Abstract
HIV-1 RNase H breaks down the intermediate RNA-DNA hybrids during reverse transcription, requiring two divalent metal ions for activity. Pyrimidinol carboxylic acid and N-hydroxy quinazolinedione inhibitors were designed to coordinate the two metal ions in the active site of RNase H. High-resolution (1.4 Å to 2.1 Å) crystal structures were determined with the isolated RNase H domain and reverse transcriptase (RT), which permit accurate assessment of the metal and water environment at the active site. The geometry of the metal coordination suggests that the inhibitors mimic a substrate state prior to phosphodiester catalysis. Surface plasmon resonance studies confirm metal-dependent binding to RNase H and demonstrate that the inhibitors do not bind at the polymerase active site of RT. Additional evaluation of the RNase H site reveals an open protein surface with few additional interactions to optimize active-site inhibitors.
INTRODUCTION
HIV-1 reverse transcriptase (RT) performs an integral role in virus replication and thus is a main target of current antiretroviral treatment. RT is a multifunctional enzyme that contains both polymerase and RNase H activities to convert the single-stranded viral RNA genome into a double-stranded DNA (dsRNA) product ready for integration. The RNase H activity is required for a number of steps in viral replication, including the generation of the polypurine tract primer, the subsequent removal of the polypurine tract and the tRNALys3 primers, and obligatory primer strand transfers (9, 56). Likewise, an important role for RNase H is the removal of the genomic RNA during the synthesis of double-stranded DNA (54, 55). Currently, two classes of RT inhibitors are used clinically. Nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) compete with the natural deoxynucleoside triphosphate (dNTP) for DNA incorporation by HIV-1 RT. After incorporation, they act as chain terminators (16). Nonnucleoside reverse transcriptase inhibitors (NNRTIs) bind to an allosteric site approximately 10 Å from the polymerase active site and disrupt RT polymerase function (44). Therapeutics targeting RNase H could be a complementary approach to the current standard of care.
Structurally, HIV-1 RT is a heterodimer originating from two molecules of a virally encoded p66 polypeptide. During viral maturation, one C-terminal p15 domain is cleaved by HIV-1 protease to produce the functional p66/p51 heterodimer. In this heterodimer, the enzymatically inactive p51 subunit serves as a scaffold for the p66 subunit that is responsible for both the polymerase and RNase H activity (Fig. 1A). With respect to a bound RNA-DNA hybrid substrate, these active sites are separated by a distance of 18 bp (45). The polymerase domain of p66 is responsible for most binding contacts with polynucleotide substrate, hence being responsible for the positioning of substrate for the RNase H active site (3, 5, 24, 42, 43, 45). This is underscored by the lack of enzymatic activity of the isolated RNase H p15, which has been largely attributed to the lack of guiding polymerase domain contacts. In the context of the isolated domain, various methods have been employed to restore activity, relying on the introduction of substrate binding sites. It has been shown that the addition of N- or C-terminal polyhistidine tags, often used in protein purification techniques, can restore activity (15, 47); the positive charge(s) of the tag likely attracts the negatively charged substrate. Similarly, epitope tags have been employed at the C terminus (49). Another creative method for restoring activity was derived from analysis of the primary sequence and crystal structures of HIV-1 RNase H and Escherichia coli RNase H. In E. coli RNase H, there is a sequence insertion which has been referred to as the basic helix-loop or basic protrusion handle (Fig. 1A and B) (26, 48). When HIV-1 p15 was engineered to contain this sequence insertion (p15-Ec), RNase H activity was restored, presumably through more efficient substrate binding. One significant caveat to all methods for restoring activity is that the enzyme exhibits activity only with manganese(II) and not with physiological magnesium(II).
Fig. 1.
(A) Ribbon diagram of HIV-1 RT with compound 2, p15-Ec with compound 1, and apo E. coli RNase H (PDB identifier 1RNH). The RT heterodimer is shown with p51 in gray, p66 palm, finger, thumb, and connection subdomains are shown in yellow, and the RNase H domain is shown in green. Both p15-Ec and E. coli proteins are colored green, with residues corresponding to the E. coli basic helix-loop, colored blue. RT polymerase and RNase H active-site residues are shown as spheres. For bound compounds and active-site residues, carbons are colored green, oxygens red, and nitrogens blue. Compounds 1 and 2 are drawn as spheres with Mn2+ bound. All figures of protein structures were generated with PyMOL (www.pymol.org). (B) Primary sequence alignment of HIV-1 p15 RNase H, E. coli RNase H, and the chimera RNase H p15-Ec. Active-site residues are shown in red, and the conserved histidine is shown in green. The E. coli basic helix-loop sequence that was inserted into HIV-1 p15 is shown in blue, and residues removed from p15 are highlighted in gray. (C) Structures of RNase H active-site inhibitors.
HIV-1 RNase H, polymerase, and integrase are known to utilize two metals, A and B, for catalysis (12, 22, 50). The most detailed structural knowledge of the RNase H dual-metal mechanism is derived from high-resolution cocrystal structures of Bacillus halodurans RNase H with RNA-DNA hybrids at different stages along the reaction pathway of phosphodiester hydrolysis (36, 38). Metal A is involved with coordinating and activating a water molecule to act as the nucleophile in an SN2-like reaction mechanism. Metal B fulfills many roles, including destabilizing the enzyme-substrate complex, stabilizing the pentavalent transition state of the scissile phosphate, and coordinating the nascent 3′-OH of the hydrolysis product. Also, it has been observed that the distance between these metals changes at different stages of the hydrolysis reaction. From approximately 4.0 Å in the substrate complex, the metals move to 3.5 Å apart in the transition state, before separating to 4.8 Å in the product complex.
There have been several reports of inhibitors that target the RNase H activity of HIV-1 RT (4, 6–8, 10, 13, 18, 27, 28, 46, 51, 53, 58, 60). To date, there have been no reports of RNase H inhibitors advancing into clinical development, despite early hits in biochemical experiments (2, 29, 57). We report here the crystal structures and biochemical evaluation of two metal-binding pharmacophores, pyrimidinol carboxylic acids and N-hydroxy quinazolinediones, which target HIV-1 RNase H (Fig. 1C). Crystal structures of the p15 RNase H protein engineered with the basic helix-loop from E. coli RNase H were determined with both chemical classes. Also, a structure of RT was derived with the NNRTI nevirapine and a pyrimidinol carboxylic acid bound in the RNase H active site. Surface plasmon resonance (SPR) was utilized to confirm the preference for these inhibitors to bind to the RNase H active site over the polymerase active site of RT.
MATERIALS AND METHODS
Protein expression and purification.
