Abstract
In the elderly, Streptococcus pneumoniae is the most common cause of pneumonia and one of the most frequently isolated pathogens in cases of acute exacerbation of chronic obstructive pulmonary disease (AECOPD). This study was conducted to compare the pneumococcal isolates obtained during episodes of AECOPD and pneumonia in patients of ≥65 years old and to analyze whether in patients with AECOPD and pneumonia within a short interval, the same isolate caused both episodes. This laboratory-based study was performed between 2005 and 2008. Pneumococcal isolates from episodes of pneumonia (n = 401) and AECOPD (n = 398), matched one-to-one by date of isolation, were characterized. The serotypes and genotypes of other pneumococcal isolates causing pneumonia and AECOPD in the same patient were compared. In patients with pneumonia, COPD as an underlying disease was not associated with more-drug-resistant pneumococci. In contrast, isolates causing AECOPD showed higher rates of resistance than those causing pneumonia. Serotypes 1, 3, and 7F were more frequent in pneumonia. The same pneumococcus was involved in 25.7% (9/35 patients) of patients with two consecutive AECOPD episodes but in only 6.3% (2/32 patients) of COPD patients with pneumonia and exacerbation (Fisher's exact test; P = 0.047). Less invasive serotypes were isolated more often in AECOPD and were more resistant to antimicrobials. The presence of a specific pneumococcal serotype in AECOPD does not predict the etiology of subsequent pneumonia.
INTRODUCTION
The elderly are generally accepted to be more vulnerable to infections than younger people. Infectious diseases are a major cause of morbidity and mortality in the geriatric population. Increased susceptibility to infections has been attributed not only to anatomical, physiological, and/or immunological aging but also to an increase in the prevalence of chronic diseases, especially cardiovascular and pulmonary diseases (18). Pneumococcal pneumonia is the leading cause of death attributable to infectious diseases in developed countries. To prevent pneumococcal disease in people over the age of 64 years, the 23-valent polysaccharide pneumococcal vaccine (PPV23) was introduced in our region (Basque Country, northern Spain) in autumn 2007. The 7-valent pneumococcal conjugate vaccine (PCV7) for children was introduced in Spain in June 2001, but the 13-valent conjugate vaccine (PCV13) was not introduced until June 2010.
Bacterial colonization in chronic obstructive pulmonary disease (COPD) contributes to airway inflammation and modulates exacerbations. The prevalence of bacterial colonization of the airways in stable COPD is high (20, 25, 28). Most exacerbations are infectious, and Streptococcus pneumoniae is frequently found both in stable periods and in exacerbations. As a consequence of acute exacerbations, patients with COPD receive frequent courses of antimicrobial treatment, which has been associated directly with a higher prevalence of resistant pneumococci (2).
The main aim of this study was to determine whether the serotypes and antimicrobial susceptibilities of S. pneumoniae isolates causing pneumonia or acute exacerbation of COPD (AECOPD) in patients over 64 years of age from the same region and in the same period were similar. A secondary aim was to discover if S. pneumoniae isolates causing pneumonia in COPD patients were related to the isolates identified during exacerbations.
MATERIALS AND METHODS
Study design.
A prospective laboratory-based study was performed in a university hospital between January 2005 and December 2008. Each year, the first 100 S. pneumoniae isolates from patients aged ≥65 years with a diagnosis of pneumonia were included (pneumonia group). These isolates were matched one-to-one with S. pneumoniae isolates from patients of the same age with AECOPD (AECOPD group). Demographic characteristics and clinical data were obtained for the patients in the pneumonia group by reviewing their medical records. Data on comorbidities (diabetes mellitus, renal failure, liver disease, malignancy, cardiopathy, nervous system diseases, obesity, and immunodeficiencies), mortality (up to 60 days), hospitalization, and antibiotic use in the month before the episode of pneumonia were collected (Table 1).
Table 1.
