patients without nephropathy. J. Nephrol. 23:478–482 [PubMed] [Google Scholar]
- 30. Shavit L., Korenfeld R., Lifschitz M., Butnaru A., Slotki I. 2009. Sodium bicarbonate versus sodium chloride and oral N-acetylcysteine for the prevention of contrast-induced nephropathy in advanced chronic kidney disease. J. Interv. Cardiol. 22:556–563 [DOI] [PubMed] [Google Scholar]
- 31. Šochmana J., et al. 2010. N-Acetylcysteine attenuates iodine contrast agent-induced nephropathy in 5/6-nephrectomized rats. Kidney Blood Press Res. 33:149–156 [DOI] [PubMed] [Google Scholar]
- 32. Spargias K., et al. 2004. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 110:2837–2842 [DOI] [PubMed] [Google Scholar]
- 33. Toso A., et al. 2009. Usefulness of atorvastatin (80 mg) in prevention of contrast-induced nephropathy in patients with chronic renal disease. Am. J. Cardiol. 105:288–292 [DOI] [PubMed] [Google Scholar]
- 34. Tumlin J., et al. 2006. Pathophysiology of contrast-induced nephropathy. Am. J. Cardiol. 98:14–20 [DOI] [PubMed] [Google Scholar]
- 35. Yasmin W., Strynadka K. D., Schulz R. 1997. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc. Res. 33:422–432 [DOI] [PubMed] [Google Scholar]
- 36. Yenicerioglu Y., et al. 2006. Effects of N-acetylcysteine on radiocontrast nephropathy in rats. Scand. J. Urol. Nephrol. 40:63–69 [DOI] [PubMed] [Google Scholar]