Residues 427 to 560 of HIV-1 RT were used to construct the isolated p15 protein. E. coli RNase H residues T79 to D102 were inserted between I506 and L517 of HIV-1 RNase H, and residues 507 to 516 from HIV-1 RNase H were removed in accordance with previous reports (26, 48). Figure 1B shows the sequence comparison of HIV-1 RNase H and E. coli RNase H, including the final amino acid sequence used in this study. This construct is termed p15-Ec to denote the E. coli basic helix-loop inserted into the p15 sequence. The construct was cloned into the pET30b vector (Novagen) and expressed in E. coli.
Overexpression of p15-Ec was induced with IPTG (isopropyl-β-d-thiogalactopyranoside) at 37°C. Cells were harvested by centrifugation after 3 h and were resuspended in buffer containing 20 mM Tris (pH 7.5), 10% glycerol, and 1 mM dithiothreitol (DTT) followed by lysis with a Microfluidizer (Microfluidics). Centrifugation removed the nonsoluble cell lysate from the soluble fraction containing p15-Ec. The soluble lysate was passed over an SP HiTrap column (GE Healthcare) and eluted with the above-described buffer containing 1 M NaCl in a linear gradient. Fractions containing p15-Ec were pooled and mixed with a highly concentrated (NH4)2SO4 and glycerol buffer to bring the final concentration of buffer components to 1.5 M (NH4)2SO4, 10% glycerol, 25 mM Tris (pH 7.5), 10 μM ZnSO4, and 1 mM DTT. The sample was loaded onto a butyl Sepharose HiTrap column (GE Healthcare) and eluted with a linear gradient in a buffer containing 25 mM Tris (pH 7.5), 10% glycerol, 10 μM ZnSO4, and 1 mM DTT. Last, the sample was passed over a KW2003 gel filtration column (GE Healthcare) in a 10 mM HEPES (pH 7.5) buffer. The purified sample was collected and concentrated to 25 mg/ml for crystallization trials.
The RT p66/p51 heterodimer was expressed and purified as described previously (31, 61).
RNase H enzymatic assay.
The polymerase-independent RNase H cleavage assay was performed as previously described (27, 41). The substrate was identical to that described except that the 18-nucleotide (nt) RNA strand had 3′-DABCYL [4-(4-dimethylaminophenylazo)benzoic acid] while the DNA strand had 5′-FAM (6-carboxyfluorescein). Reactions were initiated by mixing 50 μl of RT (or human RNase H) in reaction buffer (50 mM Tris [pH 8.0], 50 mM KCl, 5 mM MgCl2, 1 mM DTT) with 50 μl of substrate in reaction buffer. The final reaction mix (100 μl) contained 50 mM Tris (pH 8.0), 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 1% dimethyl sulfoxide (DMSO), 200 nM substrate, and either 3 nM RT or 10 nM human RNase H. All compound stocks were prepared in DMSO. To generate dose response curves, serially diluted compounds were preincubated with RT for 2 min prior to initiating the reaction. Assays were performed in kinetic mode over a 15-min incubation period at 37°C.
Surface plasmon resonance binding assay.
All surface plasmon resonance (SPR) experiments were performed using a GE T100 system (GE Healthcare). RT and p15-Ec were minimally biotinylated and captured onto a CM5 sensor chip to which Neutravidin had been coupled using standard amine methodology. Neutravidin immobilization levels were typically ∼11,000 response units (RUs), and the captured protein levels were ∼10,000 for the RT wild-type (WT) and mutant proteins and ∼3,000 for the p15-Ec proteins. All unused biotin sites on the Neutravidin surface were blocked using polyethylene oxide (PEO)-biotin after capture of the RT and p15-Ec proteins. The reference surface (Fc1) was coupled with Neutravidin, blocked with biotin, and used during data analysis for data normalization of bulk refractive effects. Experiments were performed in 10 mM HEPES (pH 7.5), 100 mM NaCl, 0.01% p20, and 3% DMSO, with 10 mM MgCl2, 3 mM EDTA, or 2 mM MnCl2 included. All experiments were performed at a temperature of 25°C. Surfaces were regenerated with a 15-s pulse of buffer containing 3 mM EDTA. Data processing included double referencing using the reference surface and blanks included throughout the run. The kinetic and binding parameters were extracted using a global fit of a 1:1 binding model performed with Scrubber software (Biologic Software Pty Ltd., Camberra, Australia).
Protein crystallization.
All crystals were grown by the hanging drop vapor diffusion method at 20°C. Cocrystals with compound 1 were obtained by combining p15-Ec at 20 mg/ml with 5 mM MnCl2, 5 mM Tris(2-carboxyethyl) phosphine (TCEP), and 1.5 mM compound 1. This solution was mixed in equal parts with mother liquor containing 1.8 M (NH4)2SO4, 100 mM HEPES (pH 7.5), and 3% polyethylene glycol (PEG) 400. Crystals were cryocooled by introducing them into a solution containing the mother liquor plus 25% glycerol, and then they were placed into a stream of nitrogen vapor at 100 K. Compound 3 was cocrystallized with p15-Ec by mixing 20 mg/ml protein with 5 mM MnCl2, 5 mM TCEP, and 1.5 mM compound 3. Crystals were obtained by mixing equal parts of the protein-inhibitor complex with 15% PEG 3350, 100 mM HEPES (pH 7.5), and 200 mM LiSO4. Crystals of this complex grew remarkably fast, often visible in less than 1 min after mixing the protein-Mn2+-inhibitor solution with well solution. A solution containing the mother liquor plus 20% glycerol was used as a cryoprotectant. Crystals were introduced into this solution and then plunged into liquid nitrogen and stored for data collection.
RT was crystallized by mixing protein at 20 mg/ml with 0.4 mM nevirapine, 5 mM MnCl2, 5 mM TCEP, and 1 mM compound 2. Crystallization mother liquor was 1.3 M (NH4)2SO4, 5 mM sodium malonate, and 100 mM cacodylate (pH 6.5). Crystals were cryocooled by adding 30% glycerol to the crystallization mother liquor and flash cooling in liquid nitrogen. Crystals were then stored for data collection.
Data collection and phase determination.
The X-ray source for each data set is listed in Table 1. All diffraction data were processed with HKL2000 (39). The structure of apo p15-Ec was determined by molecular replacement using the program AMoRe (34). The crystal structure of the isolated p15 domain of RT was used as a search model (Protein Data Bank [PDB] identifier 1HRH) to determine the structure of p15-Ec–Mn2+–compound 1. This structure was subsequently used as a search model for the p15-Ec–Mn2+–compound 3 structure. The structure of RT with nevirapine and compound 2 was determined by molecular replacement with PDB identifier 1VRT.
Table 1.