Characteristics and comorbidities of patients with pneumonia, with and without COPD as an underlying disease
| Variable | Value |
P value | |
|---|---|---|---|
| Non-COPD group (230 patients) | COPD group (171 patients) | ||
| No. (%) of males | 144 (62.6) | 146 (85.4) | <0.0001 |
| Mean age (yr) (SD) | 76.8 (7.3) | 77.6 (6.1) | 0.29 |
| No. (%) of patients with comorbidity | |||
| Diabetes mellitus | 46 (20) | 38 (22.2) | 0.59 |
| Chronic renal failure | 10 (4.3) | 5 (2.9) | 0.60 |
| Chronic liver disease | 4 (1.7) | 6 (3.5) | 0.34 |
| Malignancy (solid) | 32 (13.9) | 17 (9.9) | 0.23 |
| Malignancy (hematologic) | 9 (3.9) | 4 (2.3) | 0.57 |
| Heart failure or cardiopathy | 55 (23.9) | 58 (33.9) | 0.03 |
| HIV infection | 1 (0.4) | 0 (0) | 1 |
| Rheumatoid arthritis | 2 (0.9) | 0 (0) | 0.51 |
| Nervous system diseases (Parkinson's or Alzheimer's disease) | 3 (1.3) | 1 (0.6) | 0.64 |
| Obesity | 9 (3.9) | 9 (5.3) | 0.63 |
| No. (%) of patients with hospitalization in the last month | 19 (8.3) | 21 (12.3) | 0.18 |
| No. (%) of patients with antibiotic use in the last month | 11 (4.8) | 23 (13.5) | 0.002 |
| No. (%) of patients with mortality of < 60 days | 17 (7.4) | 10 (5.8) | 0.69 |
Diagnosis of COPD was made using spirometric pulmonary function testing. For patients without recorded spirometric evaluation, the diagnosis of COPD was established according to the clinical diagnosis made by their pulmonologists. The following measures of lung function were used: forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC). All COPD patients had an FEV1/FVC ratio of <70% (postbronchodilator) and a previous clinical history of coughing and sputum production on most days for at least 3 months per year for 2 consecutive years. AECOPD was defined as intensification of preexisting dyspnea, with an increase in the sputum production volume and/or purulence.
Patients and isolates.
All patients included in the pneumonia group had radiologically confirmed pneumonia (new infiltrate on chest radiography) and an S. pneumoniae isolate recovered from blood, pleural effusion, or a lower respiratory tract specimen (bronchoalveolar lavage fluid, tracheobronchial aspirate, or sputum). Isolates obtained from the same patient were considered distinct episodes when specimens were recovered at least 2 months apart. For the AECOPD group, only expectorated sputum samples were obtained. For all sputum samples, fewer than 10 squamous epithelial cells and more than 25 polymorphonuclear cells were observed per low-power field (magnification, ×10).
All S. pneumoniae isolates were kept at −80°C, allowing all isolates from the same patient obtained on different dates to be compared. When more than one pneumococcal strain was isolated from the same patient in an interval of between 2 months and 1 year, these isolates were compared by means of serotyping, pulsed-field gel electrophoresis (PFGE), and antimicrobial susceptibility patterns. The isolate causing the pneumonia was compared with the isolate closest in time to the AECOPD episode. Pneumococci isolated during the two closest episodes of exacerbation were also compared.
Microbiological procedures.
S. pneumoniae isolates were identified on the basis of their typical colony morphology, alpha-hemolysis on blood agar, optochin sensitivity, and bile solubility. Serotyping was performed using the recently developed Pneumoarray (17), and serotypes were confirmed by the Quellung reaction, using polyclonal antisera (Statens Serum Institut, Copenhagen, Denmark), and/or by a multiplex PCR of specific capsular genes (17). PFGE was performed as previously described (16).
Antimicrobial susceptibility testing was performed by the broth microdilution method, using Clinical and Laboratory Standards Institute (CLSI) guidelines and criteria (8). An isolate was considered multidrug resistant if it was oral penicillin nonsusceptible (MIC ≥ 0.12 μg/ml) and nonsusceptible to another two or more antimicrobial classes. Ertapenem susceptibility was studied in all S. pneumoniae isolates from patients with pneumonia by use of the Etest method (AB Biodisk, Solna, Sweden).
Statistical methods.
Statistical analysis was performed using GraphPad InStat, version 3.05 (GraphPad, San Diego, CA). The chi-square test or Fisher's exact test was used to compare proportions. Means were compared by the unpaired t test. The chi-square test for trend was used to analyze trends. In all tests, P values of <0.05 were considered statistically significant.
RESULTS
Patients with pneumonia.
A total of 401 episodes of pneumococcal pneumonia corresponding to 394 patients of ≥65 years of age were included. The average age per episode was 77.1 years, and 111 (27.7%) episodes occurred in women; 304 (75.8%) isolates were obtained from lower respiratory tract specimens, 93 (23.2%) from blood, and 4 (1%) from pleural effusion.
Of the 401 pneumonias, 171 (42.6%) episodes occurred in 164 patients with COPD as an underlying disease. The remaining 230 pneumonia episodes occurred in the same number of patients without COPD (Table 1). Although both groups (with and without COPD) showed similar demographic data and comorbidities, there were more males, more patients with cardiopathy, and more instances of previous antibiotic use in the last month in the group of patients with COPD.
No significant differences were found in antimicrobial resistance rates (Table 2) or in serotype distributions of pneumococci from invasive (blood or pleural liquid) and respiratory samples for patients with pneumonia stratified according to the presence or absence of COPD. Ertapenem susceptibility testing showed that all pneumococcal isolates from patients with pneumonia were ertapenem susceptible. The ertapenem MIC50 and MIC90 were 0.006 μg/ml and 0.19 μg/ml, respectively (range, ≤0.003 μg/ml to 0.75 μg/ml).
Table 2.
Nonsusceptibility of S. pneumoniae isolates from patients with pneumonia and with AECOPD
| Antibiotic | No. (%) of nonsusceptible isolates from patients with pneumonia |
P valuea | Total no. (%) of nonsusceptible isolates |
P valueb | ||
|---|---|---|---|---|---|---|
| COPD (n = 171) | Non-COPD (n = 230) | Patients with pneumonia (n = 401) | Patients with AECOPD (n = 398) | |||
| Oral benzylpenicillin | 19 (11.1) | 35 (15.2) | 0.3 | 54 (13.5) | 81 (20.4) | 0.01 |
| Parenteral penicillin | 0 (0) | 0 (0) | 0 (0) | 4 (1) | 0.061 | |
| Amoxicillin | 3 (1.8) | 5 (2.2) | 1 | 8 (2) | 10 (2.5) | 0.64 |
| Cefotaxime | 2 (1.2) | 2 (0.9) | 1 | 4 (1) | 9 (2.3) | 0.17 |
| Ertapenemc | 0 (0) | 0 (0) | 0 (0) | NDd | ||
| Erythromycin | 24 (14) | 26 (11.3) | 0.4 | 50 (12.5) | 79 (19.8) | 0.005 |
| Azithromycin | 24 (14) | 26 (11.3) | 0.4 | 50 (12.5) | 79 (19.8) | 0.005 |
| Quinupristin-dalfopristin | 0 (0) | 1 (0.4) | 1 (0.2) | 1 (0.3) | ||
| Clindamycin | 16 (9.4) | 16 (7) | 0.5 | 32 (8) | 63 (15.8) | <0.001 |
| Tetracycline | 17 (9.9) | 20 (8.7) | 0.6 | 37 (9.2) | 71 (17.8) | <0.001 |
| Trimethoprim-sulfamethoxazole | 29 (17) | 39 (17) | 1 | 68 (17) | 79 (19.8) | 0.18 |
| Chloramphenicol | 2 (1.2) | 1 (0.4) | 3 (0.7) | 10 (2.5) | 0.055 | |
| Rifampin | 0 (0) | 1 (0.4) | 1 (0.2) | 0 (0) | ||
| Ciprofloxacin | 13 (7.6) | 14 (6.1) | 0.7 | 27 (6.7) | 38 (9.5) | 0.15 |
| Levofloxacin | 3 (1.8) | 0 (0) | 3 (0.7) | 16 (4.0) | 0.002 | |
P values between patients with pneumonia and with and without COPD as an underlying disease.
P values between all patients with pneumonia and patients with acute exacerbation of COPD.
Ertapenem susceptibility was determined by Etest.
ND, not determined.
Comparison of pneumococci isolated from episodes of pneumonia and AECOPD.
Overall, 799 nonduplicated S. pneumoniae isolates were included in this part of the study, including isolates from the above-mentioned 401 episodes of pneumonia and isolates from 398 episodes (371 patients) of AECOPD. The average age per AECOPD episode was 75.9 years, and 58 (14.6%) episodes occurred in women. Given the microbiological information, cultures were monomicrobial (only the pneumococcus was reported) in 295/398 cases (74.1%). In the remaining 103 cases (25.9%), another species besides S. pneumoniae was recorded, mainly Haemophilus influenzae (47 episodes [11.8%]) and Pseudomonas aeruginosa (27 episodes [6.8%]). However, based on Gram staining and the colony counts in culture, only pneumococcal isolates were taken into consideration.
S. pneumoniae isolates from patients with AECOPD showed higher prevalences of resistance to penicillin, erythromycin, azithromycin, clindamycin, tetracycline, and levofloxacin than did isolates from patients with pneumonia. Multidrug resistance, defined as nonsusceptibility to oral penicillin (MIC ≥ 0.12 μg/ml) and nonsusceptibility to another two antimicrobials, was found in 4% (16/401 isolates) of isolates from patients with pneumonia and in 8.3% (33/398 isolates) of those from patients with AECOPD (P = 0.01). In both groups, the most frequent multidrug resistance pattern included resistance to penicillin, erythromycin, clindamycin, and tetracycline.
From 2005 to 2008, there were significant decreases in the percentages of resistance to oral penicillin (18.6% to 10.4%; P value for trend = 0.036), trimethoprim-sulfamethoxazole (20.6% to 10.4%; P = 0.028), and ciprofloxacin (10.3% to 3.1%; P = 0.015) among isolates from patients with pneumonia; however, a decrease in the percentage of resistance to ciprofloxacin only (14.6% to 6.3%; P = 0.009) was found for isolates from patients with AECOPD (Table 3). No increase in resistance rates was observed for any of the antimicrobials studied for isolates from the two groups of patients between these years.
Table 3.