X-ray data collection and refinement statistics
| Data collection and refinement statistics | Value/result |
||
|---|---|---|---|
| p15-Ec–Mn2+–compound 1 | p15-Ec–Mn2+–compound 3 | RT-Mn2+-compound 2 | |
| Data collection | |||
| X-ray source | Rotating Cu anode | ALS BL5.0.1 | ALS BL5.0.2 |
| Wavelength (Å) | 1.54 | 0.98 | 1.00 |
| Space group | I212121 | P212121 | C2221 |
| Unit cell: a, b, c (Å) | 38.8, 90.4, 112.9 | 42.9, 56.4, 64.0 | 118.9, 154.9, 153.1 |
| Resolutiona (Å) | 50.0–1.7 (1.73–1.70) | 50.0–1.4 (1.43–1.40) | 50.0–2.1 (2.14–2.10) |
| No. of observations | 66,404 | 162,767 | 463,180 |
| No. of unique reflections | 21,540 | 30,610 | 82,059 |
| I/σa | 10.6 (3.1) | 19.3 (1.7) | 29.3 (2.1) |
| Rmergea,b (%) | 5.1 (33.0) | 6.0 (49.7) | 4.6 (50.0) |
| Completenessa (%) | 97.0 (95.0) | 98.0 (83.1) | 98.9 (87.9) |
| Refinement statistics | |||
| Resolution (Å) | 30.0–1.7 | 30.0–1.4 | 30.0–2.1 |
| No. of reflections (F ≥ 0) | 20,866 | 29,060 | 76,324 |
| Rcrystc | 22 | 19.6 | 21.6 |
| Rfreec | 25.3 | 22.8 | 26.7 |
| RMS bond lengths (Å) | 0.008 | 0.005 | 0.008 |
| RMS bond angles (°) | 1.14 | 0.93 | 1.09 |
Values in parentheses represent the highest-resolution shell.
Rmerge = [∑h∑i|Ih − Ihi|/∑h∑iIhi], where Ih is the mean of Ihi observations of reflection h.
Rcryst and Rfree = ∑||Fobs| − |Fcalc||/∑|Fobs| × 100 for 95% of recorded data (Rcryst) or 5% of data (Rfree).
Model building and refinement.
Rigid body, simulated annealing, energy minimization, and B-factor refinement were performed with the refinement package PHENIX (1). Model building was carried out with the molecular graphics programs O (25) and Coot (14). The final R-factor and Rfree values for the refined structures are listed in Table 1.
Protein structure accession numbers.
Atomic coordinates and structure factors have been deposited in the Protein Data Bank. Accession numbers are 3QIN, 3QIO, and 3QIP for compound 1 bound to p15-Ec, compound 3 bound to p15-Ec, and HIV-1 RT with compound 2, respectively.
RESULTS
Both active sites of RT exert their respective activity via dual metal coordination. Hence, a physical separation of the two enzymes is desirable for detailed mechanistic studies of a metal-chelating inhibitor. In this report, the isolated p15 RNase H domain from HIV-1 RT (residues 427 to 560) was engineered with the E. coli RNase H basic helix-loop insertion (Fig. 1) to restore enzymatic activity as described previously (26, 48). We refer to this chimeric protein as p15-Ec to denote the p15 RNase H domain containing the inserted E. coli amino acid sequence (see Materials and Methods). RNases H contain a spatially conserved active-site tetrad of carboxylate-containing amino acids (DEDD) (36). In the case of HIV-1 RNase H, these active-site residues are D443, E478, D498, and D549. Additionally, H539 plays an important role in catalysis and is highly conserved among RNase H from diverse organisms (36, 38, 56). Also, the metal nomenclature used here is consistent with that of previous structural RNase H efforts; thus, metal A activates the water nucleophile and metal B coordinates to the nascent 3′ hydroxyl group (36, 38).
Cocrystal structure of p15-Ec with a pyrimidinol carboxylic acid.
Pyrimidinol carboxylic acids have previously been explored as inhibitors of hepatitis C virus (HCV) NS5B polymerase, which were proposed to coordinate two metals in the active site (30). Structurally, this chemical class is related to raltegravir, which targets HIV integrase (52). The crystal structure of the prototype foamy virus (PFV) integrase in complex with donor DNA and raltegravir confirmed that this chemical pharmacophore coordinates two magnesium or manganese ions in the active site of integrase (21).
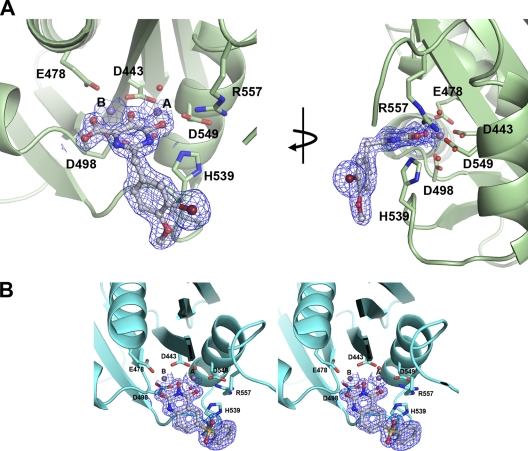
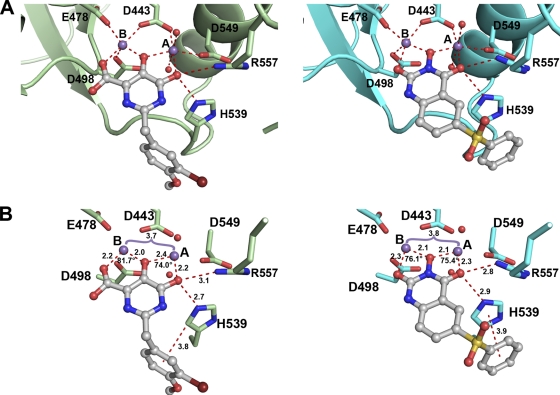
In this report, the cocrystal structure of p15-Ec with a pyrimidinol carboxylic acid (compound 1) was determined in the presence of manganese at a resolution of 1.7 Å (Fig. 2). In this complex, the coordination sphere of metal A is octahedral, with two planar coordination sites satisfied by the 5,6-hydroxypyrimidine and the remaining two satisfied by the side chain of oxygens of D443 and D549. At this resolution, two tightly bound water molecules which occupy the apical sites to metal A are observed. The distances between metal A and coordinating oxygen atoms range from 2.1 Å to 2.4 Å. In contrast, metal B is coordinated by five oxygen atoms: three from D443, E478, and D498 and two from compound 1. The distances between ligands and metal B range from 2.0 to 2.3 Å, similar to those observed for metal A. While the octahedral coordination geometry of metal A is considered to be ideal, metal B's pentacoordinate geometry is strained (19). The similarity with the B. halodurans RNase H RNA-DNA substrate complex (36) is striking: not only are the coordination geometries for both metals similar, but water coordinates to metal A in both cases, whereas metal B has no interactions with water.
Fig. 2.