Annual percentages of resistant pneumococci in patients with pneumonia and AECOPD
| Antibiotic | No. (%) of resistant isolates from patients with pneumonia |
P value for trenda | No. (%) of resistant isolates from patients with AECOPD |
P value for trenda | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 (n = 97) | 2006 (n = 104) | 2007 (n = 104) | 2008 (n = 96) | 2005 (n = 96) | 2006 (n = 103) | 2007 (n = 104) | 2008 (n = 95) | |||
| Oral benzylpenicillin | 18 (18.6) | 17 (16.3) | 9 (8.7) | 10 (10.4) | 0.036 | 21 (21.9) | 18 (17.5) | 19 (18.3) | 23 (24.2) | |
| Parenteral benzylpenicillin | 0 (0) | 0 | 0 (0) | 0 (0) | 0 (0) | 2 (1.9) | 0 (0) | 2 (2.1) | ||
| Amoxicillin | 2 (2.1) | 4 (3.8) | 2 (1.9) | 0 (0) | 2 (2.1) | 1 (1) | 4 (3.8) | 3 (3.2) | ||
| Cefotaxime | 1 (1) | 2 (1.9) | 1 (1) | 0 (0) | 0 (0) | 5 (4.9) | 1 (1) | 3 (3.2) | ||
| Erythromycin | 15 (15.5) | 12 (11.5) | 11 (10.6) | 12 (12.5) | 17 (17.7) | 24 (23.3) | 23 (22.1) | 15 (15.8) | ||
| Azithromycin | 15 (15.5) | 12 (11.5) | 11 (10.6) | 12 (12.5) | 17 (17.7) | 24 (23.3) | 23 (22.1) | 15 (15.8) | ||
| Quinupristin-dalfopristin | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | ||
| Clindamycin | 9 (9.3) | 7 (6.7) | 10 (9.6) | 7 (7.3) | 15 (15.6) | 20 (19.4) | 19 (18.3) | 9 (9.5) | ||
| Tetracycline | 15 (15.5) | 7 (6.7) | 7 (6.7) | 8 (8.3) | 19 (19.8) | 21 (20.4) | 20 (19.2) | 10 (10.5) | ||
| Trimethoprim-sulfamethoxazole | 20 (20.6) | 26 (25) | 12 (11.5) | 10 (10.4) | 0.028 | 19 (19.8) | 20 (19.4) | 21 (20.2) | 19 (20) | |
| Chloramphenicol | 2 (2.1) | 0 (0) | 1 (1) | 1 (1) | 5 (5.2) | 3 (2.9) | 2 (1.9) | 0 (0) | ||
| Rifampin | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Ciprofloxacin | 10 (10.3) | 10 (9.6) | 4 (3.8) | 3 (3.1) | 0.015 | 14 (14.6) | 14 (13.6) | 4 (3.8) | 6 (6.3) | 0.009 |
| Levofloxacin | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 6 (6.3) | 6 (5.8) | 1 (1) | 3 (3.2) | ||
Only statistically significant P values are shown.
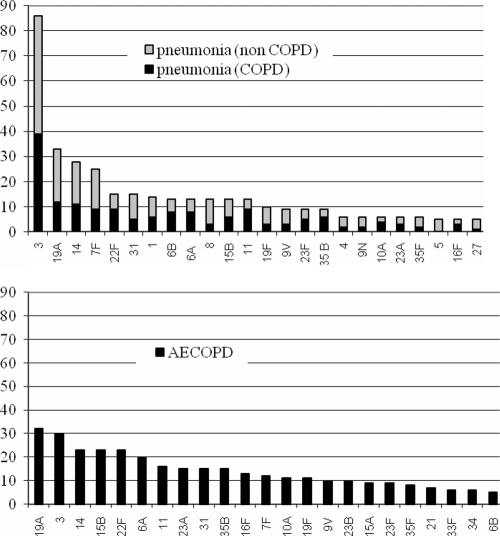
The dominant serotypes among pneumonia and AECOPD pneumococcal isolates partially overlapped, but the percentages of each serotype in the groups were dissimilar (Fig. 1 and Table 4). The most prevalent serotype in patients with pneumonia was serotype 3, which was isolated almost three times more frequently in pneumonia episodes (n = 86) than in AECOPD (n = 30). Serotypes 3, 1, and 7F were isolated more frequently from patients with pneumonia; in contrast, serotypes 15A, 21, 23A, and 33F and nontypeable isolates were isolated more frequently from patients with AECOPD. Interestingly, all isolates causing pneumonia could be typed, while 26 isolates (6.5%) causing AECOPD were nontypeable. Compared with the remaining serotypes causing AECOPD, these 26 nontypeable isolates showed non-statistically significant differences in penicillin resistance (7/26 [26.9%] isolates versus 73/372 [19.6%] isolates; P = 0.124) but were more resistant to erythromycin (9/26 [34.6%] isolates versus 70/372 [18.8%] isolates; P = 0.03).
Fig. 1.
Numbers of isolates of the most prevalent serotypes causing pneumonia in patients with and without COPD as an underlying disease and those causing AECOPD.
Table 4.
Pneumococcal serotype distributions of isolates from patients with pneumonia and AECOPD
| Serotype | No. (%) of isolates |
P value | |
|---|---|---|---|
| Pneumonia | AECOPD | ||
| 4 | 6 (1.5) | 2 (0.5) | |
| 6B | 13 (3.2) | 5 (1.3) | |
| 9V | 9 (2.2) | 10 (2.5) | |
| 14 | 28 (7.0) | 23 (5.8) | |
| 18C | 3 (0.7) | 4 (1) | |
| 19F | 10 (2.5) | 11 (2.8) | |
| 23F | 9 (2.2) | 9 (2.3) | |
| Total for PCV7 serotypes | 78 (19.5) | 64 (16.1) | |
| 1 | 14 (3.5) | 2 (0.3) | 0.004 |
| 5 | 5 (1.2) | 0 (0) | |
| 7F | 25 (6.2) | 12 (3) | 0.030 |
| 3 | 86 (21.4) | 30 (7.5) | <0.001 |
| 6Aa | 13 (3.2) | 20 (5) | |
| 19A | 33 (8.2) | 32 (8) | |
| Total for PCV13 serotypes | 254 (63.3) | 160 (40.2) | <0.001 |
| 2 | 0 (0) | 0 (0) | |
| 8 | 13 (3.2) | 3 (0.8) | |
| 9N | 6 (1.5) | 2 (0.5) | |
| 10A | 6 (1.5) | 11 (2.8) | |
| 11A | 2 (0.5) | 1 (0.3) | |
| 12F | 0 (0) | 1 (0.3) | |
| 15B | 13 (3.2) | 23 (5.8) | |
| 17F | 4 (1.0) | 3 (0.8) | |
| 20 | 1 (0.2) | 2 (0.5) | |
| 22F | 15 (3.7) | 23 (5.8) | |
| 33F | 0 (0) | 6 (1.5) | 0.015 |
| Total for PPV23 serotypes | 301 (75.1) | 215 (54) | <0.001 |
| 11b | 13 (3.2) | 16 (4.0) | |
| 15A | 0 (0) | 9 (2.3) | 0.002 |
| 16F | 5 (1.2) | 13 (3.3) | |
| 21 | 1 (0.2) | 7 (1.8) | 0.038 |
| 23A | 6 (1.5) | 15 (3.8) | 0.049 |
| 23B | 3 (0.7) | 10 (2.5) | |
| 27 | 5 (1.2) | 0 (0) | |
| 31 | 15 (3.7) | 15 (3.8) | |
| 34 | 1 (0.2) | 6 (1.5) | |
| 35 B | 9 (2.2) | 15 (3.8) | |
| 35F | 6 (1.5) | 8 (2) | |
| Nontypeable isolates | 0 (0) | 26 (6.5) | <0.001 |
| Otherc | 23 (5.7) | 23 (5.8) | |
| Total | 401 | 398 | |
Not included in the PPV23.
Serotypes 11B, 11C, 11D, or 11F.
Serotypes with fewer than five isolates.
Overall, no difference in the distributions of serotypes included in the 7-valent conjugate vaccine (PCV7 serotypes) was found between the two groups of patients, but the percentages of PCV13 and PPV23 serotypes were higher for patients with pneumonia (Table 4). Additionally, among patients with pneumonia, the prevalences of serotypes included in PCV7, PCV13, and PPV23 decreased throughout the 4 years of the study, from 31%, 71%, and 88% to 7%, 57%, and 66%, respectively (P < 0.05 for all trends).
No difference in the percentages of nonsusceptibility was observed between isolates of the same serotype obtained from patients with pneumonia or with AECOPD. Nonsusceptibility was associated mainly with certain serotypes, such as serotype 14, which showed percentages of oral penicillin nonsusceptibility above 75% and was frequently accompanied by resistance to other antimicrobials. The highest percentages of nonsusceptibility to erythromycin, clindamycin, and tetracycline were observed with serotype 19F. The most frequent serotypes, serotypes 3, 1, 7F, and 22F, were generally susceptible to most of the antimicrobials tested. In contrast, nontypeable isolates, which were isolated exclusively from patients with AECOPD, showed high percentages of nonsusceptibility.
Comparison of S. pneumoniae isolates from the same patient.
We compared the serotypes and PFGE patterns of two pneumococcal strains isolated from the same patient in two consecutive episodes of the following: (i) one episode of exacerbation and one of pneumonia or (ii) two consecutive episodes of exacerbation. The interval separating these episodes was between 2 and 12 months for both groups, with an average of 4.7 (95% confidence interval [95% CI], 3.6 to 5.8 months) months for the pneumococci isolated from exacerbation and pneumonia episodes and an average of 4.9 (95% CI, 4.2 to 5.7 months) months for the pneumococci isolated in two consecutive episodes of exacerbation.
There were 32 COPD patients who met the criterion of having a pneumococcal isolate from one episode of exacerbation and another from an episode of pneumonia in a period of less than 1 year. For 30 (93.75%) of these patients, the serotypes isolated in the exacerbation and the pneumonia episodes were different. For the remaining two patients, both isolates had the same serotype (serotypes 3 and 31) and the same antimicrobial susceptibility and PFGE patterns.