Crystal structure of p15-Ec with manganese and electron density of compound 1 or 3. (A) p15-Ec is colored green, with active-site amino acid side chains that coordinate metal shown. Compound 1 is colored gray, and two manganese atoms, labeled A and B, are shown as purple spheres. Two tightly bound water atoms to metal A are shown as red spheres. The simulated annealing omit map electron density is shown as blue mesh around compound 1 and contoured at 1.0σ. The panel on the right shows the structure rotated about the y axis. (B) Stereodiagram of p15-Ec with manganese and compound 3. The omit map is shown in blue mesh and contoured at 1.0σ (standard deviation).
The 2-benzyl substituent of compound 1 was found to increase specificity and activity for this series (27). The conformational flexibility conferred by the methylene linker enables an interaction between the second aromatic ring and the loop containing H539, effectively locking the loop in position. This locked conformation is similar to structural elements observed in RT complexes with dsDNA or RNA-DNA hybrids (24, 45). The benzyl ring forms an edge-on π stacking interaction with the H539 side chain (Fig. 3) in which the center of the ring is 3.7 Å away from the edge of H539. Additional interactions are made between compound 1 and the protein. The 6-hydroxy oxygen atom of the chelator is 2.7 Å from the Nε2 of the H539 side chain, indicative of a hydrogen bond. Also in close proximity to this outer oxygen of compound 1 is R557. R557 interacts with D549 in a head-to-head fashion, akin to the behavior observed in the recently reported structure of β-thujaplicinol bound to isolate HIV-1 RNase H (23). Due to the close proximity of the R557 side chain to the active site, a nitrogen from the guanidinium head group of R557 is 3.1 Å from the 6-hydroxy oxygen atom of compound 1.
Fig. 3.
Structure comparison of p15-Ec in complex with pyrimidinol carboxylic acid and N-hydroxy quinazolinedione inhibitors. (A) Compounds 1 (left) and 3 (right) are observed binding to both active-site metals. The contacts made between metals and protein, water, or inhibitor are shown as dashed lines. (B) Distances and angles observed for the chelator pharmacophore in the vicinity of the metals.
Cocrystal structure of p15-Ec with an N-hydroxy quinazolinedione.
The N-hydroxy quinazolinedione structure class closely resembles N-hydroxyimides reported in early efforts to inhibit the RNase H function of HIV-1 RT (28). In accordance with the earlier structure-activity relationship (SAR) for N-hydroxyimides, the presence of at least one hydrogen atom at the α-carbonyl position was deemed necessary. In addition, the introduction of the second nitrogen to form the quinazoline ring was reasoned to reduce electrophilicity at the carbonyl group, a possible source of compound instability (27). This class of compounds has also been described as inhibitors of FLAP endonuclease, a mechanistically related enzyme (59).
Compound 3 was cocrystallized with p15-Ec in a similar fashion as compound 1, utilizing manganese as the divalent metal. While the protein-Mn2+-inhibitor complex with compound 3 crystallized under different buffer conditions and in a different space group than compound 1, the overall protein structures are nearly identical, with a root mean square deviation (RMSD) of 0.39 Å between p15-Ec structures. Analysis of p15-Ec with bound compound 3 illustrates that the 3-hydroxy-H-quinazoline-2,4-dione scaffold coordinates two manganese ions the same way as compound 1 (Fig. 2). Metal A maintains an octahedral coordination sphere created by oxygen atoms from the inhibitor, D443 and D549, and two water molecules. Metal B is pentacoordinated, interacting only with inhibitor and protein atoms. Also, R557 is observed interacting with D549 and the oxygen at the 4 position of compound 3 (2.8 Å), similar to compound 1.
Whereas compound 1 incorporates a carboxylic acid for coordination to metal B, the scaffold of compound 3 contains a carbonyl in place of the carboxylic acid, resulting in a subtle difference in coordination geometry (Fig. 3). The distances between metal B and the coordinating oxygens are similar for both inhibitors, 2.2 Å and 2.3 Å for compounds 1 and 3, respectively. However, the angle generated between the outer coordinating oxygen, metal B, and the central coordinating oxygen is 82° for compound 1 but is reduced to 76° for compound 3. In contrast, the corresponding geometry at metal A is relatively unchanged (74 to 75°) between the structures. Similarly to compound 1, a phenyl-containing substituent is attached to compound 3, which engages H539 but in a significantly different way. Unlike the edge-on π interaction for compound 1, the phenyl substituent of compound 3 interacts in a parallel displaced arrangement with the imidazole group of the amino acid side chain. In other words, the two π ring systems lie parallel to each other when compound 3 is bound to RNase H.
Structure of a pyrimidinol carboxylic acid with RT.
To help confirm that these inhibitors target the RNase H active site in the context of RT, a structure of compound 2 was derived with RT in complex with the NNRTI nevirapine. Despite many attempts, crystallization of RT alone with compound 2 did not yield crystals. The crystallization of RT is greatly enhanced in the presence of NNRTIs; occupancy of the allosteric pocket near the polymerase active site apparently primes the system for conformational rigidity. Analysis of several published crystal structures of HIV-1 RT with bound NNRTIs reveals that the RNase H domains generally superimpose well, although the C-terminal helix is occasionally disordered (40). A modified crystallization strategy was successful when nevirapine, MnCl2, and compound 2 were included in the crystallization mother liquor (Materials and Methods). The structure of the RT-nevirapine complex reveals electron density consistent with compound 2 binding in the RNase H active site and coordinating two manganese ions. The electron density for the pyrimidinol chelator is good, although it is much weaker for the 2-phenyl indole substituent (Fig. 4A). This indicates movement of the pendant group and the possibility that variable conformations are adopted in the crystal; no specific interactions are evident between the phenyl moiety and the protein. Similarly to the higher-resolution structures with p15-Ec, the electron density around the metal A indicates that there are two waters closely coordinated. It is noteworthy that in this space group for RT crystals, the crystal packing brings two RNase H domains from neighboring proteins into close proximity. It is possible that the binding conformation of the 2-phenyl indole group is affected by these crystal contacts. Overall, this structure still agrees well with compound 1 bound to p15-Ec (Fig. 4B).
Fig. 4.
Structure of RT with compound 2 bound in the RNase H active site. (A) Simulated annealing omit electron density map contoured at 1.0σ around compound 2 and Mn2+. RT is colored yellow. (B) Comparison of compound 2 binding to RT and compound 1 binding to p15-Ec (green). (C) Comparison of compound 2 with β-thujaplicinol (PDB identifier 3IG1; brown) and MK2 (PDB identifier 3LP1; gray) bound to RNase H of RT.
Biochemical evaluation.
Compounds 1 to 3 were assayed in an RNase H cleavage assay (41) in the presence of 5 mM MgCl2 (Table 2). All three inhibitors demonstrated metal-dependent inhibition of RNase H. The 50% inhibitory concentration (IC50) for compound 1 was 1.18 μM, in agreement with a previous report (27). Compounds 2 and 3 are more potent, with compound 3 showing the highest potency tested at 0.23 μM (IC50). Previously, compound 1 was tested for its ability to inhibit human RNase H1 (hRNase H) and showed no activity against the enzyme (27). Likewise, compound 3 (containing a different metal-chelating warhead) was tested, and the activity was >5 μM. Additionally, compounds 1 to 3 showed no activity against RT in polymerization assays.
Table 2.
Biochemical and biophysical dataa
| Compound | WT RT |
D185N RT |
WT p15-Ec |
D443N p15-Ec |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) (MgCl2) | ka (M−1 s−1) | kd (s−1) | KD (nM) | ka (M−1 s−1) | kd (s−1) | KD (nM) | ka (M−1 s−1) | kd (s−1) | KD (nM) | KD (nM) | |
| 1 | 1.18 ± 0.16 | 5.54 × 104 ± 1.21 × 104 | 0.016 ± 0.001 | 293 ± 81 | 3.1 × 105 ± 3.25 × 105 | 0.025 ± 0.001 | 384 ± 116 | 1.80 × 105 ± 0.85 × 105 | 0.024 ± 0.01 | 136 ± 11 | NB |
| 2 | 0.80 ± 0.06 | 1.45 × 105 ± 0.36 × 105 | 0.018 ± 0.001 | 129 ± 43 | 3.01 × 105 ± 0.42 × 105 | 0.027 ± 0.007 | 93 ± 17 | 3.44 × 105 ± 0.98 × 105 | 0.020 ± 0.004 | 59 ± 5 | NB |
| 3 | 0.23 ± 0.01 | 1.02 × 105 ± 0.25 × 105 | 0.018 ± 0.001 | 181 ± 57 | 1.49 × 105 ± 0.41 × 105 | 0.024 ± 0.002 | 171 ± 62 | 5.00 × 105 ± 3.1 × 105 | 0.070 ± 0.039 | 144 ± 11 | NB |
Values are the means ± standard deviations of results from three independent experiments. SPR data represent parameterization from globally analyzed duplicate experiments, averages and standard deviations reported from two independent experiments, or independent data processing. NB, no binding detected.
In order to study the metal-mediated binding of compounds 1 to 3 further, SPR was utilized in which protein was immobilized to a BIAcore chip. All three compounds show clear binding to wild-type RT, utilizing MnCl2 as the divalent metal, with KDs (equilibrium dissociation constants) ranging from 129 to 293 nM (Table 2). The association rate for binding to wild-type RT (ka) tracks well with the overall KD. Also, all three compounds showed comparable dissociation constants.
The binding of compounds 1 to 3 to the RT containing polymerase active-site mutation D185N was investigated next. The D185N mutation abrogates polymerase activity by altering metal binding at the polymerase active site. Since polymerase utilizes two metals for its enzymatic function, it is important to test both active sites of RT to determine where these dual metal-chelating compounds are binding. An RNase H inhibitor targeting metal binding at the RNase H active site would be expected to bind to the D185N enzyme with a similar affinity as wild-type RT. Indeed, the KD for binding to RT D185N protein is similar to that of the wild-type enzyme (Table 2), strongly arguing against binding at the polymerase site of RT.
Finally, inhibitor binding to the isolated p15-Ec RNase H domain was tested (Table 2). Fully consistent with the structural work, these compounds bind to p15-Ec with affinities similar to but slightly higher than that of RT. The RNase H active-site mutation D443N in p15-Ec was also tested to confirm whether binding of these compounds to RNase H is metal dependent (Fig. 5B). It has previously been shown that D443N prohibits RNase H activity and can affect the binding of metal-dependent inhibitors to the enzyme (33, 46). The D443N mutation also makes structural sense since D443 is the only active-site residue which interacts with both metal ions. As expected, none of these compounds showed binding to this protein. Thus, these inhibitors exhibit metal-dependent binding to the RNase H domain of RT, showing no indication of interactions at the polymerase metal-binding site.
Fig. 5.
Composite SPR analysis of inhibitor binding to RNase H active site. (A) Dose response of compound 2 binding to wild-type RT and to RT containing polymerase active-site mutation D185N. (B) Dose response of binding to isolated p15-Ec RNase H domain and to p15-Ec containing RNase H active-site mutation D443N.
Strikingly, in the SPR experiments, no binding is detected for compounds 1 to 3 in the presence of MgCl2 up to a concentration of 50 mM using either the RT or p15-Ec. This may be due to the fact that RNA-DNA substrate is not present, which may increase the affinity of the RNase H active site for the more physiologically relevant magnesium. Manganese has less stringent coordination requirements than magnesium, and thus it is often able to substitute for magnesium in divalent metal-requiring reactions and crystal structures. Cirino et al. have previously shown that for RT, RNase H can utilize either Mg2+ or Mn2+ (11). Importantly, biochemical assays measuring the IC50 of these inhibitors were performed in the presence of MgCl2, where the inhibitors show clear metal dependence for inhibition (27). Additionally, these inhibitors did not demonstrate anti-HIV activity in cell-based assays. The pyrimidinol carboxylic acids were generally highly protein bound and showed very low membrane permeability as determined by Caco-2 experiments. N-Hydroxy quinazolinediones showed improved properties but did not inhibit viral replication.
DISCUSSION
Publications by Nowotny and coworkers have helped define how RNase H enzymes interact with divalent metals and substrate (35–38). By sequestering metals from the scissile phosphate and physically blocking the active site of RNase H, compounds 1 to 3 can prevent catalysis. The distance between metals and their individual coordination spheres has been postulated to indicate different steps within the catalytic cycle (36, 38). According to this proposal, all the structures discussed here represent a stage akin to the enzyme-substrate complex, prior to catalysis, in which the metals are separated by about 4.0 Å. Metal B is coordinated by five oxygen atoms from the protein and inhibitor but not water. Metal A exhibits the ideal coordination geometry for Mn2+ (or the physiologically relevant Mg2+), with an octahedral ligand sphere and the optimal 2.0 to 2.3 Å distance to ligand oxygens (20, 32).