In the same period, 35 COPD patients with two pneumococcal strains isolated in two consecutive episodes of exacerbation were included. Among these, the same serotype was isolated in both episodes for 9 (25.71%) patients, a percentage much higher than the 6.25% (2/32 patients) incidence observed for patients with pneumonia and exacerbations (P = 0.047 by Fisher's exact test).
DISCUSSION
Streptococcus pneumoniae isolates obtained from AECOPD patients were more resistant to the antimicrobial agents generally used in the treatment of pneumococcal infections than those isolated from patients with pneumonia. Statistically significant differences were found in susceptibilities to penicillin, macrolides, tetracycline, and levofloxacin and in the prevalence of multidrug-resistant isolates. This result was expected, as patients with COPD usually receive antimicrobial treatments because of frequent acute bacterial exacerbations and the association between antibiotic consumption and antimicrobial resistance has been demonstrated widely (2, 23, 26).
A striking finding was that for patients with pneumonia, no difference in the antimicrobial resistance of isolates from patients with or without COPD as the underlying disease was observed. We could have expected a higher frequency of nonsusceptible pneumococci in COPD patients with pneumonia. In fact, gender, previous antibiotic treatment, and cardiopathy were the only differences between patients with pneumonia with and without COPD. Another two studies performed in Spain found no differences in the antimicrobial resistance of pneumococci causing pneumonia in COPD and non-COPD patients (7, 31).
Pneumococcal serotypes causing pneumonia differed from those causing AECOPD. The different serotype distributions in patients with AECOPD and pneumonia explain the differences in antimicrobial resistance, as no difference in antimicrobial resistance was observed for a given serotype, regardless of the origin of the isolate (pneumonia or AECOPD). In contrast, the distributions of serotypes causing pneumonia did not differ in patients with and without COPD, suggesting that the different serotype distributions seem to be related to the disease caused by the serotype rather than to the patient's history of COPD. Our results suggest that some serotypes are less “alveoli invasive” (they have a lower capacity to reach the alveoli) and thus cause pneumonia less frequently than AECOPD.
To determine whether the differences in the pneumococci involved in pneumonia and AECOPD observed in the two groups of patients could also be found in individual patients, we compared the isolates from the two closest episodes of AECOPD and pneumonia that occurred within a short interval in the same patient. Typically, the serotype of the AECOPD episode was unrelated to that of the pneumonia episode. In contrast, we observed that different episodes of AECOPD were more frequently caused by the same serotype.
Pneumonia can be considered a more disseminated or invasive disease (affecting alveoli and bronchioles) than AECOPD, which is characterized by inflammation that normally affects only the bronchi. Several studies have shown that isolates causing invasive disease are usually more susceptible to antimicrobial agents than noninvasive isolates (11, 13, 15). It is not clear whether this apparent “fitness cost of resistance” decreases the ability of resistant isolates to cause invasive—or at least more severe—disease or whether the development of resistance is caused by a greater ability to remain commensal. The results of this study suggest that it is due to the latter possibility, that is, that certain serotypes have a greater capacity to remain as commensals in COPD patients during periods without clinical manifestations of infection, thus facilitating their participation in new episodes of AECOPD. This persistence of isolates in the airways of COPD patients during cycles of antimicrobial treatment of acute bacterial exacerbations is known to favor the development of resistance (2, 23). An inverse correlation between the prevalence of carriage and invasiveness has been reported (4, 5, 27, 29). In the present study, serotypes 1 and 7F, two commonly invasive serotypes, were isolated more frequently from patients with pneumonia. In contrast, serotypes rarely responsible for invasive infection, or typical colonizers such as serotype 15A, as well as nontypeable isolates, were isolated more frequently from patients with AECOPD. Possibly, the pneumococci that remain in the bronchi of patients with COPD have greater difficulty in producing pneumonia. Apart from the decreased ability of some pneumococcal serotypes to evade the local innate immune response, the greater immune stimulus produced by the capsular polysaccharide in causing the pneumonia would hamper the continued presence of the same serotype in the bronchial tree after the disease has been overcome. One limitation of our study is that medical records were collected retrospectively and some spirometric data were not available, making it impossible to establish a possible relationship between specific serotypes and GOLD (NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease) (21) status.
Several studies of pneumonia found that serotype 3, the most frequent serotype causing pneumonia in Spain, was very often associated with severe disease in the elderly (3, 12, 14). Although serotype 3 was isolated very frequently in the present study, both from patients with pneumonia and from those with AECOPD, it was isolated more often from patients with pneumonia. Among the main problems hampering the choice of an appropriate empirical antibiotic treatment of S. pneumoniae infections are the current high levels of antimicrobial resistance. In the present study, overall levels of nonsusceptibility to oral benzylpenicillin (13.4%), amoxicillin (2%), cefotaxime (1%), erythromycin (12%), and levofloxacin (0.8%) in S. pneumoniae isolates from elderly patients with pneumonia were lower than the percentages of resistance described in other studies of pneumococcal pneumonia performed in Spain (7, 14, 30, 31). Additionally, and in agreement with recent studies (10, 24, 31), the trend toward a decrease in nonsusceptible S. pneumoniae isolates seems to be continuing and is associated with a decrease in episodes of pneumonia caused by PCV7 serotypes. PCV7 childhood vaccination in our region seems to have had a beneficial effect in diminishing the incidence of vaccine serotypes (22). The role of PPV23 vaccination of people over 64 years of age, introduced in our region in 2007, probably had little effect due to the limited implementation period.
Considering the new breakpoints for parenteral penicillin for nonmeningeal infections (8), none of the isolates causing pneumonia were resistant and only 1% of the isolates obtained during AECOPD episodes showed resistance. Among beta-lactam antimicrobial agents, amoxicillin, cefotaxime, and ertapenem demonstrated good in vitro activity. In our study, none of the strains were resistant to ertapenem, and the MICs obtained for this antimicrobial correlated with those obtained for amoxicillin, although the intrinsic activity of ertapenem was higher. Ertapenem is a bactericidal antibiotic that has shown efficacy against elderly human pneumonia (19) and in a murine model of acute pneumonia (9). Given that only 8 of 401 (2%) isolates showed an ertapenem MIC of >0.25 μg/ml, a daily dose of 1 g a day would be adequate (6).
Macrolide nonsusceptibility levels were similar to those for oral benzylpenicillin and were associated with both the PCV7 serotypes 19F and 14 and the non-PCV7 serotypes 19A and 15B. However, unlike the case for oral benzylpenicillin, no decreasing trend in macrolide resistance was observed throughout the study period. The decrease in the prevalence of PCV7 macrolide-resistant serotypes was balanced by the increase of macrolide-resistant non-PCV7 serotypes.
Since the introduction of fluoroquinolones for the treatment of respiratory infections, the increase in fluoroquinolone resistance has been linked to an increase in its use (1, 23). In our study, levofloxacin resistance levels were very low, but we also observed a significant decrease in ciprofloxacin-resistant isolates from patients with pneumonia from 2005 to 2008, which, as in the case of penicillin, was associated with a decrease in the incidence of serotype 14 infections.
In conclusion, we observed differences in serotype distribution and drug resistance among S. pneumoniae isolates causing AECOPD and pneumonia over a 4-year period in elderly patients of the same age living in the same region. Further investigations will be needed to determine the extent to which the pneumococcal capsular type (serotype) plays a role in the clinical manifestations of the disease and to identify its relationship with host immunity.
ACKNOWLEDGMENTS
This work was supported in part by a grant from Merck Sharp & Dome, Spain, and by grant GIU09-59 from the University of the Basque Country, UPV/EHU, Spain.
E.P.-T. has received research grants from Merck Sharp & Dome, Pfizer, and GlaxoSmithKline (GSK) and lecture sponsorships from Pfizer and GSK. All other authors report no conflicts of interest.
Footnotes
Published ahead of print on 14 March 2011.
REFERENCES
- 1. Adam H. J., Hoban D. J., Gin A. S., Zhanel G. G. 2009. Association between fluoroquinolone usage and a dramatic rise in ciprofloxacin-resistant Streptococcus pneumoniae in Canada, 1997–2006. Int. J. Antimicrob. Agents 34:82–85 [DOI] [PubMed] [Google Scholar]
- 2. Albrich W. C., Monnet D. L., Harbarth S. 2004. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg. Infect. Dis. 10:514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aspa J., et al. 2004. Drug-resistant pneumococcal pneumonia: clinical relevance and related factors. Clin. Infect. Dis. 38:787–798 [DOI] [PubMed] [Google Scholar]
- 4. Brueggemann A. B., et al. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424–1432 [DOI] [PubMed] [Google Scholar]
- 5. Brueggemann A. B., et al. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203–1211 [DOI] [PubMed] [Google Scholar]
- 6. Burkhardt O., et al. 2010. Underdosing of ertapenem in critically ill patients with pneumonia confirmed by Monte Carlo simulations. Int. J. Antimicrob. Agents 35:96–97 [DOI] [PubMed] [Google Scholar]