There have been several recent reports of inhibitors that chelate divalent metals in the active site of HIV-1 RNase H (23, 27, 29, 51). Detailed structural knowledge of how these inhibitors bind to the metals and of the specific interactions that are made with the protein is only beginning to emerge. As with the two compound classes reported here, all prior reported crystal structures of HIV-1 RNase H inhibitors were determined with manganese as a surrogate for magnesium. Overall, the published structures of RT with RNase H active-site inhibitors are similar, with the RMSD of protein backbone atoms ranging from 0.4 to 0.5 Å (Fig. 4C). The coordinations of the chelating pharmacophore to the metals A and B are similar for pyrimidinol carboxylic acid, N-hydroxy quinazolinedione, β-thujaplicinol, and naphthyridinone inhibitors. However, waters are not observed coordinating to metal A for β-thujaplicinol and naphthyridinone inhibitors. The resolution of these structures may not have permitted modeling of waters in this region. This is important, since the coordination sphere of the metals is necessary for understanding the electronic environment of a chelator binding to metal. Also, none of the reported structures have a pendant group that engages H539 via potential π-π interactions. Incorporation of these types of interactions may increase the activity against HIV-1 RNase H as they do for pyrimidinol carboxylic acids and N-hydroxy quinazolinediones.
Crystal structures of human RNase H1 (hRNase H) with RNA-DNA substrates are available (37). As expected from the high sequence similarity, the active site of hRNase H shares an architecture similar to that of HIV-1 RNase H. Yet, when tested, compounds 1 and 3 did not inhibit hRNase H. Closer examination of the hRNase H crystal structures reveals subtle differences which may account for the selectivity of these inhibitors to HIV-1 RNase H over hRNase H (Fig. 6). Compounds 1 to 3 have two significant interactions with H539: a polar interaction with the outer carbonyl when engaged to metal A and a π-π interaction with the imidazole side chain. The position of H264 in crystal structures of the human protein could interfere with the pendant groups of compounds 1 to 3 and possibly prevent binding. It should also be noted that the position of H264 may be influenced by the binding of the RNA-DNA substrate. Analysis of the primary sequence of the C-terminal residues of RNase H reveals another significant difference between HIV-1 and hRNase H. In the majority of RNase H amino acid sequences, there is an arginine residue one helical turn past the last conserved active-site aspartic acid. The hRNase H structures with RNA and DNA revealed that this arginine interacts with RNA. However, in HIV-1, at the equivalent position is S553. The structures of p15-Ec reveal that another arginine, R557, loops back and appears to substitute structurally for this otherwise conserved arginine. In the structures with p15-Ec and inhibitor, R557 interacts with D549 in a head-to-head fashion. It is not apparent that this interaction is possible in hRNase H. The shape of the C-terminal residues of HIV-1 RNase H is also different where HIV-1 amino acids loop back over for R557 to form the interaction with D549. This shape of the loop is still compatible with RNA/DNA substrate binding based on the hRNase H structure. In the RT structure with compound 2 bound, R557 is disordered. This is possibly due to the crystal packing, as mentioned above, where available space is constrained.
Fig. 6.
Comparison between p15-Ec (green) with Mn2+-compound 1 and hRNase H with Ca2+-RNA-DNA substrate (PDB identifier 2QKK; chain A; tan). Residues shown for comparison are labeled with the HIV-1 number, colored green, and hRNase H number, in parentheses and colored tan. Based on this model, there is a potential clash with H264 and the benzyl group of compound 1. The intimate coordination of the histidine and arginine to compound 1 in the HIV-1 active site is highlighted by dashed lines. This coordination would not be seen based on the hRNase H structures with substrate. Below, the primary sequence for the C-terminal portion of RNase H from selected sources is shown for comparison: HIV-1, human immunodeficiency virus 1 RT; Hs, human; MLV, Moloney murine leukemia virus RT; Ec, E. coli; XMRV, xenotropic murine leukemia virus-related virus RT; HBV, human hepatitis B virus pol; HTLV, human T-cell lymphotropic virus RT. Highlighted in yellow is an arginine which is highly conserved one helical turn away from the last active-site aspartic acid (D549 in HIV-1). HIV-1 is the outlier and contains a serine at this position (blue). To compensate, R577 loops back to interact with compound 1 and D549.
HIV-1 RNase H shares many structural features with RNases H from other viruses. Moloney murine leukemia virus (MMLV), xenotropic murine leukemia virus (XMRV), human T-cell lymphotropic virus (HTLV), and the more distantly related hepatitis B virus (HBV) contain RNase H domains on their respective polymerase proteins to break down RNA during reverse transcription (Fig. 6). While there are some overarching differences in sequence, the RNase H domain in these viruses contains the prototypical DEDD active-site motif. Targeting RNase H of HBV and other human retroviruses may be a viable strategy for drug development. It is worthwhile pointing out that the RNases H from these viruses contain the highly conserved arginine near the last active-site aspartic acid, similarly to hRNase H. It is unclear what effect this would have on developing selective inhibitors targeting active-site metals.
Lastly, the quality of the HIV-1 RNase H active site as a target for small-molecule inhibitors was evaluated by SiteMap (17). This program determines the hydrophobic, hydrophilic, and metal-binding potentials of a protein-binding pocket, and in combination with other elements, such as site enclosure, assigns a score for overall druggability of the site. In the case of RNase H, the chelation site is well defined, but the pocket is open and offers little else for small-molecule optimization (Fig. 7). Thus, despite a potential interaction between ligand and H539, for example, the lack of site enclosure minimizes its impact on binding energy. SiteMap assigned an overall score of 0.68 to the RNase H active site, in contrast to a score of 1.07 for the polymerase active site and a score of 1.29 for the NNRTI pocket. The higher score for the polymerase site reflects a very good balance between metal-binding, hydrophilic, and hydrophobic potentials. The allosteric NNRTI pocket, while almost entirely hydrophobic in character, benefits from its enclosed nature, and a drug such as nevirapine is exceptionally well molded to its distinctive butterfly shape. While there are numerous examples of surface-exposed sites being targeted by drug molecules, RNase H may represent an extreme case that proves to be a difficult challenge for further optimization.
Fig. 7.
SiteMap evaluation of RNase H active site (A), RT polymerase active site (B), and RT NNRTI binding site (C). The RNase H active site is very open and offers few potential interaction sites for a small molecule to bind other than the metal ions. In contrast, the polymerase site is a rich balance of hydrophobic and hydrophilic potentials, and the NNRTI site is a well-enclosed hydrophobic pocket.
The structures reported here shed more light on the active site of HIV-1 RNase H and the position of metals and waters. Metal chelators targeting RNase H demonstrate the feasibility of this mode of inhibition and at the same time highlight the challenges involved in increasing potency by establishing stronger interactions with protein residues in the vicinity.
ACKNOWLEDGMENTS
We thank the staff of The Advanced Light Source for help in data collection. We are grateful to Christian Callebaut, Todd Appleby, John Somoza, Swami Swaminathan, Marcos Hatada, and Mary McGrath for helpful discussions during data collection and preparation of the manuscript.
The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under contract no. DE-AC02-05CH11231.
Footnotes
Published ahead of print on 4 April 2011.