- 7. Calbo E., et al. 2009. Bacteraemic pneumococcal pneumonia in COPD patients: better outcomes than expected. Eur. J. Clin. Microbiol. Infect. Dis. 28:971–976 [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute (CLSI) 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S20 CLSI, Wayne, PA [Google Scholar]
- 9. de Azavedo J. C., et al. 2007. Antipneumococcal activity of ertapenem compared with gatifloxacin in a temperature-sensitive murine model of acute pneumonia. J. Chemother. 19:392–397 [DOI] [PubMed] [Google Scholar]
- 10. Fenoll A., et al. 2009. Temporal trends of invasive Streptococcus pneumoniae serotypes and antimicrobial resistance patterns in Spain from 1979 to 2007. J. Clin. Microbiol. 47:1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fenoll A., Jado I., Vicioso D., Perez A., Casal J. 1998. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996). J. Clin. Microbiol. 36:3447–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Henriques B., et al. 2000. Molecular epidemiology of Streptococcus pneumoniae causing invasive disease in 5 countries. J. Infect. Dis. 182:833–839 [DOI] [PubMed] [Google Scholar]
- 13. Lambertsen L. M., Harboe Z. B., Konradsen H. B., Christensen J. J., Hammerum A. M. 2010. Non-invasive erythromycin-resistant pneumococcal isolates are more often non-susceptible to more antimicrobial agents than invasive isolates. Int. J. Antimicrob. Agents 35:72–75 [DOI] [PubMed] [Google Scholar]
- 14. Lujan M., et al. 2010. Influence of pneumococcal serotype group on outcome in adults with bacteraemic pneumonia. Eur. Respir. J. 36:1073–1079 [DOI] [PubMed] [Google Scholar]
- 15. Marco F., Bouza E., Garcia-de-Lomas J., Aguilar L. 2000. Streptococcus pneumoniae in community-acquired respiratory tract infections in Spain: the impact of serotype and geographical, seasonal and clinical factors on its susceptibility to the most commonly prescribed antibiotics. The Spanish Surveillance Group for Respiratory Pathogens. J. Antimicrob. Chemother. 46:557–564 [DOI] [PubMed] [Google Scholar]
- 16. Marimón J. M., Iglesias L., Vicente D., Pérez-Trallero E. 2003. Molecular characterization of erythromycin-resistant clinical isolates of the four major antimicrobial-resistant Spanish clones of Streptococcus pneumoniae (Spain23F-1, Spain6B-2, Spain9V-3, and Spain14-5). Microb. Drug Resist. 9:133–137 [DOI] [PubMed] [Google Scholar]
- 17. Marimón J. M., et al. 2010. Antibody microarray typing, a novel technique for Streptococcus pneumoniae serotyping. J. Microbiol. Methods 80:274–280 [DOI] [PubMed] [Google Scholar]
- 18. Mathur S. K., Meyer K. C. 2009. Lung infections and aging, p. 95–112 In Percival S. L. (ed.), Microbiology and aging. Springer, New York, NY [Google Scholar]
- 19. Murcia J. M., et al. 2009. Clinical response to ertapenem in severe community-acquired pneumonia: a retrospective series in an elderly population. Clin. Microbiol. Infect. 15:1046–1050 [DOI] [PubMed] [Google Scholar]
- 20. Patel I. S., et al. 2002. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 57:759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pauwels R. A., et al. 2001. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 163:1256–1276 [DOI] [PubMed] [Google Scholar]
- 22. Pérez-Trallero E., Marimón J. M., Ercibengoa M., Vicente D., Pérez-Yarza E. G. 2009. Invasive Streptococcus pneumoniae infections in children and older adults in the north of Spain before and after the introduction of the heptavalent pneumococcal conjugate vaccine. Eur. J. Clin. Microbiol. Infect. Dis. 28:731–738 [DOI] [PubMed] [Google Scholar]
- 23. Pérez-Trallero E., Marimón J. M., González A., Ercibengoa M., Larruskain J. 2005. In vivo development of high-level fluoroquinolone resistance in Streptococcus pneumoniae in chronic obstructive pulmonary disease. Clin. Infect. Dis. 41:560–564 [DOI] [PubMed] [Google Scholar]
- 24. Pérez-Trallero E., et al. 2010. Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996–1997 to 2006–2007). Antimicrob. Agents Chemother. 54:2953–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosell A., et al. 2005. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch. Intern. Med. 165:891–897 [DOI] [PubMed] [Google Scholar]
- 26. Ruhe J. J., Hasbun R. 2003. Streptococcus pneumoniae bacteremia: duration of previous antibiotic use and association with penicillin resistance. Clin. Infect. Dis. 36:1132–1138 [DOI] [PubMed] [Google Scholar]
- 27. Sandgren A., et al. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785–796 [DOI] [PubMed] [Google Scholar]
- 28. Sethi S., et al. 2007. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 176:356–361 [DOI] [PubMed] [Google Scholar]
- 29. Sjostrom K., et al. 2006. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin. Infect. Dis. 42:451–459 [DOI] [PubMed] [Google Scholar]
- 30. Valles X., et al. 2006. Hospitalized community-acquired pneumonia due to Streptococcus pneumoniae: has resistance to antibiotics decreased? Chest 130:800–806 [DOI] [PubMed] [Google Scholar]
- 31. Vila-Corcoles A., et al. 2009. Drug-resistance in Streptococcus pneumoniae isolates among Spanish middle aged and older adults with community-acquired pneumonia. BMC Infect. Dis. 9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]