REFERENCES
- 1. Adams P. D., et al. 2010. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreola M. L., et al. 2002. HIV-1 integrase and RNase H activities as therapeutic targets. Expert Opin. Ther. Targets 6:433–446 [DOI] [PubMed] [Google Scholar]
- 3. Arts E. J., Le Grice S. F. 1998. Interaction of retroviral reverse transcriptase with template-primer duplexes during replication. Prog. Nucleic Acid Res. Mol. Biol. 58:339–393 [DOI] [PubMed] [Google Scholar]
- 4. Billamboz M., et al. 2008. Design, synthesis, and biological evaluation of a series of 2-hydroxyisoquinoline-1,3(2H,4H)-diones as dual inhibitors of human immunodeficiency virus type 1 integrase and the reverse transcriptase RNase H domain. J. Med. Chem. 51:7717–7730 [DOI] [PubMed] [Google Scholar]
- 5. Bohlayer W. P., DeStefano J. J. 2006. Tighter binding of HIV reverse transcriptase to RNA-DNA versus DNA-DNA results mostly from interactions in the polymerase domain and requires just a small stretch of RNA-DNA. Biochemistry 45:7628–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bokesch H. R., et al. 2008. HIV-1 ribonuclease H inhibitory phenolic glycosides from Eugenia hyemalis. J. Nat. Prod. 71:1634–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borkow G., et al. 1997. Inhibition of the ribonuclease H and DNA polymerase activities of HIV-1 reverse transcriptase by N-(4-tert-butylbenzoyl)-2-hydroxy-1-naphthaldehyde hydrazone. Biochemistry 36:3179–3185 [DOI] [PubMed] [Google Scholar]
- 8. Budihas S. R., et al. 2005. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 33:1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Champoux J. J. 1993. Reverse transcriptase, p. 103–117 In Skalka A. M., Goff S. P. (ed.), Reverse transcriptase. Cold Spring Harbor Laboratory Press, Plainview, NY: [PubMed] [Google Scholar]
- 10. Chung S., et al. 2010. Structure-activity analysis of vinylogous urea inhibitors of human immunodeficiency virus-encoded ribonuclease H. Antimicrob. Agents Chemother. 54:3913–3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cirino N. M., et al. 1995. Divalent cation modulation of the ribonuclease functions of human immunodeficiency virus reverse transcriptase. Biochemistry 34:9936–9943 [DOI] [PubMed] [Google Scholar]
- 12. Cowan J. A., et al. 2000. Metal-ion stoichiometry of the HIV-1 RT ribonuclease H domain: evidence for two mutually exclusive sites leads to new mechanistic insights on metal-mediated hydrolysis in nucleic acid biochemistry. J. Biol. Inorg. Chem. 5:67–74 [DOI] [PubMed] [Google Scholar]
- 13. Dat N. T., et al. 2007. A dimeric lactone from Ardisia japonica with inhibitory activity for HIV-1 and HIV-2 ribonuclease H. J. Nat. Prod. 70:839–841 [DOI] [PubMed] [Google Scholar]
- 14. Emsley P., Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 15. Evans D. B., Brawn K., Deibel M. R., Jr., Tarpley W. G., Sharma S. K. 1991. A recombinant ribonuclease H domain of HIV-1 reverse transcriptase that is enzymatically active. J. Biol. Chem. 266:20583–20585 [PubMed] [Google Scholar]
- 16. Goody R. S., Muller B., Restle T. 1991. Factors contributing to the inhibition of HIV reverse transcriptase by chain-terminating nucleotides in vitro and in vivo. FEBS Lett. 291:1–5 [DOI] [PubMed] [Google Scholar]
- 17. Halgren T. 2007. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 69:146–148 [DOI] [PubMed] [Google Scholar]
- 18. Hang J. Q., et al. 2004. Activity of the isolated HIV RNase H domain and specific inhibition by N-hydroxyimides. Biochem. Biophys. Res. Comm. 317:321–329 [DOI] [PubMed] [Google Scholar]
- 19. Harding M. M. 2001. Geometry of metal-ligand interactions in proteins. Acta Crystallogr. D Biol. Crystallogr. 57:401–411 [DOI] [PubMed] [Google Scholar]
- 20. Harding M. M. 1999. The geometry of metal-ligand interactions relevant to proteins. Acta Crystallogr. D Biol. Crystallogr. 55:1432–1443 [DOI] [PubMed] [Google Scholar]
- 21. Hare S., Gupta S. S., Valkov E., Engelman A., Cherepanov P. 2010. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 464:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haren L., Ton-Hoang B., Chandler M. 1999. Integrating DNA: transposases and retroviral integrases. Annu. Rev. Microbiol. 53:245–281 [DOI] [PubMed] [Google Scholar]
- 23. Himmel D. M., et al. 2009. Structure of HIV-1 reverse transcriptase with the inhibitor beta-thujaplicinol bound at the RNase H active site. Structure 17:1625–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang H., Chopra R., Verdine G. L., Harrison S. C. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669–1675 [DOI] [PubMed] [Google Scholar]
- 25. Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47(Pt. 2):110–119 [DOI] [PubMed] [Google Scholar]
- 26. Keck J. L., Marqusee S. 1995. Substitution of a highly basic helix/loop sequence into the RNase H domain of human immunodeficiency virus reverse transcriptase restores its Mn(2+)-dependent RNase H activity. Proc. Natl. Acad. Sci. U. S. A. 92:2740–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirschberg T. A., et al. 2009. RNase H active site inhibitors of human immunodeficiency virus type 1 reverse transcriptase: design, biochemical activity, and structural information. J. Med. Chem. 52:5781–5784 [DOI] [PubMed] [Google Scholar]
- 28. Klumpp K., et al. 2003. Two-metal ion mechanism of RNA cleavage by HIV RNase H and mechanism-based design of selective HIV RNase H inhibitors. Nucleic Acids Res. 31:6852–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klumpp K., Mirzadegan T. 2006. Recent progress in the design of small molecule inhibitors of HIV RNase H. Curr. Pharm. Des. 12:1909–1922 [DOI] [PubMed] [Google Scholar]
- 30. Koch U., et al. 2006. 2-(2-Thienyl)-5,6-dihydroxy-4-carboxypyrimidines as inhibitors of the hepatitis C virus NS5B polymerase: discovery, SAR, modeling, and mutagenesis. J. Med. Chem. 49:1693–1705 [DOI] [PubMed] [Google Scholar]
- 31. Lansdon E. B., et al. 2010. Crystal structures of HIV-1 reverse transcriptase with etravirine (TMC125) and rilpivirine (TMC278): implications for drug design. J. Med. Chem. 53:4295–4299 [DOI] [PubMed] [Google Scholar]
- 32. Maguire M. E., Cowan J. A. 2002. Magnesium chemistry and biochemistry. Biometals 15:203–210 [DOI] [PubMed] [Google Scholar]
- 33. Mizrahi V., Usdin M. T., Harington A., Dudding L. R. 1990. Site-directed mutagenesis of the conserved Asp-443 and Asp-498 carboxy-terminal residues of HIV-1 reverse transcriptase. Nucleic Acids Res. 18:5359–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navaza J. 1993. On the computation of the fast rotation function. Acta Crystallogr. D Biol. Crystallogr. 49:588–591 [DOI] [PubMed] [Google Scholar]
- 35. Nowotny M., et al. 2008. Specific recognition of RNA/DNA hybrid and enhancement of human RNase H1 activity by HBD. EMBO J. 27:1172–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nowotny M., Gaidamakov S. A., Crouch R. J., Yang W. 2005. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell 121:1005–1016 [DOI] [PubMed] [Google Scholar]
- 37. Nowotny M., et al. 2007. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol. Cell 28:264–276 [DOI] [PubMed] [Google Scholar]
- 38. Nowotny M., Yang W. 2006. Stepwise analyses of metal ions in RNase H catalysis from substrate destabilization to product release. EMBO J. 25:1924–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otwinowski Z. M., Minor W. 1997. Processing of X-ray diffraction data collection in oscillation mode, p. 307–326 In Carter C. W., Jr., Sweet R. M. (ed.), Methods in enzymology, vol. 276 Academic Press, New York, NY: [DOI] [PubMed] [Google Scholar]
- 40. Pari K., Mueller G. A., DeRose E. F., Kirby T. W., London R. E. 2003. Solution structure of the RNase H domain of the HIV-1 reverse transcriptase in the presence of magnesium. Biochemistry 42:639–650 [DOI] [PubMed] [Google Scholar]
- 41. Parniak M. A., Min K. L., Budihas S. R., Le Grice S. F., Beutler J. A. 2003. A fluorescence-based high-throughput screening assay for inhibitors of human immunodeficiency virus-1 reverse transcriptase-associated ribonuclease H activity. Anal. Biochem. 322:33–39 [DOI] [PubMed] [Google Scholar]
- 42. Rausch J. W., Le Grice S. F. 2004. ‘Binding, bending and bonding’: polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. Int. J. Biochem. Cell Biol. 36:1752–1766 [DOI] [PubMed] [Google Scholar]
- 43. Rausch J. W., Sathyanarayana B. K., Bona M. K., Le Grice S. F. 2000. Probing contacts between the ribonuclease H domain of HIV-1 reverse transcriptase and nucleic acid by site-specific photocross-linking. J. Biol. Chem. 275:16015–16022 [DOI] [PubMed] [Google Scholar]
- 44. Ren J., Stammers D. K. 2008. Structural basis for drug resistance mechanisms for non-nucleoside inhibitors of HIV reverse transcriptase. Virus Res. 134:157–170 [DOI] [PubMed] [Google Scholar]
- 45. Sarafianos S. G., et al. 2001. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20:1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shaw-Reid C. A., et al. 2003. Inhibition of HIV-1 ribonuclease H by a novel diketo acid, 4-[5-(benzoylamino)thien-2-yl]-2,4-dioxobutanoic acid. J. Biol. Chem. 278:2777–2780 [DOI] [PubMed] [Google Scholar]
- 47. Smith J. S., Roth M. J. 1993. Purification and characterization of an active human immunodeficiency virus type 1 RNase H domain. J. Virol. 67:4037–4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stahl S. J., Kaufman J. D., Vikic-Topic S., Crouch R. J., Wingfield P. T. 1994. Construction of an enzymatically active ribonuclease H domain of human immunodeficiency virus type 1 reverse transcriptase. Protein Eng. 7:1103–1108 [DOI] [PubMed] [Google Scholar]
- 49. Stammers D. K., et al. 1991. Rapid purification and characterisation of HIV-1 reverse transcriptase and RNaseH engineered to incorporate a C-terminal tripeptide alpha-tubulin epitope. FEBS Lett. 283:298–302 [DOI] [PubMed] [Google Scholar]
- 50. Steitz T. A., Smerdon S. J., Jager J., Joyce C. M. 1994. A unified polymerase mechanism for nonhomologous DNA and RNA polymerases. Science 266:2022–2025 [DOI] [PubMed] [Google Scholar]
- 51. Su H. P., et al. 2010. Structural basis for the inhibition of RNase H activity of HIV-1 reverse transcriptase by RNase H active site-directed inhibitors. J. Virol. 84:7625–7633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Summa V., et al. 2008. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 51:5843–5855 [DOI] [PubMed] [Google Scholar]
- 53. Takada K., et al. 2007. An HIV RNase H inhibitory 1,3,4,5-tetragalloylapiitol from the African plant Hylodendron gabunensis. J. Nat. Prod. 70:1647–1649 [DOI] [PubMed] [Google Scholar]
- 54. Tanese N., Telesnitsky A., Goff S. P. 1991. Abortive reverse transcription by mutants of Moloney murine leukemia virus deficient in the reverse transcriptase-associated RNase H function. J. Virol. 65:4387–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Telesnitsky A., Goff S. P. 1993. Two defective forms of reverse transcriptase can complement to restore retroviral infectivity. EMBO J. 12:4433–4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tisdale M., Schulze T., Larder B. A., Moelling K. 1991. Mutations within the RNase H domain of human immunodeficiency virus type 1 reverse transcriptase abolish virus infectivity. J. Gen. Virol. 72(Pt. 1):59–66 [DOI] [PubMed] [Google Scholar]
- 57. Tramontano E. 2006. HIV-1 RNase H: recent progress in an exciting, yet little explored, drug target. Mini Rev. Med. Chem. 6:727–737 [DOI] [PubMed] [Google Scholar]
- 58. Tramontano E., et al. 2005. 6-[1-(4-Fluorophenyl)methyl-1H-pyrrol-2-yl)]-2,4-dioxo-5-hexenoic acid ethyl ester, a novel diketo acid derivative which selectively inhibits the HIV-1 viral replication in cell culture and the ribonuclease H activity in vitro. Antiviral Res. 65:117–124 [DOI] [PubMed] [Google Scholar]
- 59. Tumey L. N., et al. 2005. The identification and optimization of a N-hydroxy urea series of flap endonuclease 1 inhibitors. Bioorg. Med. Chem. Lett. 15:277–281 [DOI] [PubMed] [Google Scholar]
- 60. Wendeler M., et al. 2008. Vinylogous ureas as a novel class of inhibitors of reverse transcriptase-associated ribonuclease H activity. ACS Chem. Biol. 3:635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White K. L., et al. 2005. A combination of decreased NRTI incorporation and decreased excision determines the resistance profile of HIV-1 K65R RT. AIDS 19:1751–1760 [DOI] [PubMed] [Google Scholar]